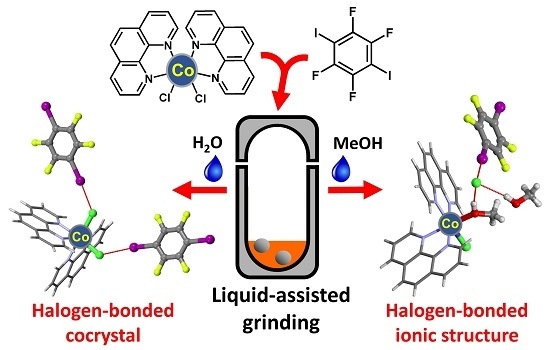
The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding
Abstract
1. Introduction
2. Results and Discussion
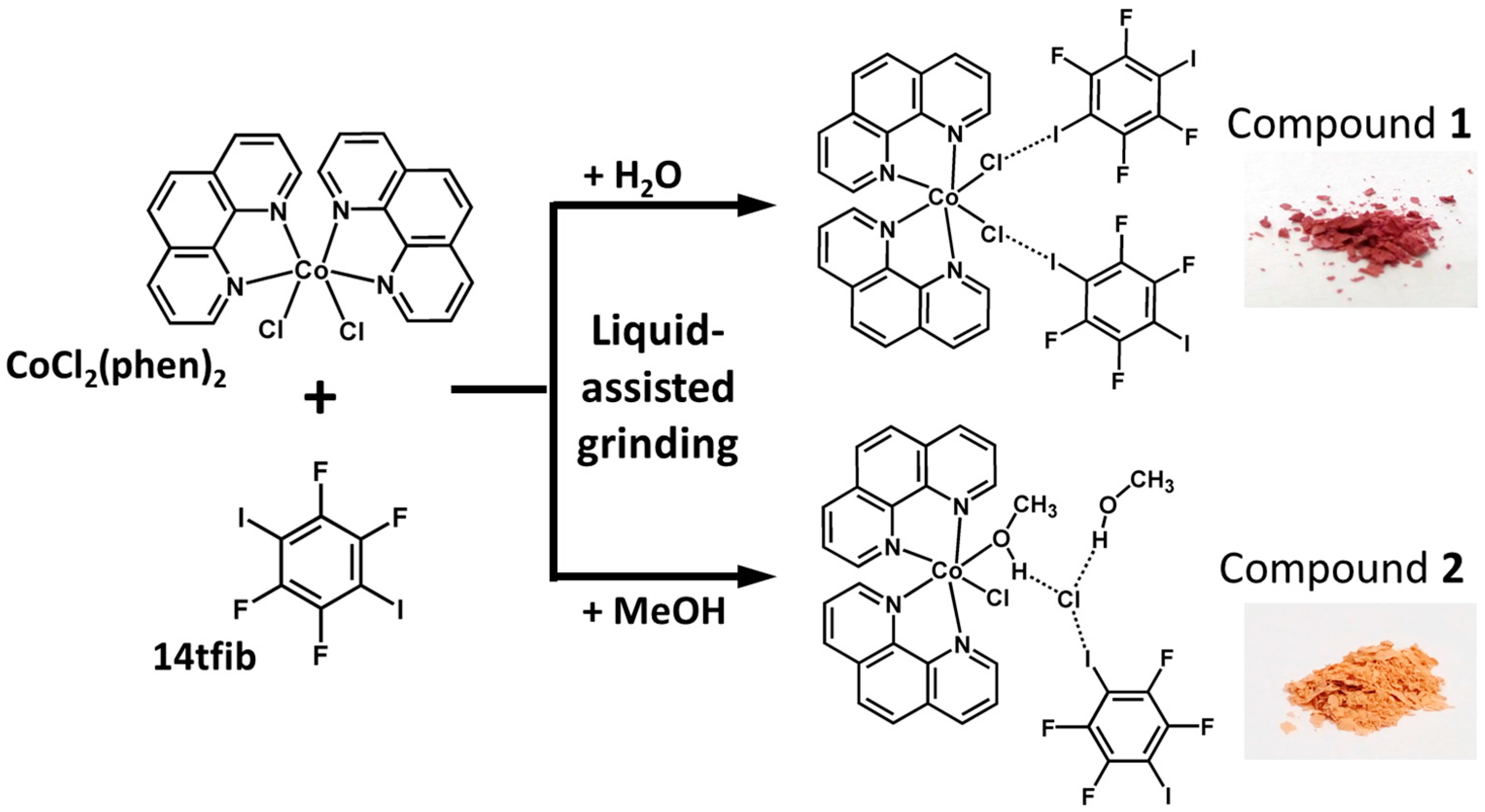
2.1. Syntheses
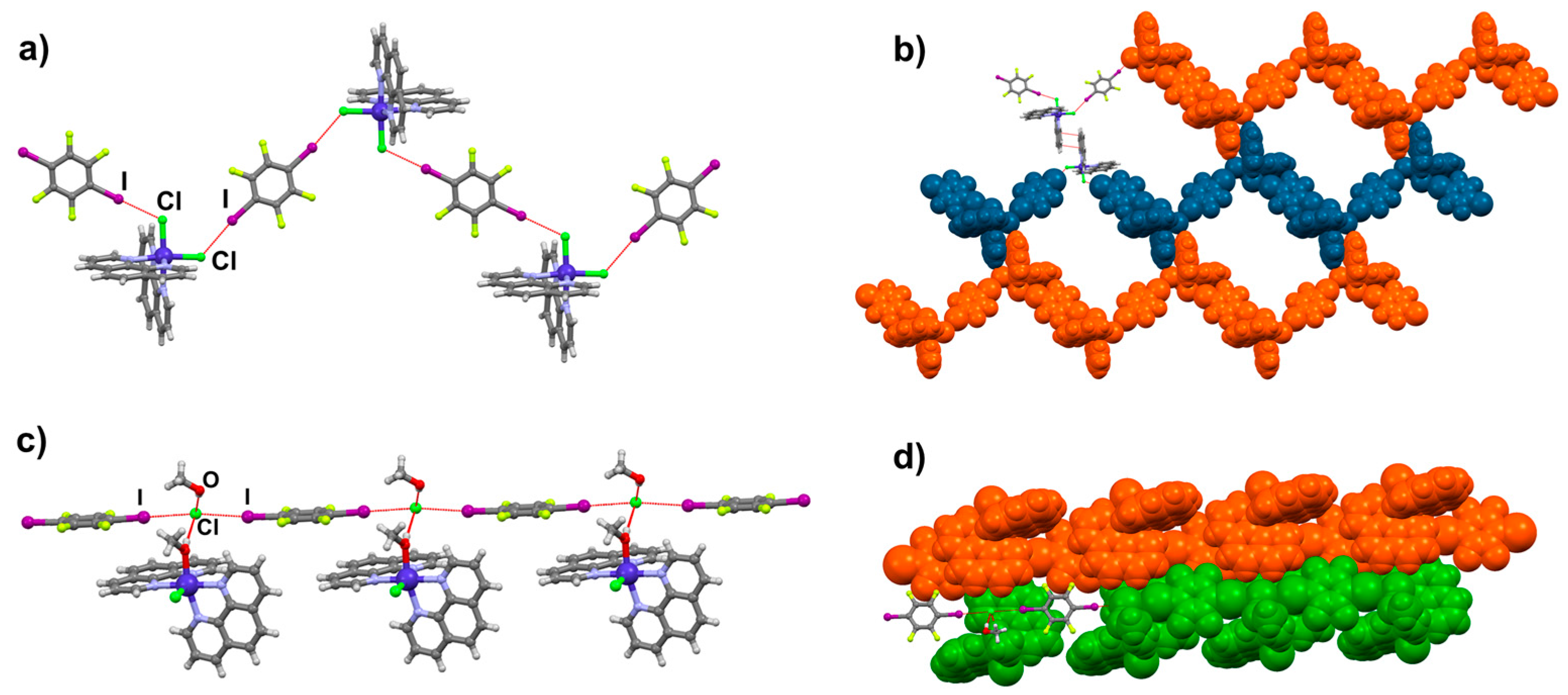
2.2. Structural Analysis
3. Materials and Methods
3.1. Synthesis of Complexes
3.2. Mechanochemical Synthesis of 1
3.3. Mechanochemical Synthesis of 2
3.4. Crystallization of 1 and 2
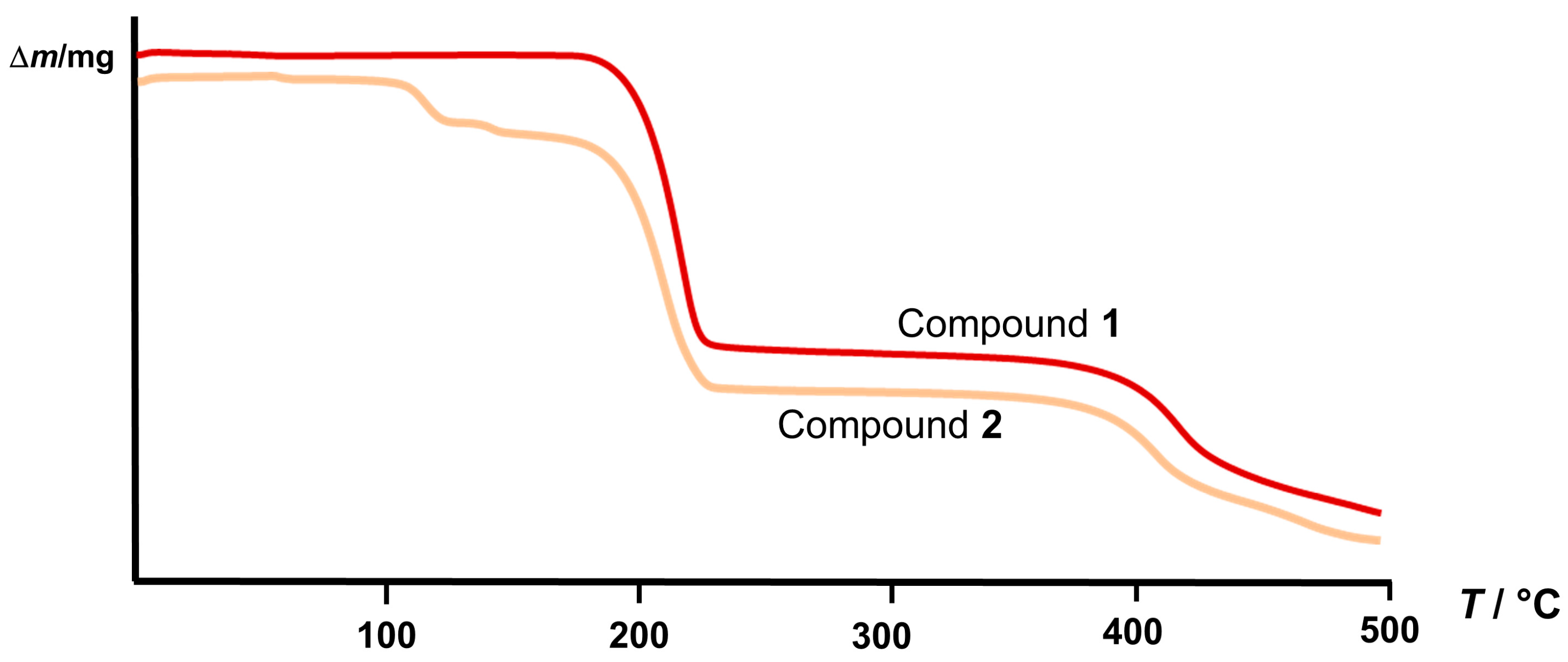
3.5. Thermal Analysis
3.6. Single Crystal X-ray Diffraction Experiments
3.7. Powder X-ray Diffraction Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimägi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Priimägi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Fourmigué, M. Halogen bonding: Recent advances. Curr. Opin. Solid State Mater. Sci. 2009, 13, 36–45. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Isostructural Materials Achieved by Using Structurally Equivalent Donors and Acceptors in Halogen-Bonded Cocrystals. Chem. Eur. J. 2008, 14, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Stilinović, V.; Horvat, G.; Hrenar, T.; Nemec, V.; Cinčić, D. Halogen and Hydrogen Bonding between (N-Halogeno)-succinimides and Pyridine Derivatives in Solution, the Solid State and In Silico. Chem. Eur. J. 2017, 22, 5244–5257. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Gamekkanda, J.C.; Sinha, A.S.; Desper, J.; Ðaković, M.; Aakeröy, C.B. The Role of Halogen Bonding in Controlling Assembly and Organization of Cu (II)-Acac Based Coordination Complexes. Crystals 2017, 7, 226. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Schultheiss, N.; Desper, J.; Moore, C. Attempted assembly of discrete coordination complexes into 1-D chains using halogen bonding or halogen⋯halogen interactions. CrystEngComm 2007, 9, 421–426. [Google Scholar] [CrossRef]
- Johnson, M.T.; Džolić, Z.; Cetina, M.; Wendt, O.F.; Ohrstrom, L.; Rissanen, K. Neutral Organometallic Halogen Bond Acceptors: Halogen Bonding in Complexes of PCPPdX (X = Cl, Br, I) with Iodine (I2), 1,4-Diiodotetrafluorobenzene (F4DIBz), and 1,4-Diiodooctafluorobutane (F8DIBu). Cryst. Growth Des. 2012, 12, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, J.-C.; Potts, K.P.; Bushuyev, O.S.; Topić, F.; Huskić, I.; Rissanen, K.; Barrett, C.J.; Friščić, T. Assembly and dichroism of a four-component halogen-bonded metal-organic cocrystal salt solvate involving dicyanoaurate(I) acceptors. Faraday Discuss. 2017, 203, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tuikka, M.J.; Hirva, P.; Kukushkin, V.Y.; Novikov, A.S.; Haukka, M. Fine-tuning halogen bonding properties of diiodine through halogen–halogen charge transfer—Extended [Ru(2,2′-bipyridine)(CO)2X2]·I2 systems (X = Cl, Br, I). CrystEngComm 2016, 18, 1987–1995. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T. Synthesis of an extended halogen-bonded metal–organic structure in a one-pot mechanochemical reaction that combines covalent bonding, coordination chemistry and supramolecular synthesis. CrystEngComm 2014, 16, 10169–10172. [Google Scholar] [CrossRef]
- Lapadula, G.; Judaš, N.; Friščić, T.; Jones, W. A Three-Component Modular Strategy to Extend and Link Coordination Complexes by Using Halogen Bonds to O, S and π Acceptors. Chem. Eur. J. 2010, 16, 7400–7403. [Google Scholar] [CrossRef] [PubMed]
- Nemec, V.; Fotović, L.; Friščić, T.; Cinčić, D. A Large Family of Halogen-Bonded Cocrystals Involving Metal–Organic Building Blocks with Open Coordination Sites. Cryst. Growth Des. 2017. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Wang, R.; Li, Y. Four supramolecular isomers of dichloridobis(1,10-phenanthroline)cobalt(II): Synthesis, structure characterization and isomerization. Acta Crystallogr. Sect. C 2016, 72, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Liu, D.-X.; Liu, T.-F. A polymorph of cis-dichloridobis(1,10-phenanthroline-κ2N,N′)cobalt(II). Acta Crystallogr. Sect. E 2007, 63, m1880. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Tong, M.; Chen, X. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Curzi, M.; Johansson, A.; Polito, M.; Rubini, K.; Grepioni, F. Simple and Quantitative Mechanochemical Preparation of a Porous Crystalline Material Based on a 1D Coordination Network for Uptake of Small Molecules. Angew. Chem. Int. Ed. 2006, 45, 148–152. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, E.V. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Kaitner, B. Schiff base derived from 2-hydroxy-1-naphthaldehyde and liquid-assisted mechanochemical synthesis of its isostructural Cu(II) and Co(II) complexes. CrystEngComm 2011, 13, 4351–4357. [Google Scholar] [CrossRef]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Effect of atmosphere on solid-state amine-aldehyde condensations: Gas-phase catalysts for solid-state transformations. Chem. Commun. 2012, 48, 11683–11685. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Juribašić, M.; Babić, D.; Molčanov, K.; Šket, P.; Plavec, J.; Ćurić, M. New insight into solid-state molecular dynamics: Mechanochemical synthesis of azobenzene/triphenylphosphine palladacycles. Chem. Commun. 2011, 47, 11543–11545. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; José Gotor, F.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Stilinović, V.; Cinčić, D.; Zbačnik, M.; Kaitner, B. Controlling solvate formation of a Schiff base by combining mechanochemistry with solution synthesis. Croat. Chem. Acta 2012, 85, 485–493. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Making crystals from crystals: A green route to crystal engineering and polymorphism. Chem. Commun. 2005, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Springuel, G.; Robeyns, K.; Norberg, B.; Wouters, J.; Leyssens, T. Cocrystal Formation between Chiral Compounds: How Cocrystals Differ from Salts. Cryst. Growth Des. 2014, 14, 3996–4004. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A Stepwise Mechanism for the Mechanochemical Synthesis of Halogen-Bonded Cocrystal Architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Structural Equivalence of Br and I Halogen Bonds: A Route to Isostructural Materials with Controllable Properties. Chem. Mater. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Mavračić, J.; Cinčić, D.; Kaitner, B. Halogen bonding of N-bromosuccinimide by grinding. CrystEngComm 2016, 18, 3343–3346. [Google Scholar] [CrossRef]
- Nemec, V.; Cinčić, D. Uncommon halogen bond motifs in cocrystals of aromatic amines and 1,4-diiodotetrafluorobenzene. CrystEngComm 2016, 18, 7425–7429. [Google Scholar] [CrossRef]
- Brammer, L.; Bruton, E.A.; Sherwood, P. Understanding the Behavior of Halogens as Hydrogen Bond Acceptors. Cryst. Growth Des. 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Stilinović, V.; Užarević, K.; Cvrtila, I.; Kaitner, B. Bis(morpholine) hydrogen bond pincer—A novel series of heteroleptic Cu(II) coordination compounds as receptors for electron rich guests. CrystEngComm 2012, 14, 7493–7501. [Google Scholar] [CrossRef]
- Pfrunder, M.C.; Micallef, A.S.; Rintoul, L.; Arnold, D.P.; McMurtrie, J. Interplay between the Supramolecular Motifs of Polypyridyl Metal Complexes and Halogen Bond Networks in Cocrystals. Cryst. Growth Des. 2016, 16, 681–695. [Google Scholar] [CrossRef]
- Cavallo, G.; Biella, S.; Lu, J.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halide anion-templated assembly of di- and triiodoperfluorobenzenes into 2D and 3D supramolecular networks. J. Fluor. Chem. 2010, 131, 1165–1172. [Google Scholar] [CrossRef]
- Raatikainen, K.; Rissanen, K. Modulation of N···I and +N−H···Cl−···I Halogen Bonding: Folding, Inclusion, and Self-Assembly of Tri- and Tetraamino Piperazine Cyclophanes. Cryst. Growth Des. 2016, 10, 3638–3646. [Google Scholar] [CrossRef]
- STARe Software v.14.00; MettlerToledo GmbH: Giessen, Germany, 2015.
- Oxford Diffraction. Xcalibur CCD System, CrysAlis CCD and CrysAlis RED, version 1.171; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.V.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Philips X’Pert Data Collector 1.3e; Philips Analytical B. V.: Almelo, The Netherlands, 2001.
- Philips X’Pert Graphic & Identify 1.3e Philips; Analytical B. V.: Almelo, The Netherlands, 2001.
- Philips X’Pert Plus 1.0; Philips Analytical B. V.: Almelo, The Netherlands, 1999.


| Compound 1 | Compound 2 | |
|---|---|---|
| Molecular Formula | (CoCl2C24H16N4)(C6F4I2) | [(CoClC25H20N4O)]Cl(CH3OH)(C6F4I2) |
| Mr | 892.10 | 956.18 |
| Crystal system | triclinic | triclinic |
| Space group | P | P |
| Crystal data: | ||
| a/Å | 9.9129(5) | 9.1111(3) |
| b/Å | 12.8302(5) | 11.1583(5) |
| c/Å | 14.0185(6) | 17.8104(8) |
| α/° | 116.357(4) | 77.661(4) |
| β/° | 103.424(4) | 76.942(4) |
| γ/° | 96.119(4) | 81.311(3) |
| V/Å3 | 1508.31(13) | 1713.04(13) |
| Z | 2 | 2 |
| Dcalc/g cm−3 | 1.964 | 1.854 |
| λ(MoKα)/Å | 0.71073 | 0.71073 |
| T/K | 295 | 295 |
| Crystal size/mm3 | 0.46 × 0.25 × 0.11 | 0.60 × 0.55 × 0.31 |
| µ/mm−1 | 2.846 | 2.517 |
| F(000) | 854 | 926 |
| Refl. collected/unique | 6632/4212 | 5872/4242 |
| Parameters | 388 | 430 |
| Δρmax, Δρmin/e Å−3 | 0.547; −0.632 | 0.586; −0.449 |
| R[F2 > 4σ(F2)] | 0.0294 | 0.0297 |
| wR(F2) | 0.0772 | 0.0655 |
| Goodness-of-fit, S | 0.879 | 0.940 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisac, K.; Cinčić, D. The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals 2017, 7, 363. https://doi.org/10.3390/cryst7120363
Lisac K, Cinčić D. The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals. 2017; 7(12):363. https://doi.org/10.3390/cryst7120363
Chicago/Turabian StyleLisac, Katarina, and Dominik Cinčić. 2017. "The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding" Crystals 7, no. 12: 363. https://doi.org/10.3390/cryst7120363
APA StyleLisac, K., & Cinčić, D. (2017). The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals, 7(12), 363. https://doi.org/10.3390/cryst7120363