Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations
Abstract
:1. Introduction
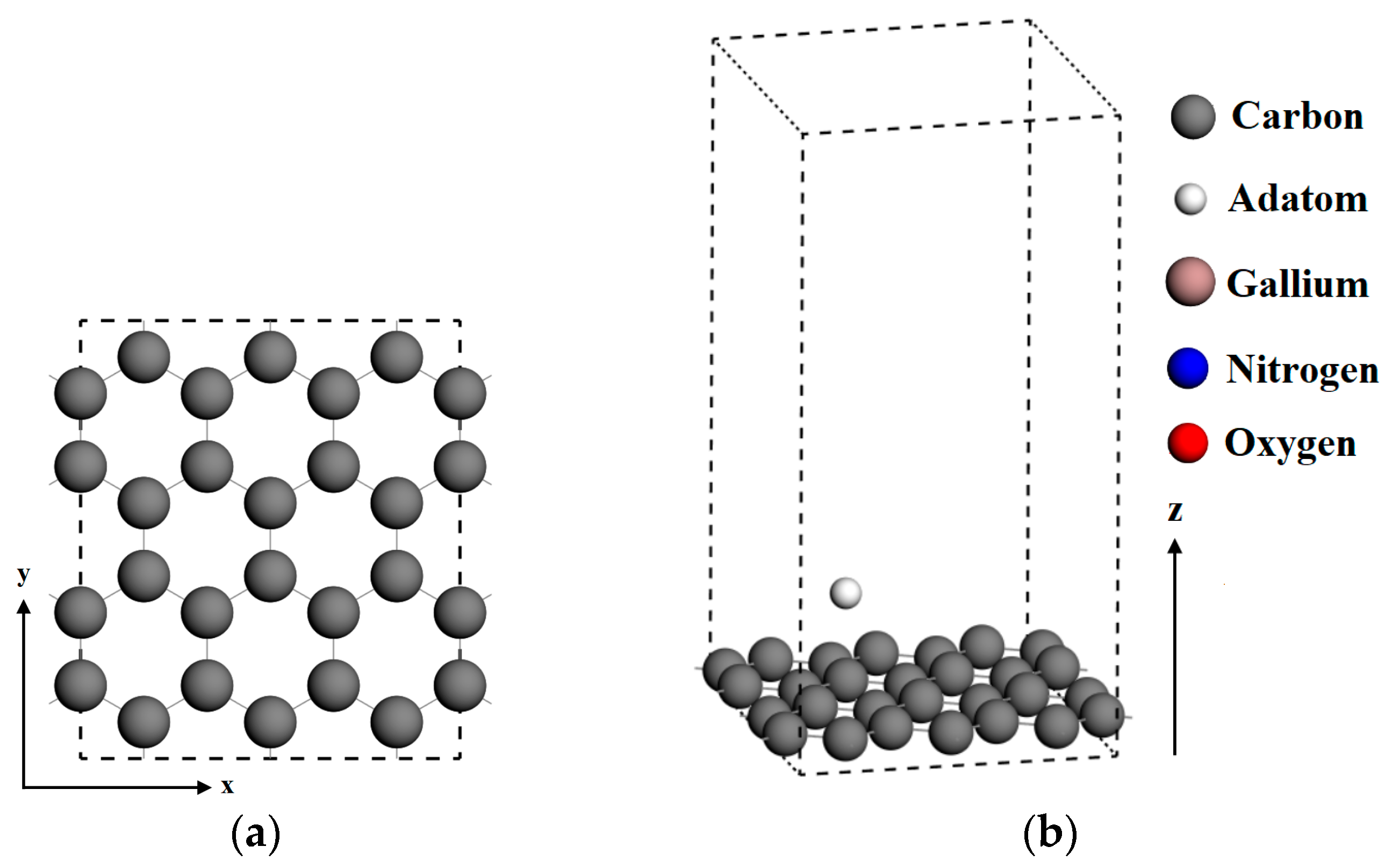
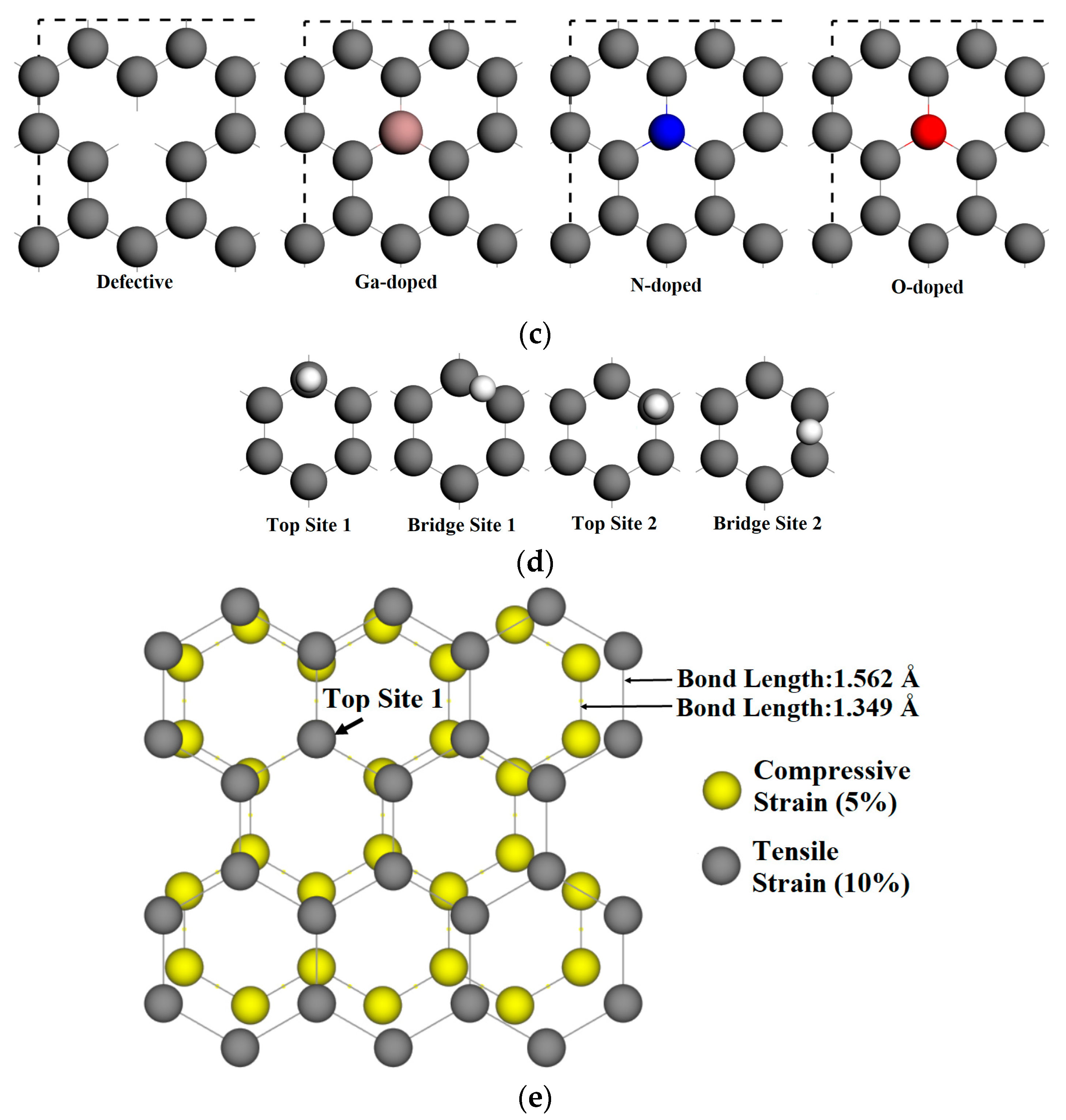
2. Method and Model
3. Results and Discussion
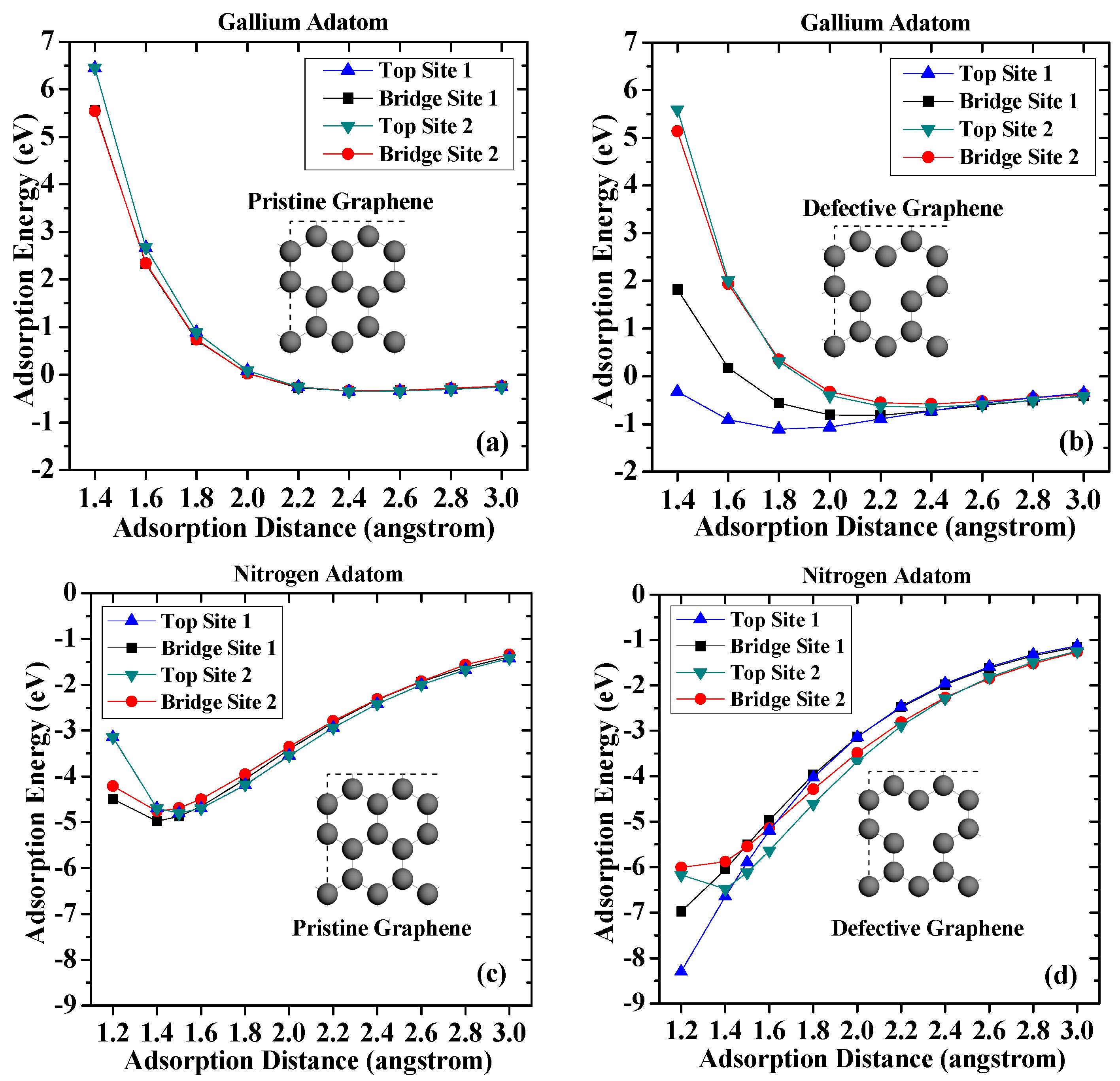
3.1. Adsorption on Unstrained Pristine and Vacancy-Defected Graphene
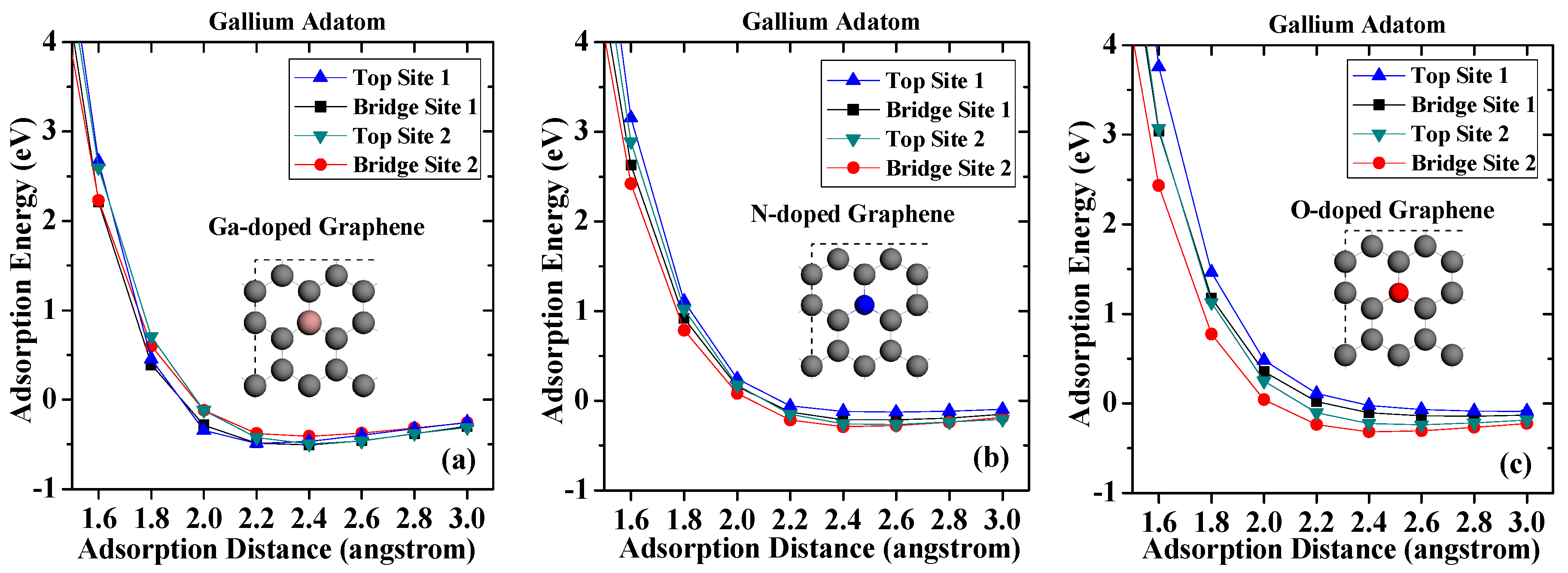
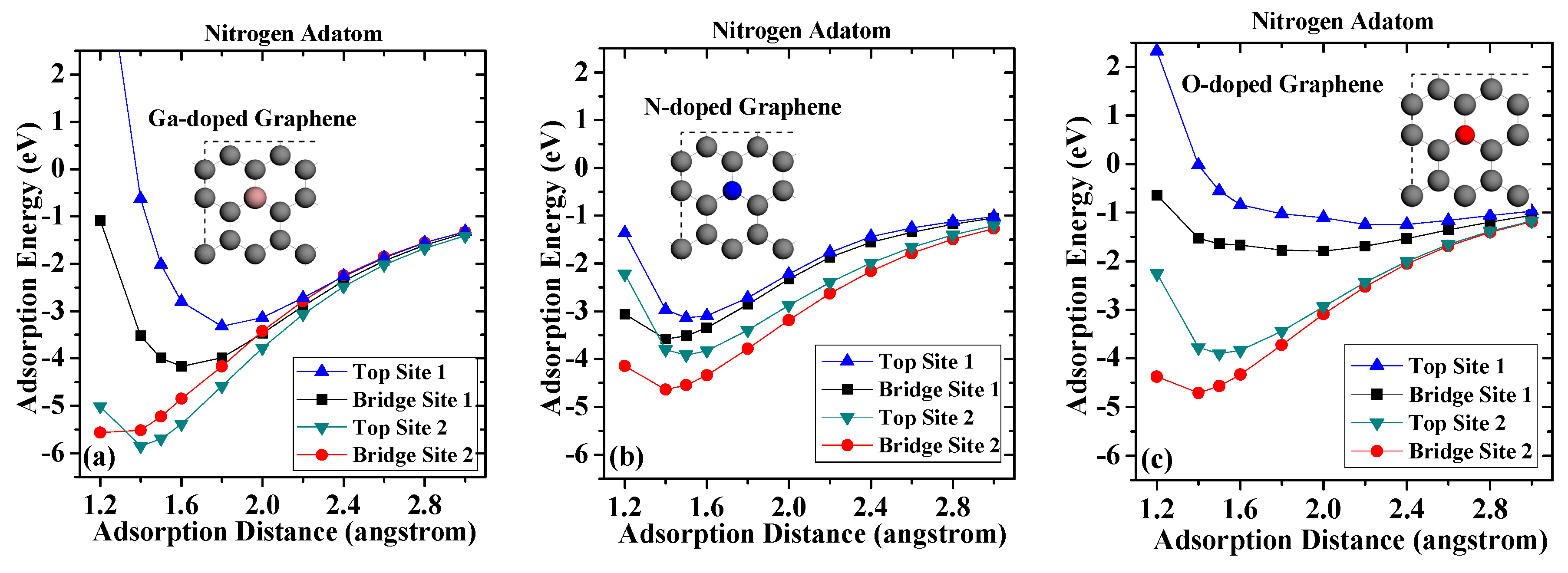
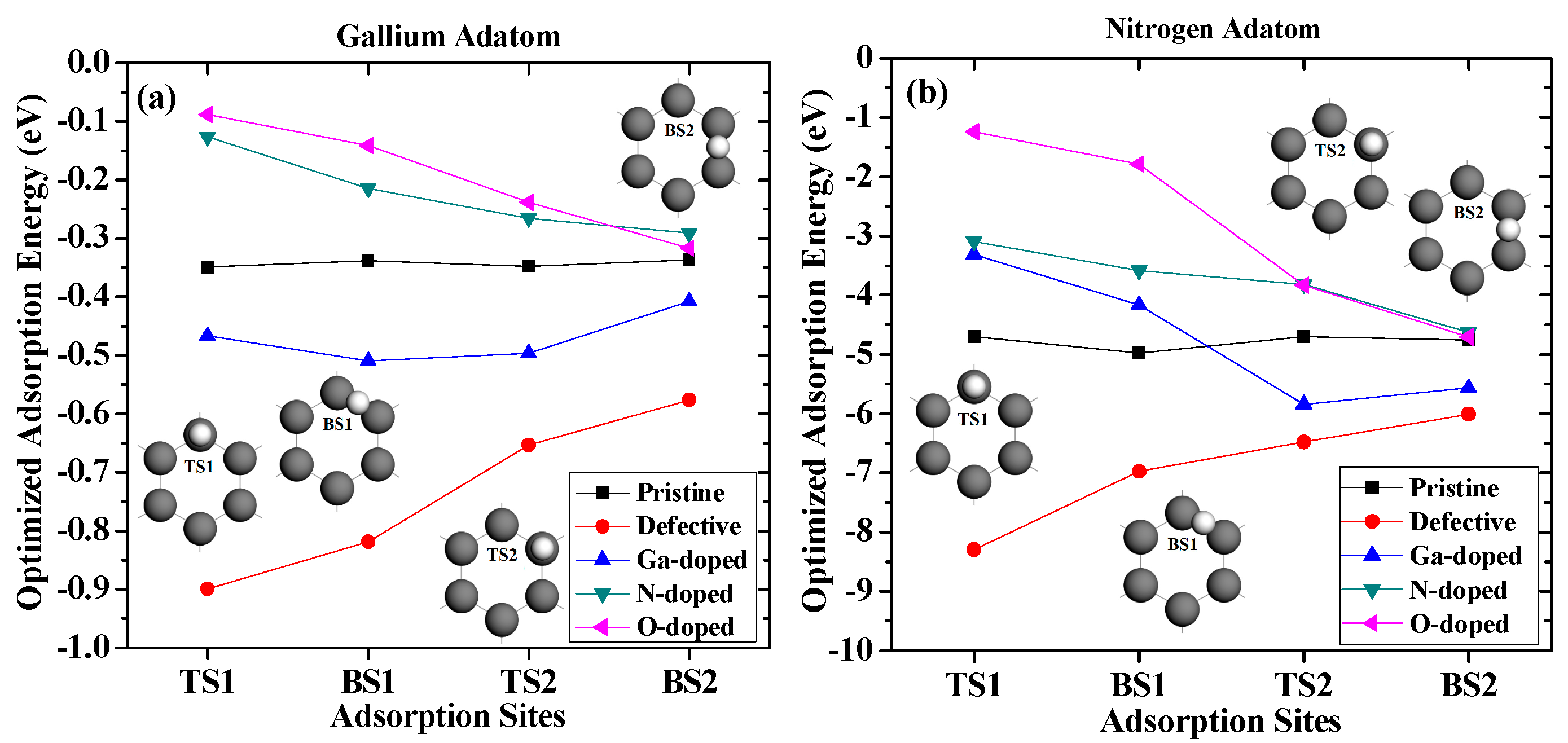
3.2. Adsorption on Doped Graphene
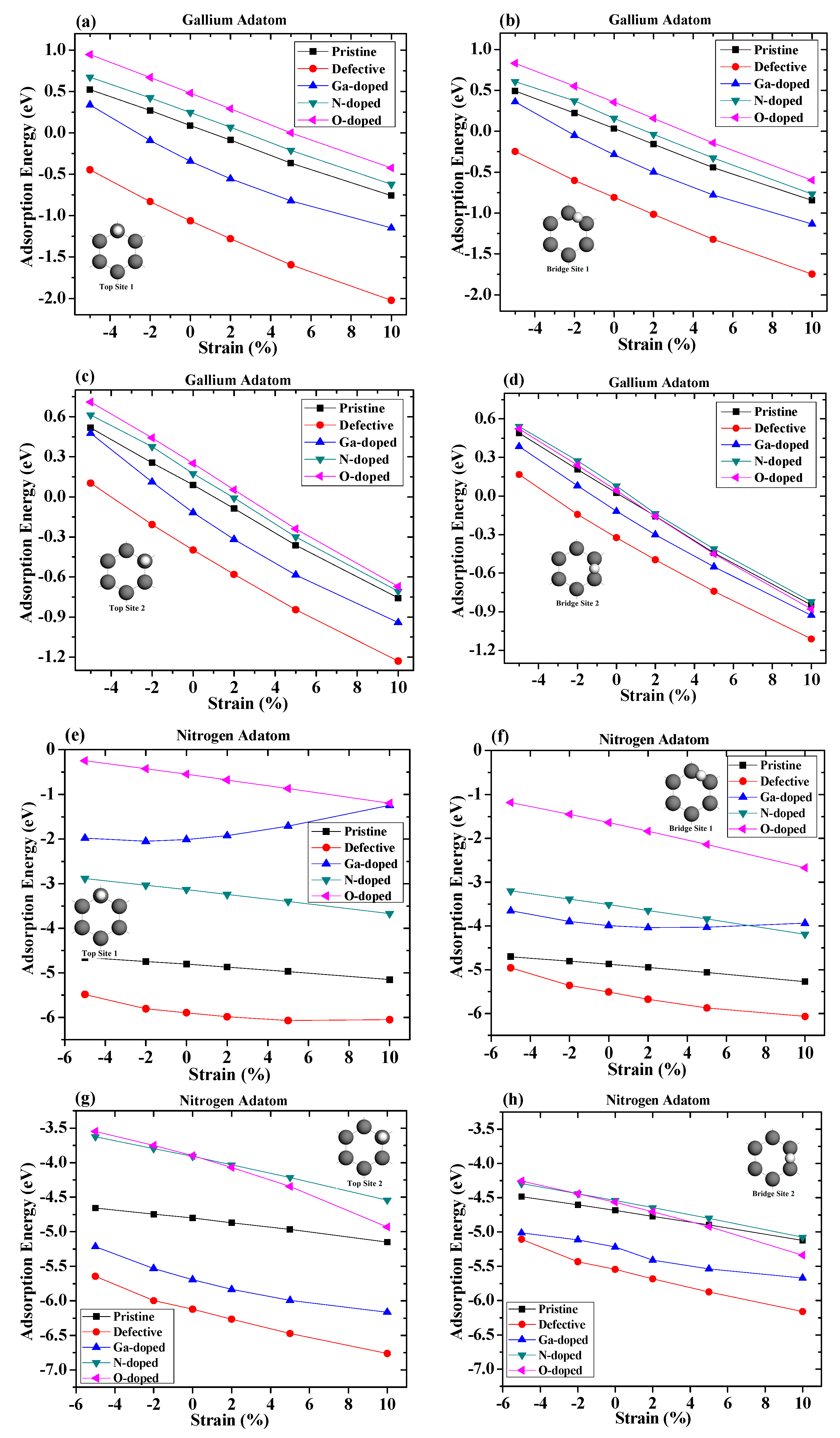
3.3. Adsorption on Strained Graphene
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Lee, C.-H.; Yi, G.-C. Transferable GaN layers grown on ZnO-coated graphene layers for optoelectronic devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, X. Effect of v-pits embedded InGaN/GaN superlattices on optical and electrical properties of GaN-based green light-emitting diodes. Phys. Status Solidi (A) 2017, 214. [Google Scholar] [CrossRef]
- Agata, B.; Grzegorz, M.; Czesław, S.; Ewa, G.; Przemysław, W.; Irina, M.; Robert, C.; Tadek, S.; Piotr, P. Influence of the growth method on degradation of InGaN laser diodes. Appl. Phys. Express 2017, 10, 091001. [Google Scholar]
- Cheng, Z.; Zhao, Z.; Ke, H.; Liu, G.; Dong, Z.; Gao, S. High efficiency broadband GaN HEMT power amplifier based on three-frequency point matching method. Microw. Opt. Technol. Lett. 2017, 59, 1850–1855. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, S.; Liu, X.; Gao, Y.; Gui, C.; Liu, S. Effects of GaN/AlGaN/sputtered AlN nucleation layers on performance of GaN-based ultraviolet light-emitting diodes. Sci. Rep. 2017, 7, 44627. [Google Scholar] [CrossRef] [PubMed]
- Al Balushi, Z.Y.; Miyagi, T.; Lin, Y.-C.; Wang, K.; Calderin, L.; Bhimanapati, G.; Redwing, J.M.; Robinson, J.A. The impact of graphene properties on GaN and AlN nucleation. Surf. Sci. 2015, 634, 81–88. [Google Scholar] [CrossRef]
- Kumaresan, V.; Largeau, L.; Madouri, A.; Glas, F.; Zhang, H.; Oehler, F.; Cavanna, A.; Babichev, A.; Travers, L.; Gogneau, N.; et al. Epitaxy of GaN nanowires on graphene. Nano Lett. 2016, 16, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bayram, C.; Park, H.; Cheng, C.-W.; Dimitrakopoulos, C.; Ott, J.A.; Reuter, K.B.; Bedell, S.W.; Sadana, D.K. Principle of direct van der Waals epitaxy of single-crystalline films on epitaxial graphene. Nat. Commun. 2014, 5, 4836. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Kim, Y.-J.; Hong, Y.J.; Jeon, S.-R.; Bae, S.; Hong, B.H.; Yi, G.-C. Flexible inorganic nanostructure light-emitting diodes fabricated on graphene films. Adv. Mater. 2011, 23, 4614–4619. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Beak, H.; Tchoe, Y.; Oh, H.; Yoo, H.; Kim, M.; Yi, G.-C. Growth and characterizations of GaN micro-rods on graphene films for flexible light emitting diodes. APL Mater. 2014, 2, 092512. [Google Scholar] [CrossRef]
- Gupta, P.; Rahman, A.A.; Hatui, N.; Gokhale, M.R.; Deshmukh, M.M.; Bhattacharya, A. Movpe growth of semipolar III-nitride semiconductors on CVD graphene. J. Cryst. Growth 2013, 372, 105–108. [Google Scholar] [CrossRef]
- Ohta, J.; Shon, J.W.; Ueno, K.; Kobayashi, A.; Fujioka, H. Gan-based light-emitting diodes with graphene buffers for their application to large-area flexible devices. IEICE Trans. Electron. 2017, E100.C, 161–165. [Google Scholar] [CrossRef]
- Shunyu, H.; Yu, X.; Lin, Q.; Zongyao, L.; Bing, C.; Chinhua, W.; Jicai, Z.; Jianfeng, W.; Ke, X. Growth of low-threading-dislocation-density GaN on graphene by hydride vapor phase epitaxy. Jpn. J. Appl. Phys. 2017, 56, 030308. [Google Scholar]
- Hwang, S.W.; Choi, S.-H. Successful fabrication of GaN epitaxial layer on non-catalytically-grown graphene. Bull. Korean Chem. Soc. 2016, 37, 1004–1009. [Google Scholar] [CrossRef]
- Heilmann, M.; Munshi, A.M.; Sarau, G.; Göbelt, M.; Tessarek, C.; Fauske, V.T.; van Helvoort, A.T.J.; Yang, J.; Latzel, M.; Hoffmann, B.; et al. Vertically oriented growth of GaN nanorods on Si using graphene as an atomically thin buffer layer. Nano Lett. 2016, 16, 3524–3532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Jin, N.; Han, J.; Wang, H.; Zhu, X.; Ge, Q. A DFT study of oxygen reduction reaction mechanism over O-doped graphene-supported Pt4, Pt3Fe and Pt3V alloy catalysts. Int. J. Hydrogen Energy 2015, 40, 5126–5134. [Google Scholar] [CrossRef]
- Guinea, F.; Katsnelson, M.I.; Geim, A.K. Energy gaps and a zero-field quantum hall effect in graphene by strain engineering. Nat. Phys. 2009, 6, 30–33. [Google Scholar] [CrossRef]
- Yang, M.; Nurbawono, A.; Zhang, C.; Wu, R.; Feng, Y.; Ariando. Manipulating absorption and diffusion of H atom on graphene by mechanical strain. AIP Adv. 2011, 1, 032109. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Wlazło, M.; Majewski, J. First principles study of gas adsorption dynamics on pristine and defected graphene. Acta Phys. Pol. A 2016, 129. [Google Scholar] [CrossRef]
- Yan, H.; Gan, Z.; Song, X.; Lv, Q.; Xu, J.; Liu, S. Car–Parrinello simulation of initial growth stage of gallium nitride on carbon nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2009, 41, 1143–1146. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef]
- Bungaro, C.; Rapcewicz, K.; Bernholc, J. Ab initio phonon dispersions of wurtzite AlN, GaN, and InN. Phys. Rev. B 2000, 61, 6720–6725. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Krasheninnikov, A.V.; Lehtinen, P.O.; Foster, A.S.; Nieminen, R.M. Bending the rules: Contrasting vacancy energetics and migration in graphite and carbon nanotubes. Chem. Phys. Lett. 2006, 418, 132–136. [Google Scholar] [CrossRef]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E. Experimental review of graphene. ISRN Condens. Matter Phys. 2012, 2012, 501686. [Google Scholar] [CrossRef]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217–1224. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Su, X.; Yong, Y.; Li, X. Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J. Phys. Chem. Solids 2017, 109, 40–45. [Google Scholar] [CrossRef]
- Huang, L.F.; Ni, M.Y.; Zhang, G.R.; Zhou, W.H.; Li, Y.G.; Zheng, X.H.; Zeng, Z. Modulation of the thermodynamic, kinetic, and magnetic properties of the hydrogen monomer on graphene by charge doping. J. Chem. Phys. 2011, 135, 064705. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, M.; Yi, C.; Suvorova, A.; Rubanov, S.; Kim, T.-H.; Giangregorio, M.M.; Jiao, W.; Bergmair, I.; Bruno, G.; Brown, A.S. Demonstrating the capability of the high-performance plasmonic gallium–graphene couple. ACS Nano 2014, 8, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mavrikakis, M. Adsorption and dissociation of O2 on gold surfaces: Effect of steps and strain. J. Phys. Chem. B 2003, 107, 9298–9307. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Ku, P.-C.; Gan, Z.-Y.; Liu, S.; Li, P. Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations. Crystals 2018, 8, 58. https://doi.org/10.3390/cryst8020058
Yan H, Ku P-C, Gan Z-Y, Liu S, Li P. Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations. Crystals. 2018; 8(2):58. https://doi.org/10.3390/cryst8020058
Chicago/Turabian StyleYan, Han, Pei-Cheng Ku, Zhi-Yin Gan, Sheng Liu, and Peng Li. 2018. "Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations" Crystals 8, no. 2: 58. https://doi.org/10.3390/cryst8020058




