Effect of Ionic Liquids on the Separation of Sucrose Crystals from a Natural Product Using Crystallization Techniques
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
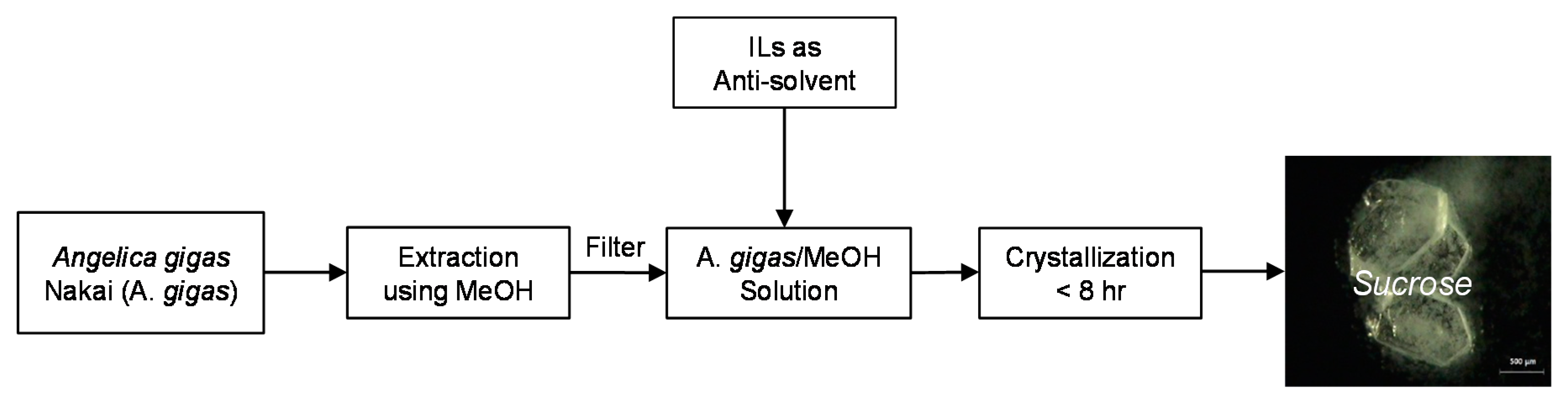
3.2. Crystallization Process of Sucrose
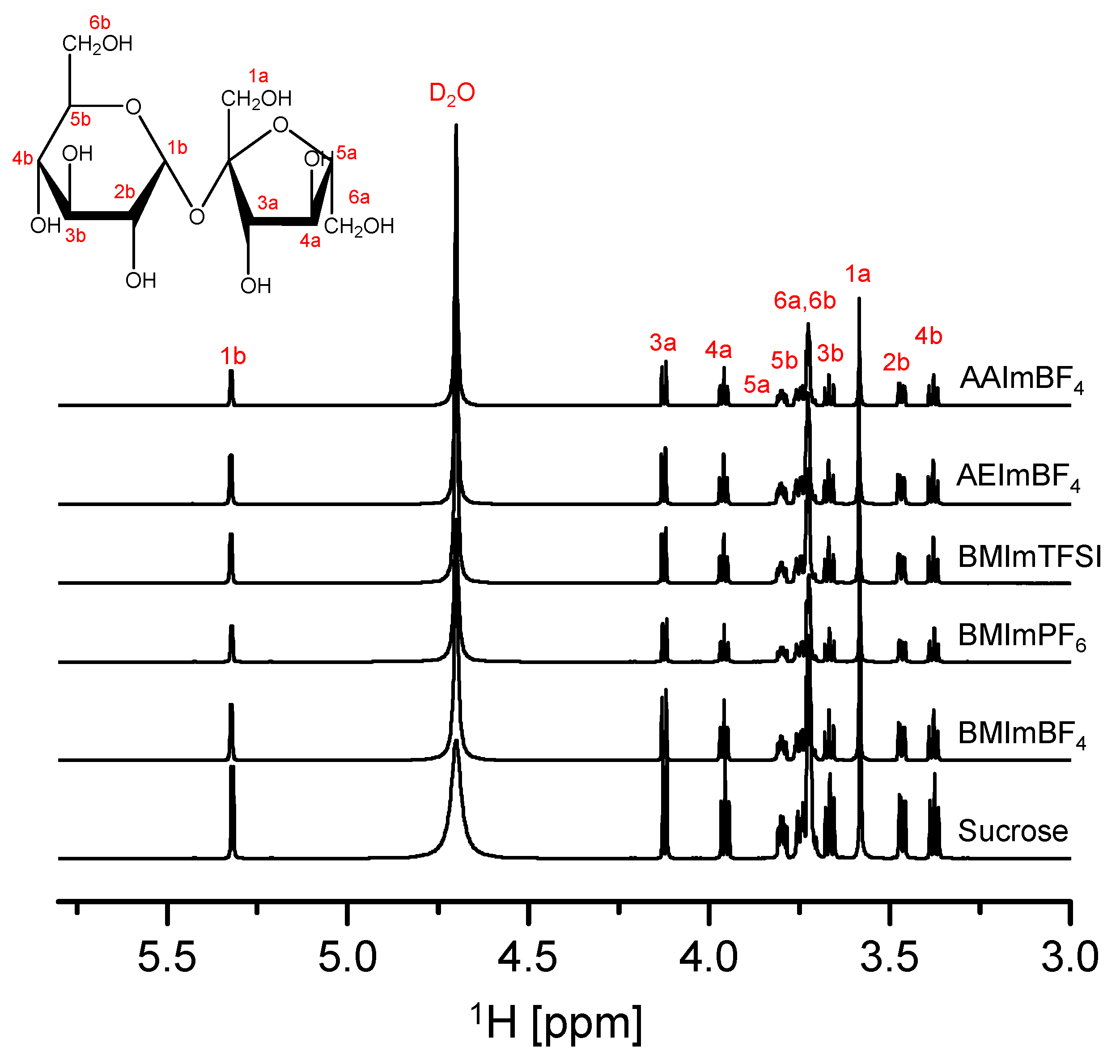
3.3. Nuclear Magnetic Resonance Spectroscopy (Solution-State NMR)
3.4. Powder X-ray Diffraction (PXRD)
3.5. Morphological Study
3.6. Single-Crystal X-ray Diffraction (SXD)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mullin, J.W. Crystallization; Lightning Source: La Vergne, TN, USA, 2001; Chapter 1; pp. 1–3. [Google Scholar]
- Davey, R.; Garside, J. Form Molecules to Crystallizers an Introduction to Crystallization; Oxford University Press: Oxford, UK, 2000; Chapter 1; pp. 1–5. [Google Scholar]
- Modi, S.R.; Dantuluri, A.K.R.; Puri, V.; Pawar, Y.B.; Nandekar, P.; Sangamwar, A.T.; Perumalla, S.R.; Sun, C.C.; Bansal, A.K. Impact of Crystal Habit on Biopharmaceutical Performance of Celecoxib. Cryst. Growth Des. 2013, 13, 2824–2832. [Google Scholar] [CrossRef]
- Thakur, A.; Thipparaboina, R.; Kumar, D.; Gouthami, K.S.; Shastri, N.R. Crystal engineered albendazole with improved dissolution and material attributes. CrystEngComm 2016, 18, 1489–1494. [Google Scholar] [CrossRef]
- Cashell, C.; Corcoran, D.; Hodnett, B.K. Effect of Amino Acid Additives on the Crystallization of l-Glutamic Acid. Cryst. Growth Des. 2005, 5, 593–597. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids-Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D. Reflections on ionic liquids. Nature 2007, 447, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Reichert, W.M.; Holbrey, J.D.; Vigour, K.B.; Morgan, T.D.; Broker, G.A.; Rogers, R.D. Approaches to crystallization from ionic liquids: Complex solvents–complex results, or, a strategy for controlled formation of new supramolecular architectures? Chem. Commun. 2006, 46, 4767–4779. [Google Scholar] [CrossRef]
- Kunov-Kruse, A.J.; Weber, C.C.; Rogers, R.D.; Myerson, A.S. The A Priori Design and Selection of Ionic Liquids as Solvents for Active Pharmaceutical Ingredients. Chem. Eur. J. 2017, 23, 5498–5508. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Solubilities of Pharmaceutical Compounds in Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. [Google Scholar] [CrossRef]
- Resende de Azevedo, J.; Letourneau, J.J.; Espitalier, F.; Re, M.I. Solubility of a New Cardioactive Prototype Drug in Ionic Liquids. J. Chem. Eng. Data 2014, 59, 1766–1773. [Google Scholar] [CrossRef]
- Weber, C.C.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. Manipulation of ionic liquid anion–solute–antisolvent interactions for the purification of acetaminophen. Chem. Commun. 2015, 51, 4294–4297. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Bridson, R.H.; Leeke, G.A. Crystallisation control of paracetamol from ionic liquids. CrystEngComm 2014, 16, 10797–10803. [Google Scholar] [CrossRef]
- Weber, C.C.; Kulkarni, S.A.; Kunov-Kruse, A.J.; Rogers, R.D.; Myerson, A.S. The Use of Cooling Crystallization in an Ionic Liquid System for the Purification of Pharmaceuticals. Cryst. Growth Des. 2015, 15, 4946–4951. [Google Scholar] [CrossRef]
- Karthika, S.; Radhakrishnan, T.K.; Kalaichelvi, P. The role of hydrogen bonding propensity in tuning the morphology of crystals obtained from imidazolium based ionic liquids. J. Cryst. Growth 2017, 463, 168–175. [Google Scholar] [CrossRef]
- Resende de Azevedo, J.; Espitalier, F.; Letourneau, J.J.; Ré, M.I. Antisolvent crystallization of a cardiotonic drug in ionic liquids: Effect of mixing on the crystal properties. J. Cryst. Growth 2017, 472, 29–34. [Google Scholar] [CrossRef]
- Pusey, M.L.; Paley, M.S.; Turner, M.B.; Rogers, R.D. Protein Crystallization Using Room Temperature Ionic Liquids. Cryst. Growth Des. 2007, 7, 787–793. [Google Scholar] [CrossRef]
- Judge, R.A.; Takahashi, S.; Longenecker, K.L.; Fry, E.H.; Zapatero, C.A.; Chiu, M.L. The Effect of Ionic Liquids on Protein Crystallization and X-ray Diffraction Resolution. Cryst. Growth Des. 2009, 9, 3463–3469. [Google Scholar] [CrossRef]
- Kennedy, D.E.; Drummond, C.J.; Peat, T.S.; Newman, J. Evaluating Protic Ionic Liquids as Protein Crystallization Additives. Cryst. Growth Des. 2011, 11, 1777–1785. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Feng, Y.D.J.; Ge, L.; Zhang, M. The crystallization of lysozyme in the system of ionic liquid [BMIm][BF4]-water. Cryst. Res. Technol. 2008, 43, 1062–1068. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Wang, H.; Wang, J. Crystallization Control of CaCO3 by Ionic Liquids in Aqueous Solution. Cryst. Growth Des. 2009, 9, 4984–4986. [Google Scholar] [CrossRef]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A position paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef] [PubMed]
- An, J.-H.; Kim, J.-M.; Chang, S.-M.; Kim, W.-S. Application of Ionic Liquid to Polymorphic Design of Pharmaceutical Ingredients. Cryst. Growth Des. 2010, 10, 3044–3050. [Google Scholar] [CrossRef]
- An, J.-H.; Kim, W.-S. Antisolvent Crystallization Using Ionic Liquids As Solvent and Antisolvent for Polymorphic Design of Active Pharmaceutical Ingredient. Cryst. Growth Des. 2013, 13, 31–39. [Google Scholar] [CrossRef]
- An, J.-H.; Jin, F.; Kim, H.S.; Ryu, H.C.; Kim, J.S.; Kim, H.M.; Kim, K.H.; Kiyonga, A.N.; Jung, K. Investigation of the Polymorphic Transformation of the Active Pharmaceutical Ingredient Clopidogrel Bisulfate Using the Ionic Liquid AEImBF4. Cryst. Growth Des. 2016, 16, 1829–1836. [Google Scholar] [CrossRef]
- An, J.H.; Jin, F.; Kim, H.S.; Ryu, H.C.; Kim, J.S.; Kim, H.M.; Kiyonga, A.N.; Min, D.S.; Youn, W.; Kim, K.H.; et al. Application of ionic liquid to polymorphic transformation of antiviral/HIV drug adefovir dipivoxil. Arch. Pharm. Res. 2016, 39, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Shimpi, M.R.; Velaga, S.P.; Shah, F.U.; Antzutkin, O.N. Pharmaceutical Crystal Engineering Using Ionic Liquid Anion–Solute Interactions. Cryst. Growth Des. 2017, 17, 1729–1734. [Google Scholar] [CrossRef]
- Pedireddi, V.R.; Shimpi, M.R.; Yakhmi, J.V. Room-Temperature Ionic Liquids: For a Difference in the Supramolecular Synthesis. Macromol. Symp. 2006, 241, 83–87. [Google Scholar] [CrossRef]
- Lapkin, A.A.; Piucinski, P.K.; Cutler, M. Comparative Assessment of Technologies for Extraction of Artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, H.-K.; Kim, J.-Y.; Homg, J.-W.; Cho, S.-I. A Review of Pharmacological Effects of Angelica gigas, Angelica sinensis, Angelica acutiloba and their Bioactive Compounds. J. Korean Orient. Med. 2011, 32, 1–24. [Google Scholar]
- Son, C.Y.; Baek, I.H.; Song, G.Y.; Kang, J.S.; Kwon, K.I. Pharmacological Effect of Decursin and Decursinol Angelate from Angelica gigas Nakai. Yakhak Hoeji. 2009, 53, 303–313. [Google Scholar]
- Xu, H.; Park, J.H.; Kim, Y.K.; Park, N.I.; Lee, S.Y.; Park, S.U. Optimization of growth and pyranocoumarins production in hairy root culture of Angelica gigas Nakai. J. Med. Plant. Res. 2009, 3, 978–981. [Google Scholar]
- Ferreira, L.; Vidal, M.M.; Geraldes, C.F.G.C.; Gil, M.H. Preparation and characterisation of gels based on sucrose modified with glycidyl methacrylate. Carb. Pol. 2000, 41, 15–24. [Google Scholar] [CrossRef]
- Brown, B.G.M.; Levy, H.A. Further Refinement of the Structure of Sucrose Based on Neutron-Diffraction Data. Acta. Cryst. 1973, B29, 790–797. [Google Scholar] [CrossRef]
- Aquilano, D.; Angela, M.F.; Rubbo, M. Growth morphology of polar crystals: A comparison between theory and experiment in sucrose. J. Crystal Growth 1983, 61, 369–376. [Google Scholar] [CrossRef]
- Woensdregt, C.F. Hartman-Perdok Theory: Influence of Crystal Structure and Crystalline Interface on Crystal Growth. Farafay Discuss. 1993, 95, 97–107. [Google Scholar] [CrossRef]
- Sounart, T.L.; Liu, J.; Voigt, J.A.; Huo, M.; Spoerke, E.D.; Mckenzie, B. Secondary Nucleation and Growth of ZnO. J. Am. Chem. Soc. 2007, 129, 15786–15793. [Google Scholar] [CrossRef] [PubMed]
- Wiemers-Meyer, S.; Winter, M.; Nowak, S. Mechanistic insights into lithium ion battery electrolyte degradation—A quantitative NMR study. Phys. Chem. Chem. Phys. 2016, 18, 26595–26601. [Google Scholar] [CrossRef] [PubMed]
- Plakhotnyk, A.V.; Ernst, L.; Schmutzler, R. Hydrolysis in the system LiPF6—propylene carbonate—dimethyl carbonate—H2O. J. Fluorine Chem. 2005, 126, 27–31. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2324–A2337. [Google Scholar] [CrossRef]
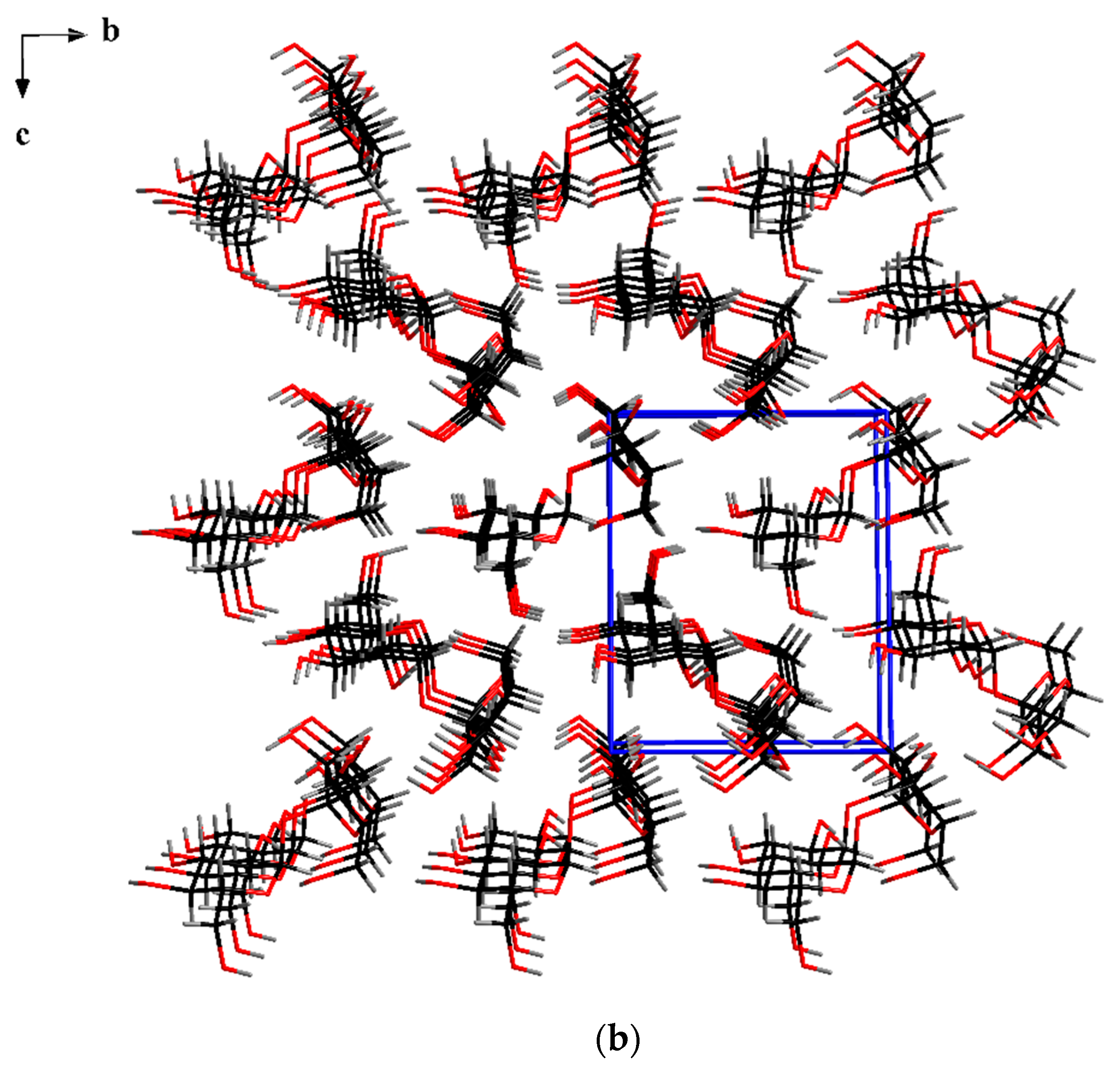



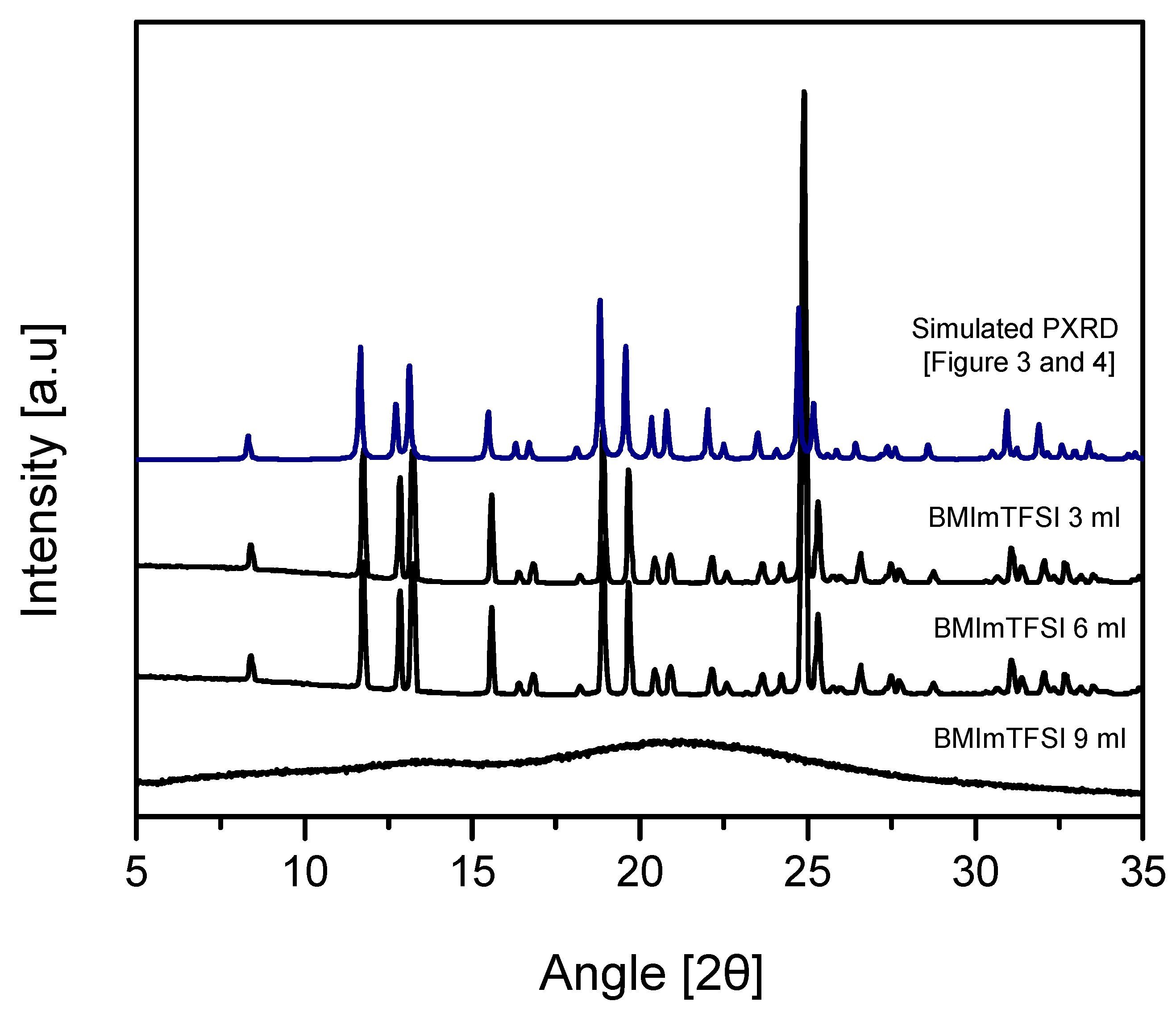
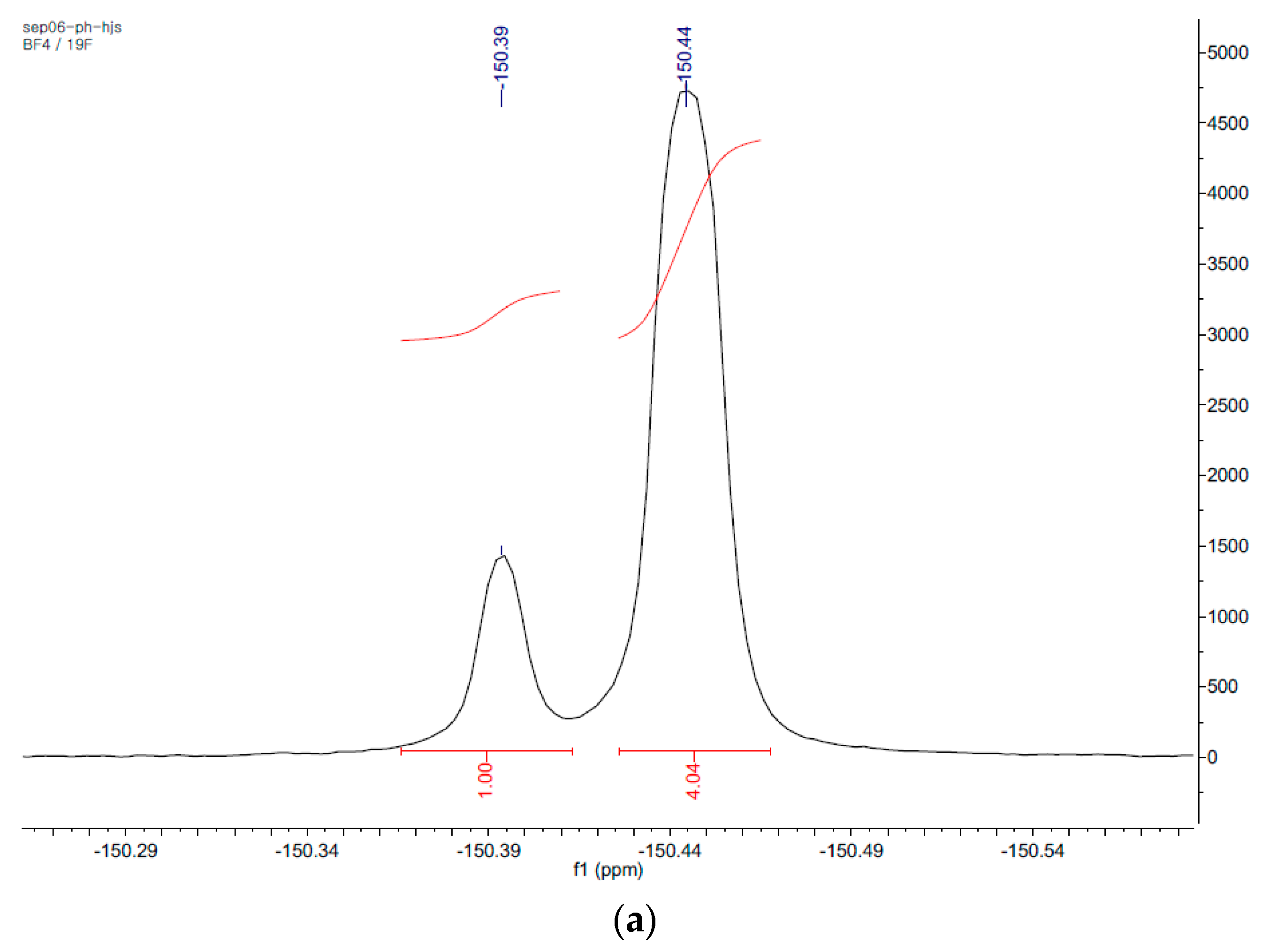
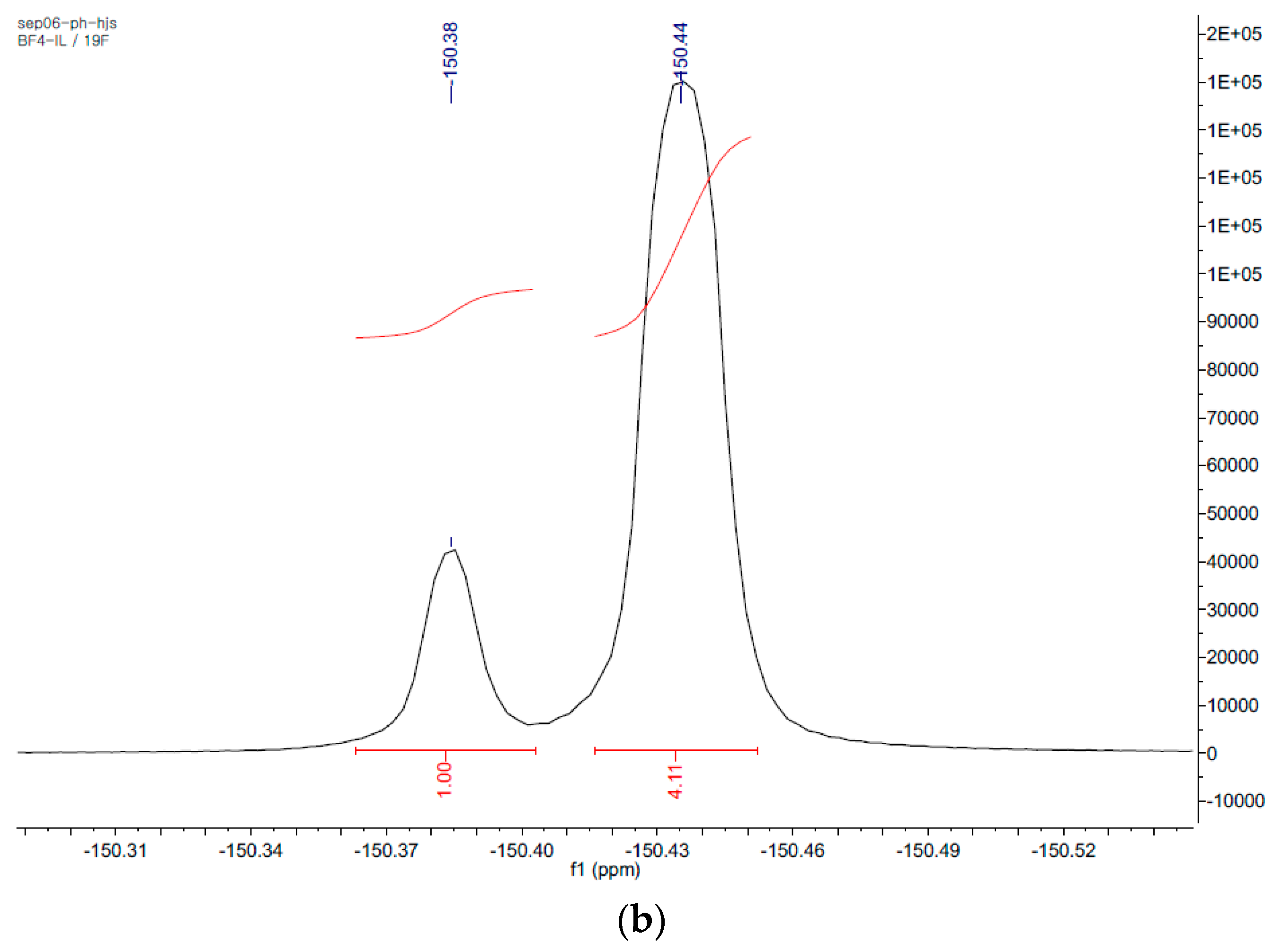
| Ionic Liquids (3mL) | BMImBF4 | BMImPF6 | AAImBF4 | AEImBF4 | BMImTFSI |
|---|---|---|---|---|---|
| a (Å) | 7.751 | 7.734 | 7.778 | 7.760 | 7.767 |
| b (Å) | 8.775 | 8.697 | 8.722 | 8.712 | 8.725 |
| c (Å) | 10.804 | 10.826 | 10.884 | 10.853 | 10.883 |
| β (°) | 103.060 | 103.006 | 102.967 | 102.946 | 102.912 |
| Cell Vol. (Å3) | 715.8 | 709.5 | 719.5 | 715.1 | 718.9 |
| Space group | P21 | P21 | P21 | P21 | P21 |
| Z | 2 | 2 | 2 | 2 | 2 |
| Crystal system | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic |
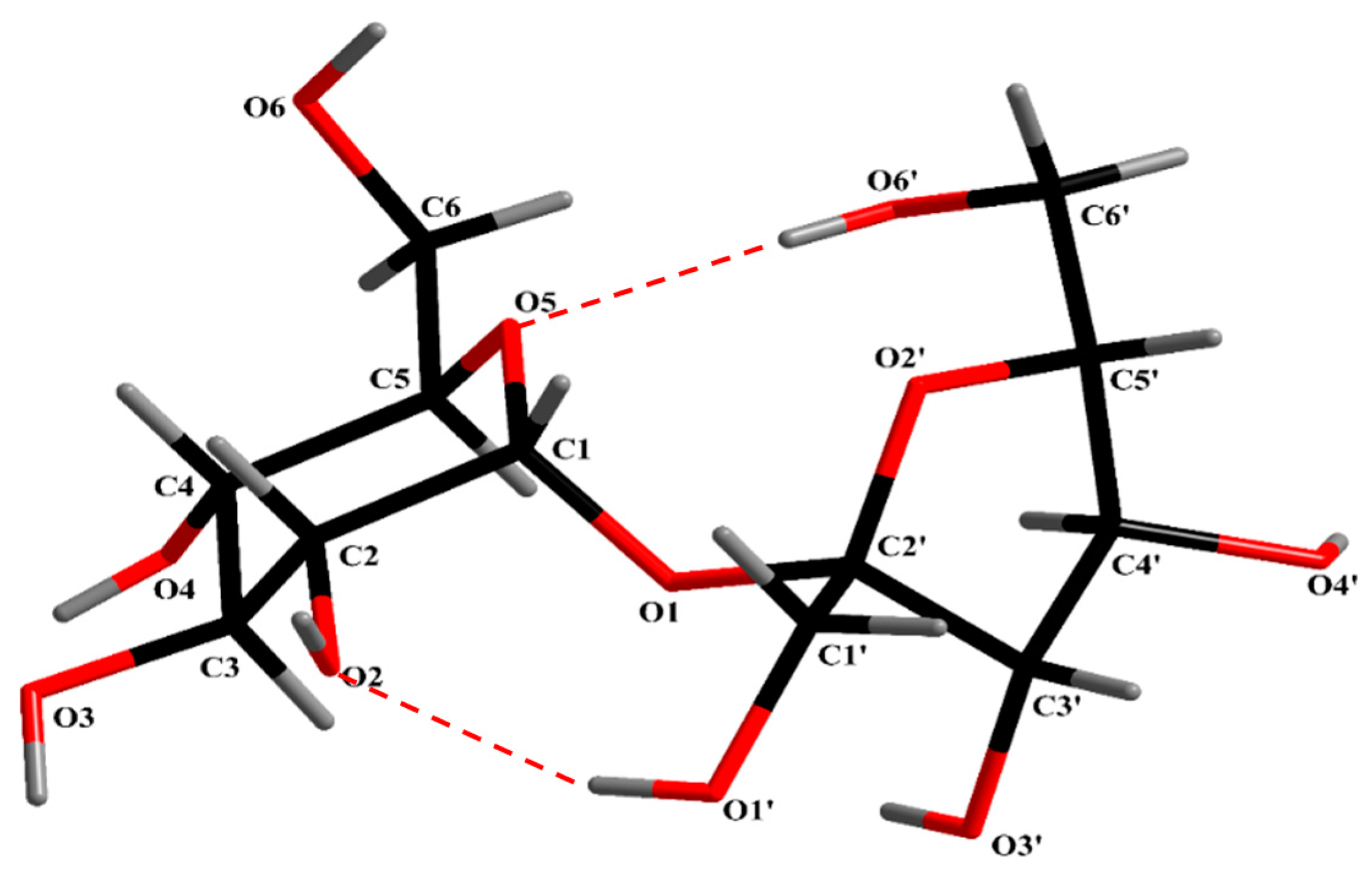
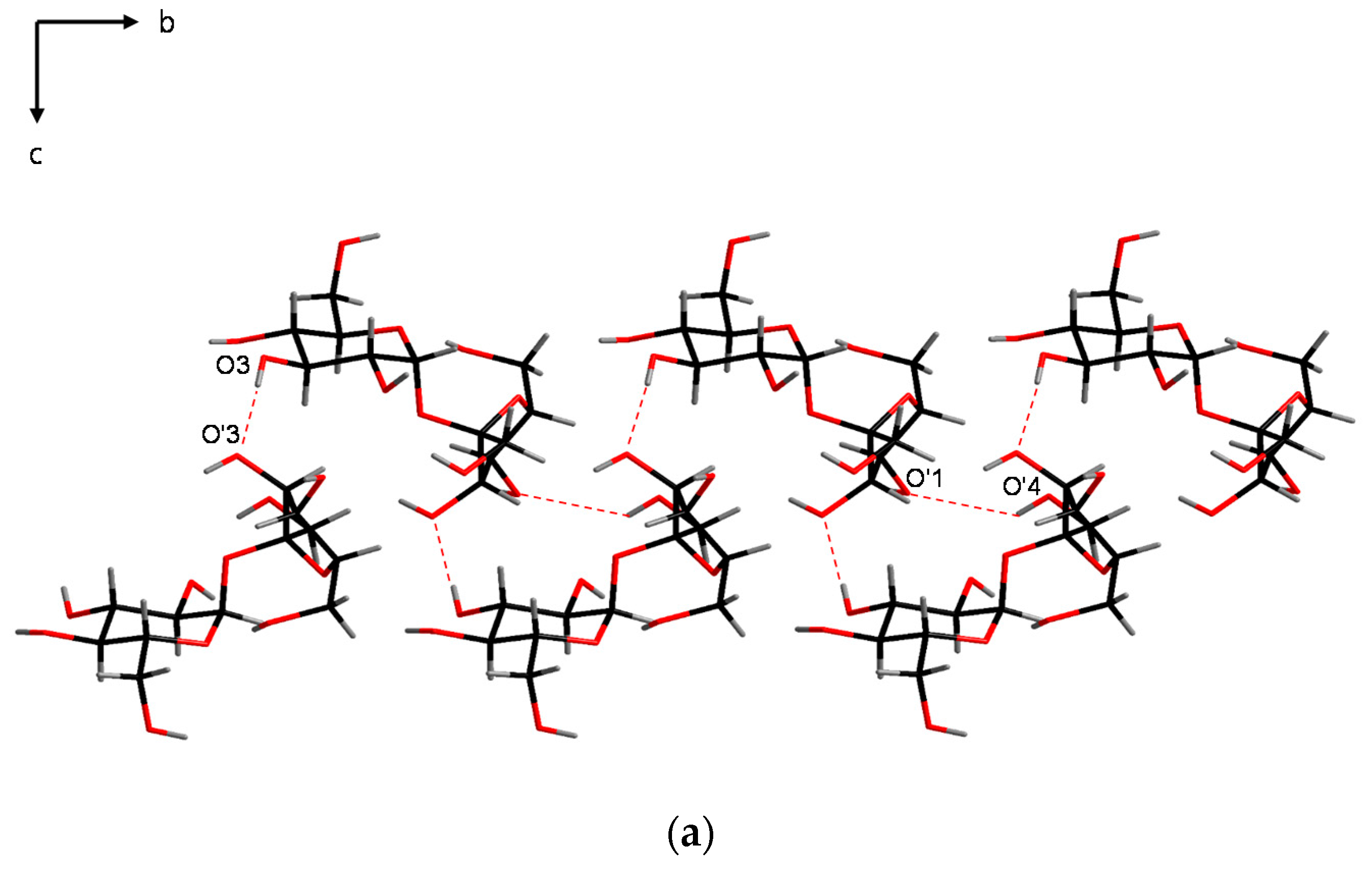
| Intramolecular | Intermolecular Periodic Bonds Chains (PBCs) |
|---|---|
| O’ (1)–H···O (2) O’ (6)–H···O (5) | α: O (3)–H···O’ (3) |
| β: O (6)–H···O (3) | |
| γ: O (2)–H···O’ (6) | |
| δ: O’ (3)–H···O’ (4) | |
| ε: O’ (4)–H···O’ (1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.-H.; Kiyonga, A.N.; Yoon, W.; Park, M.; Lim, C.; Yun, Y.; Park, G.-H.; Jung, K. Effect of Ionic Liquids on the Separation of Sucrose Crystals from a Natural Product Using Crystallization Techniques. Crystals 2017, 7, 284. https://doi.org/10.3390/cryst7100284
An J-H, Kiyonga AN, Yoon W, Park M, Lim C, Yun Y, Park G-H, Jung K. Effect of Ionic Liquids on the Separation of Sucrose Crystals from a Natural Product Using Crystallization Techniques. Crystals. 2017; 7(10):284. https://doi.org/10.3390/cryst7100284
Chicago/Turabian StyleAn, Ji-Hun, Alice Nguvoko Kiyonga, Woojin Yoon, Minho Park, Changjin Lim, Younghwi Yun, Gyu-Hwan Park, and Kiwon Jung. 2017. "Effect of Ionic Liquids on the Separation of Sucrose Crystals from a Natural Product Using Crystallization Techniques" Crystals 7, no. 10: 284. https://doi.org/10.3390/cryst7100284







