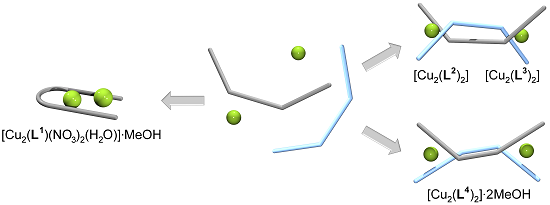
Spacer-Controlled Supramolecular Assemblies of Cu(II) with Bis(2-Hydroxyphenylimine) Ligands. from Monoligand Complexes to Double-Stranded Helicates and Metallomacrocycles
†
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis of the Ligands and Their Cu(II) Complexes
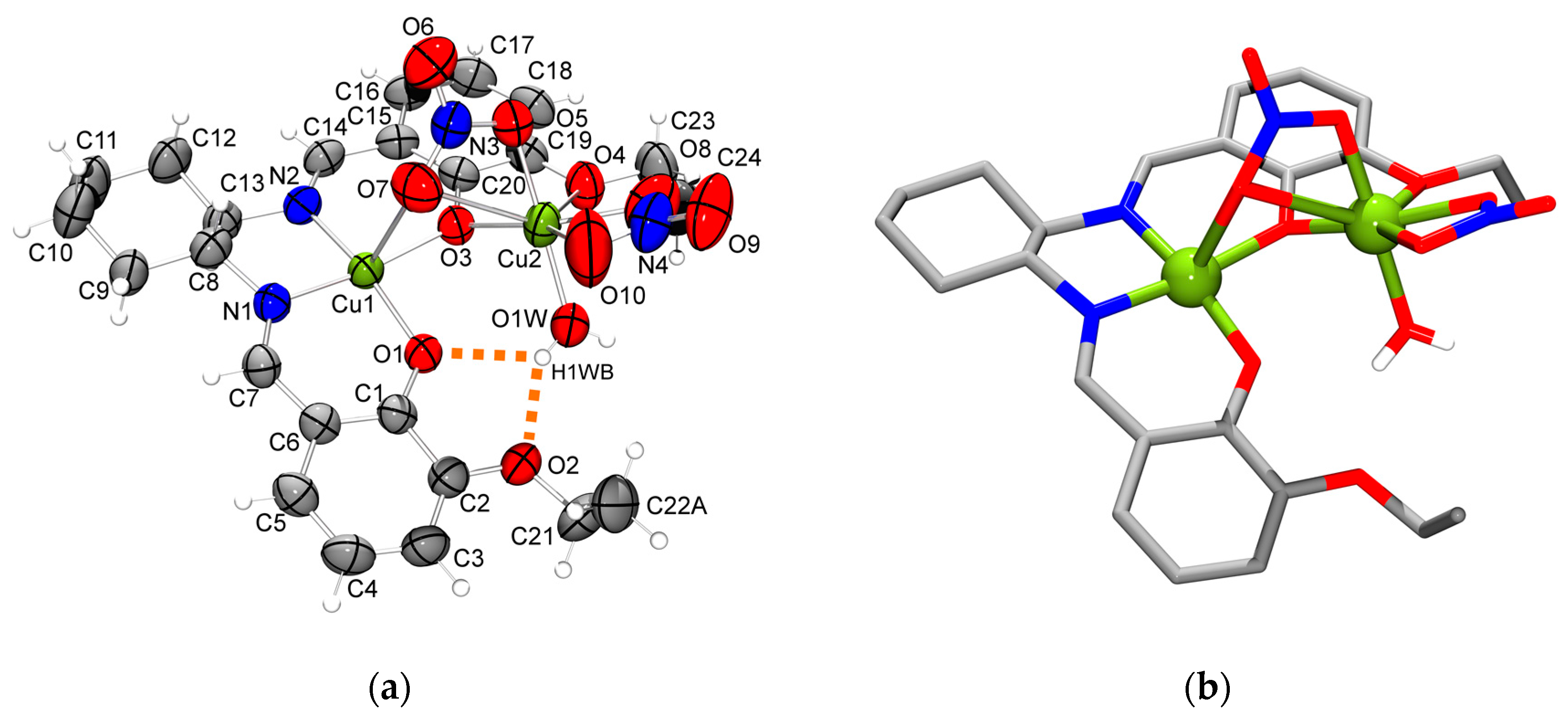
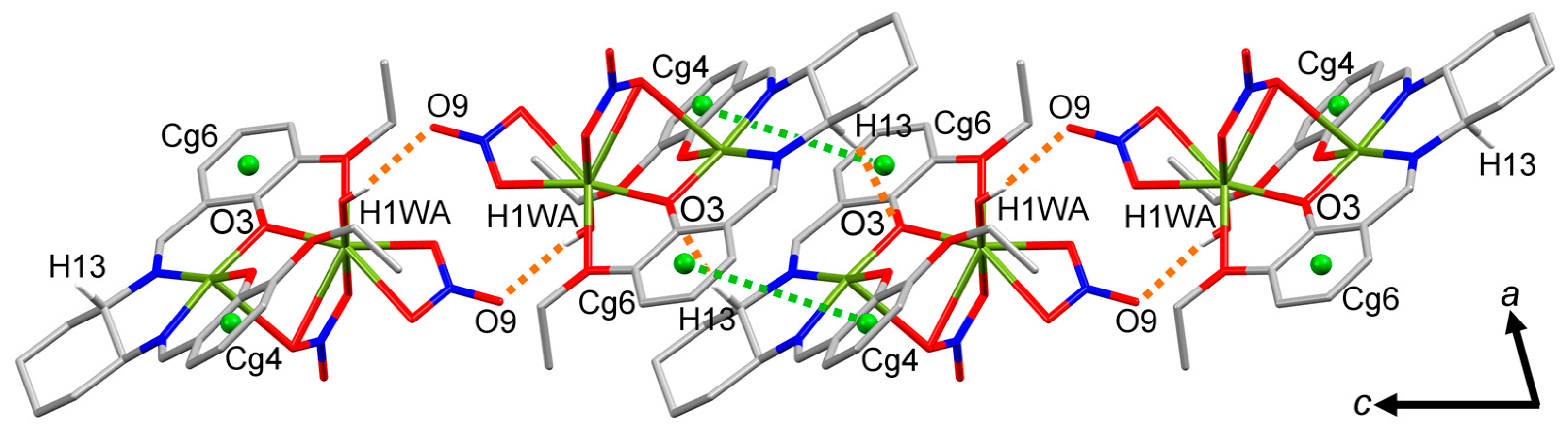
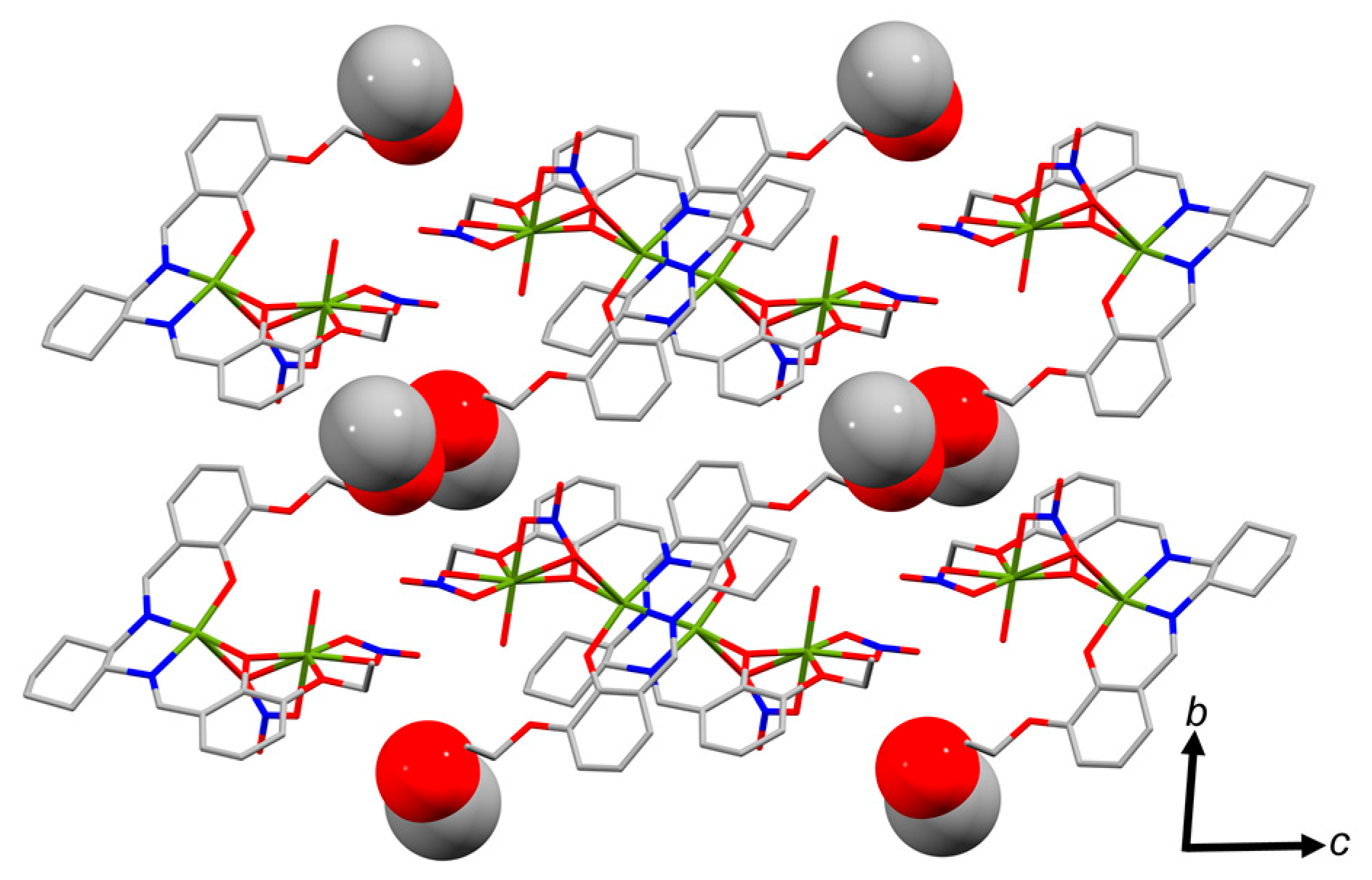
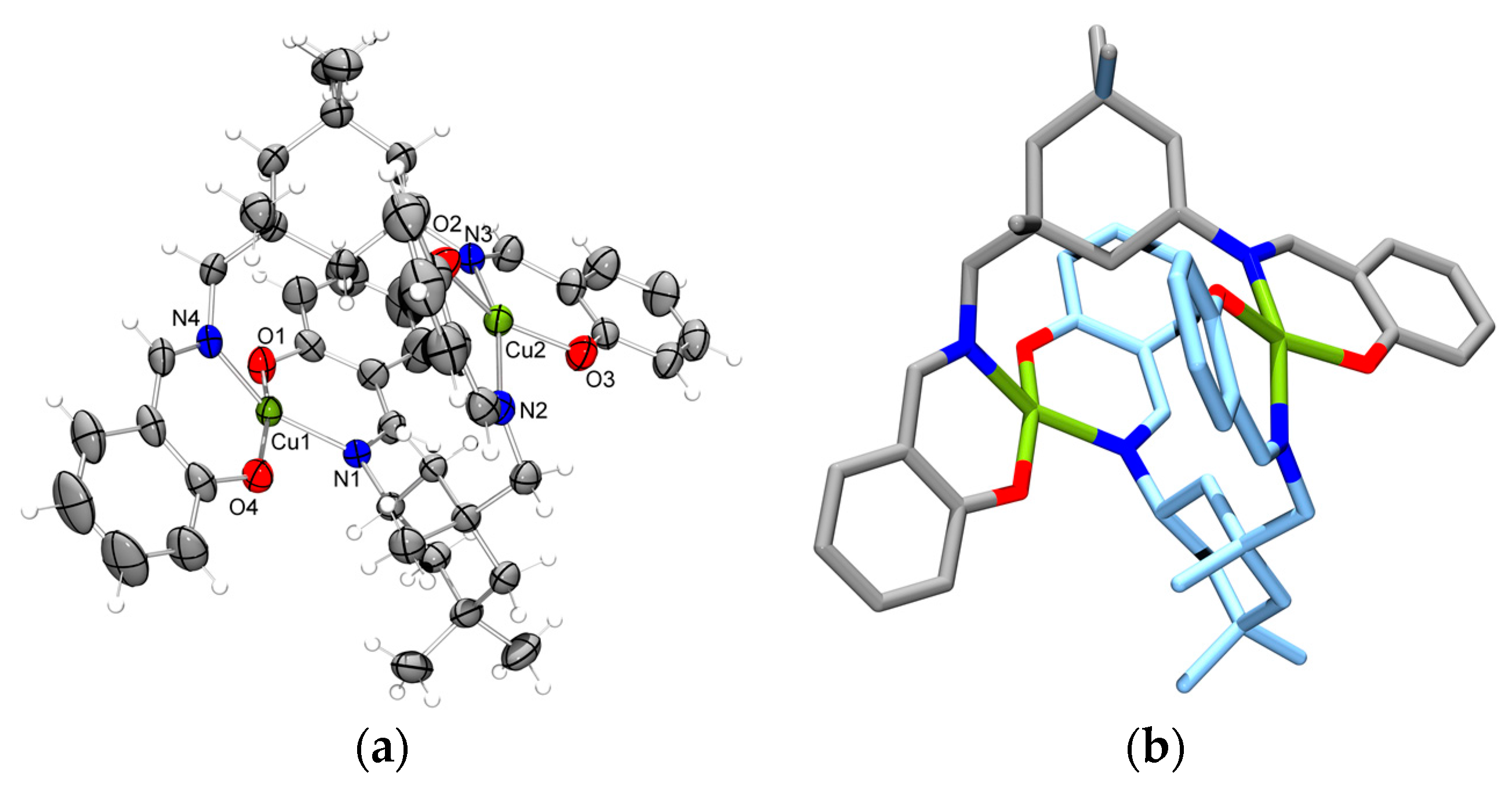
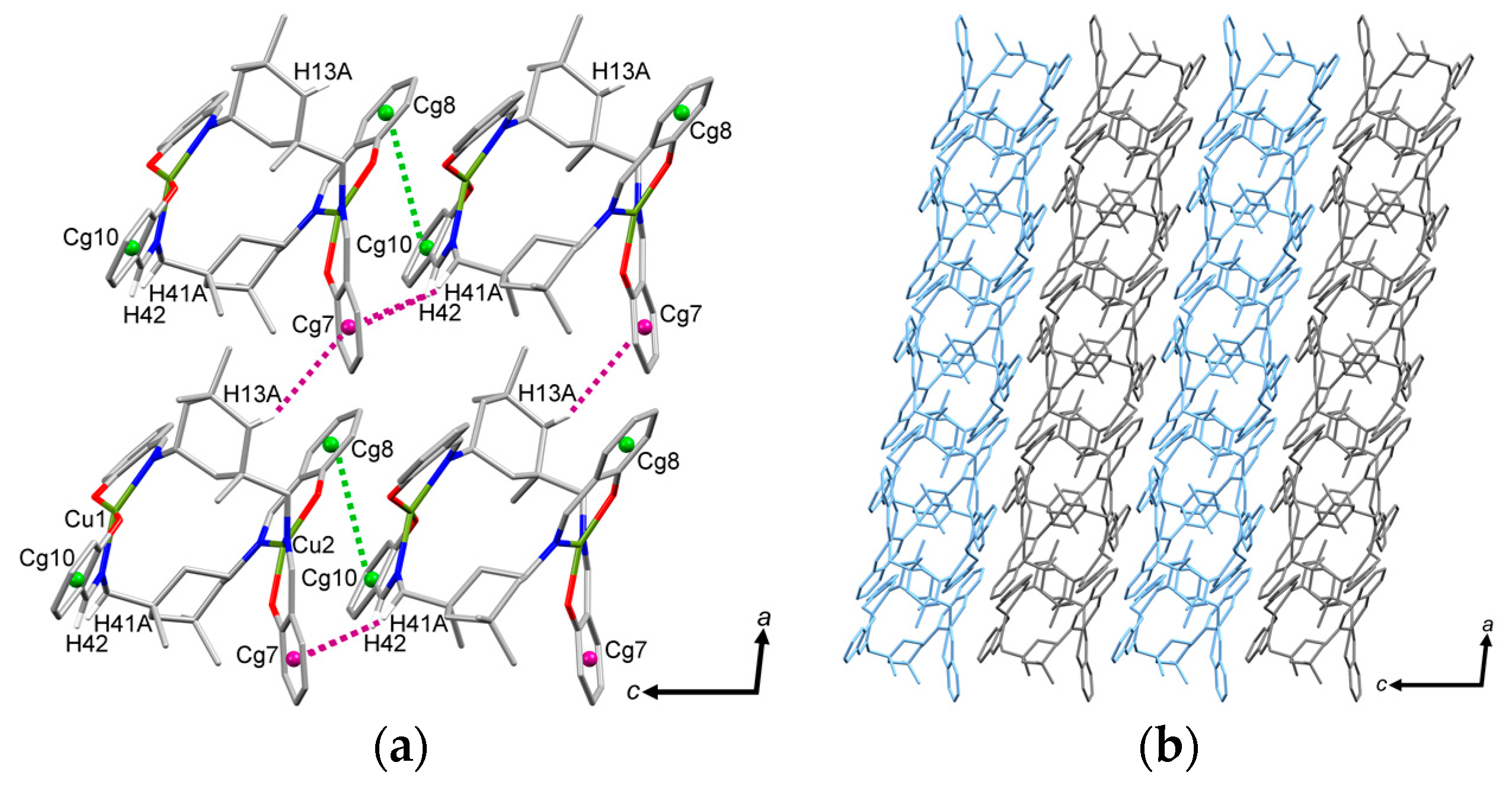
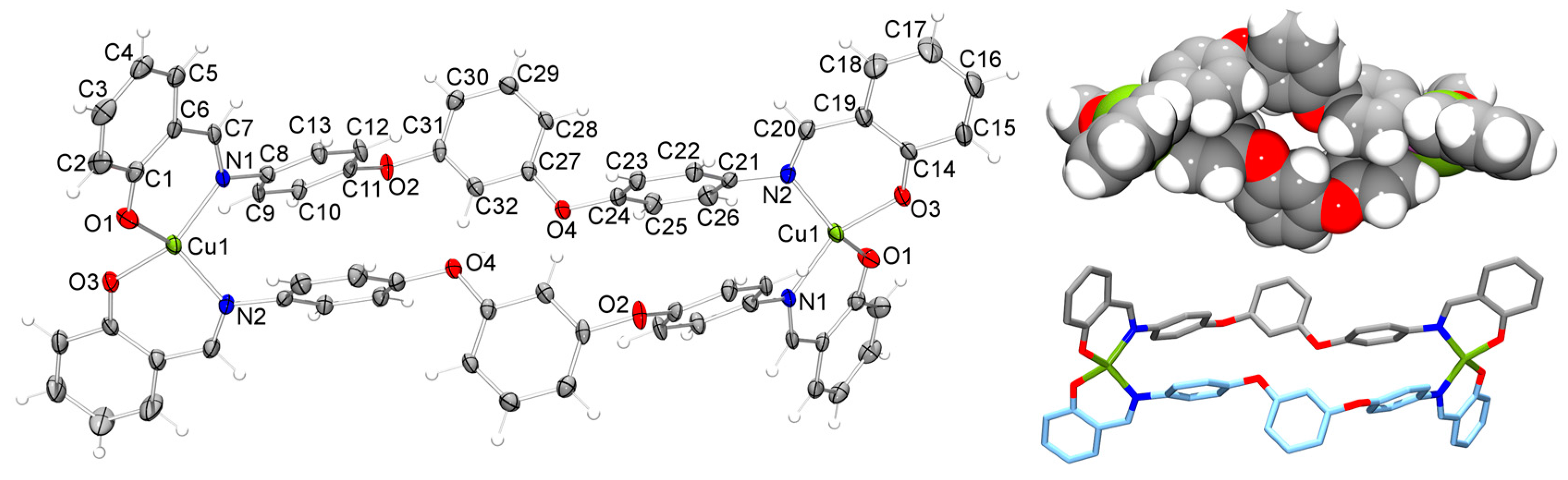
2.2. Molecular Structure of the Monoligand Cu(II) Complex with H2L1
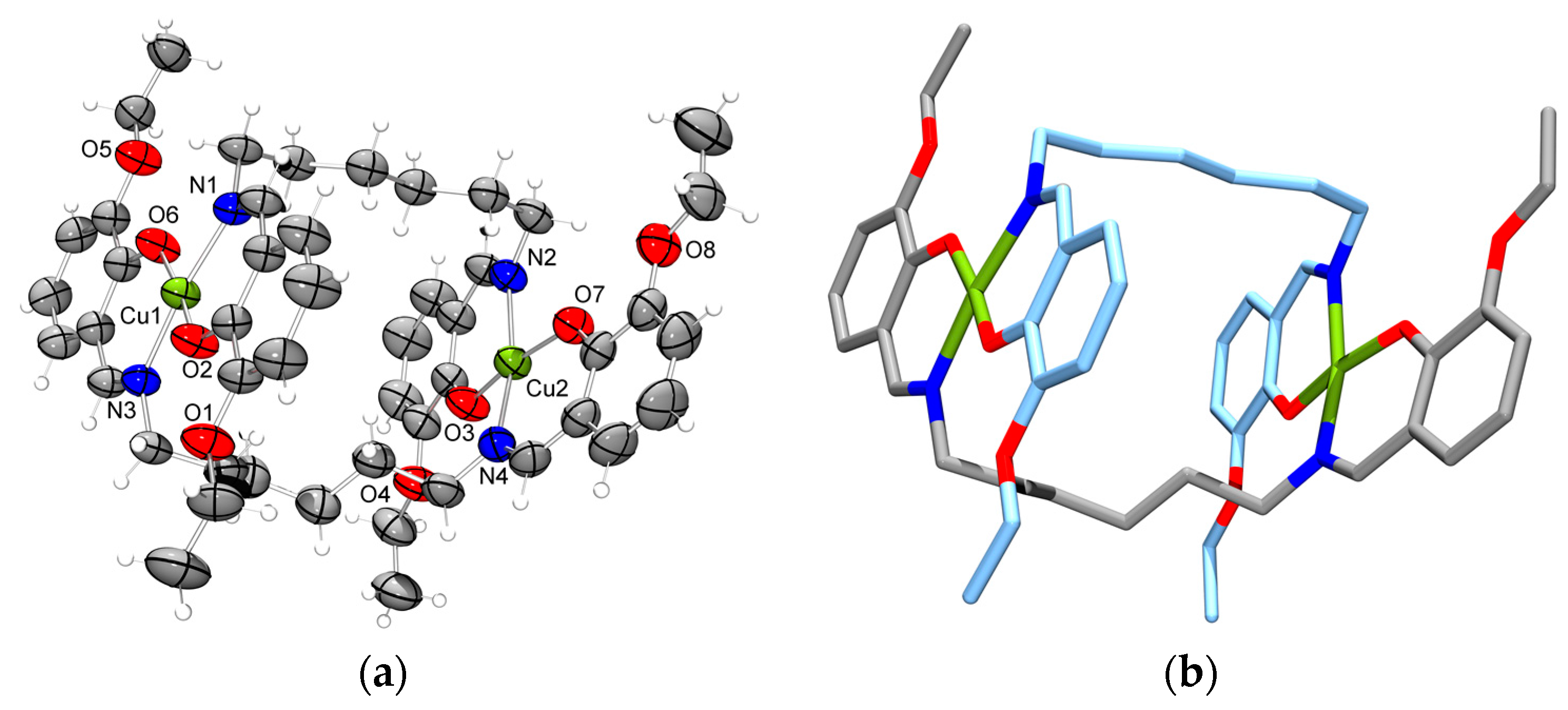
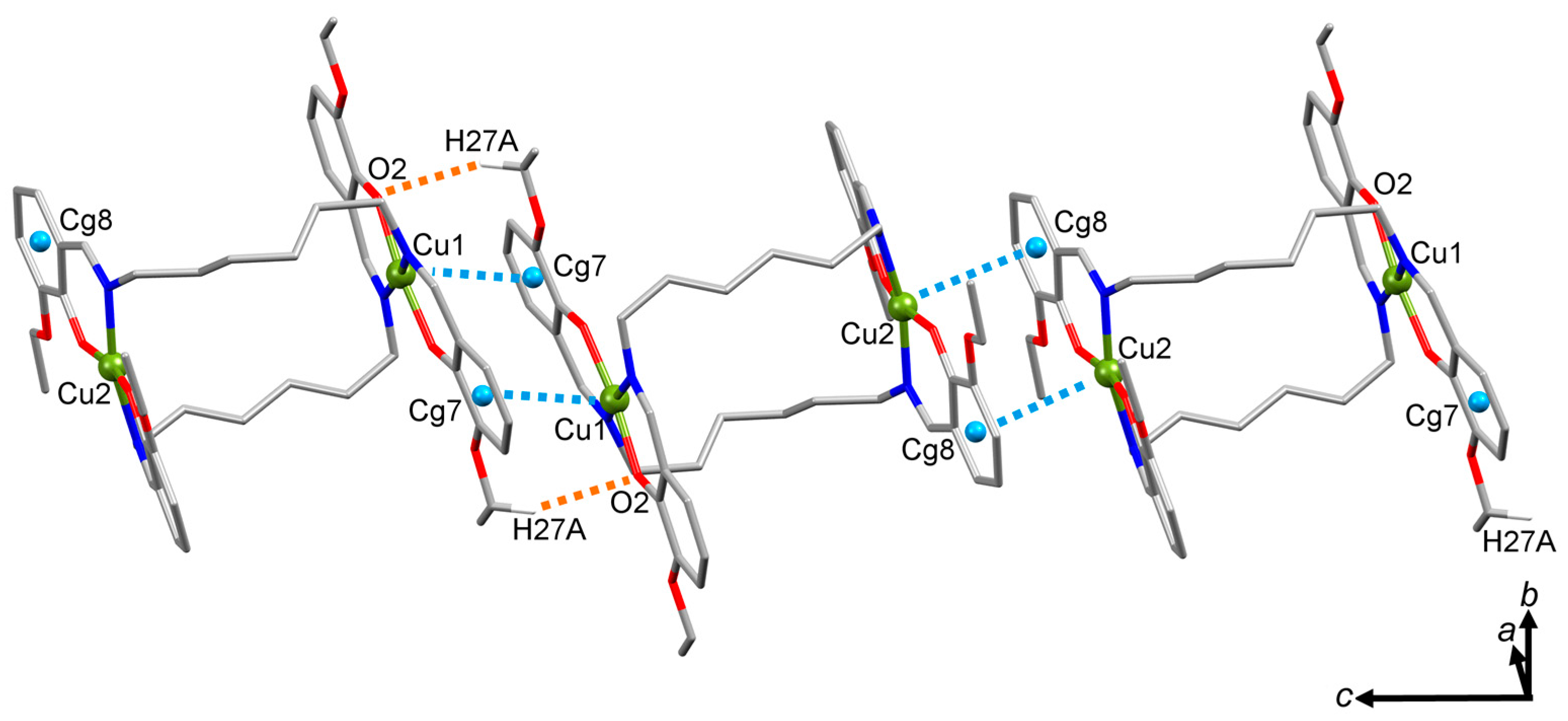
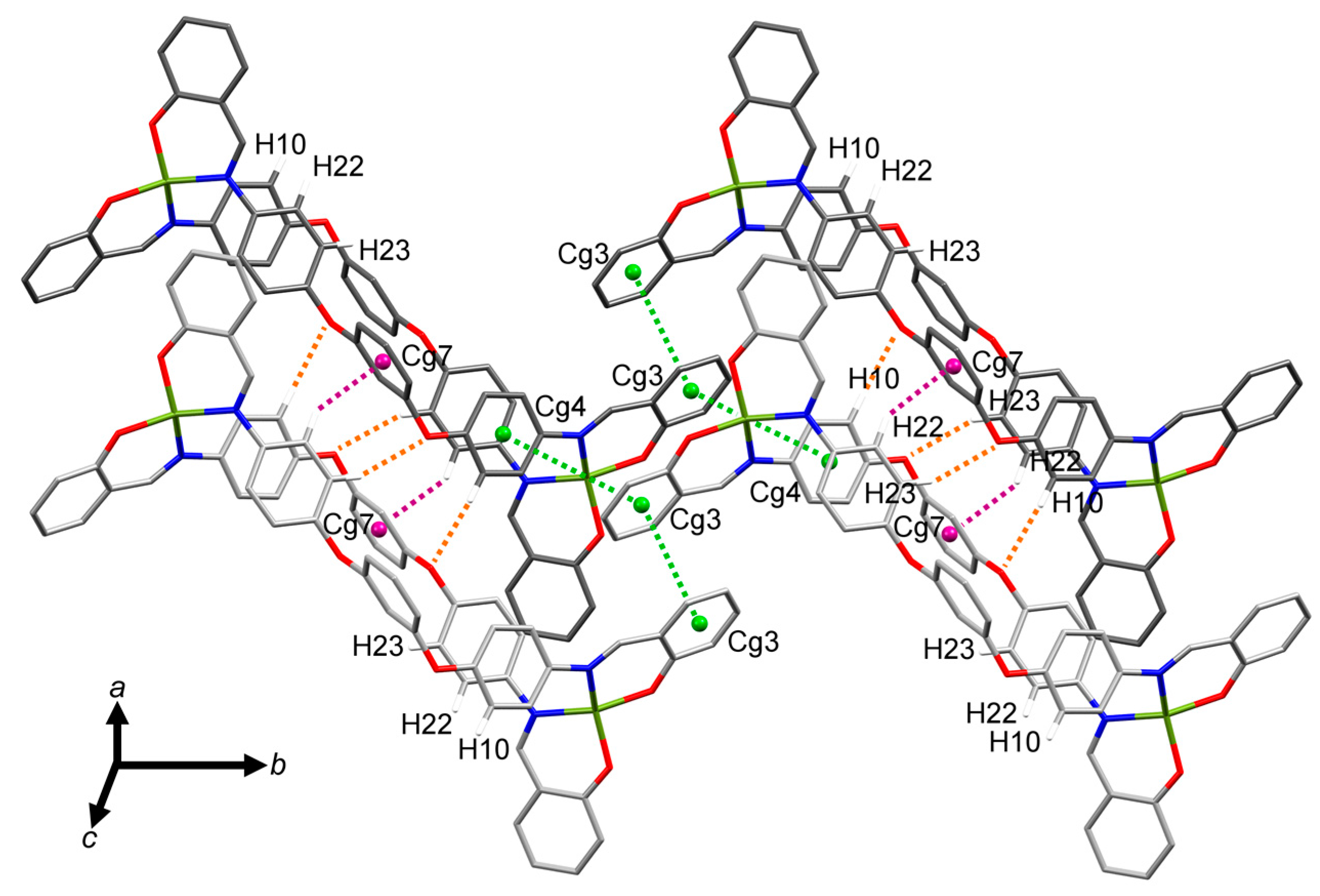
2.3. Molecular Structure of the Double-Stranded Cu(II) Helicates with H2L2 and H2L3
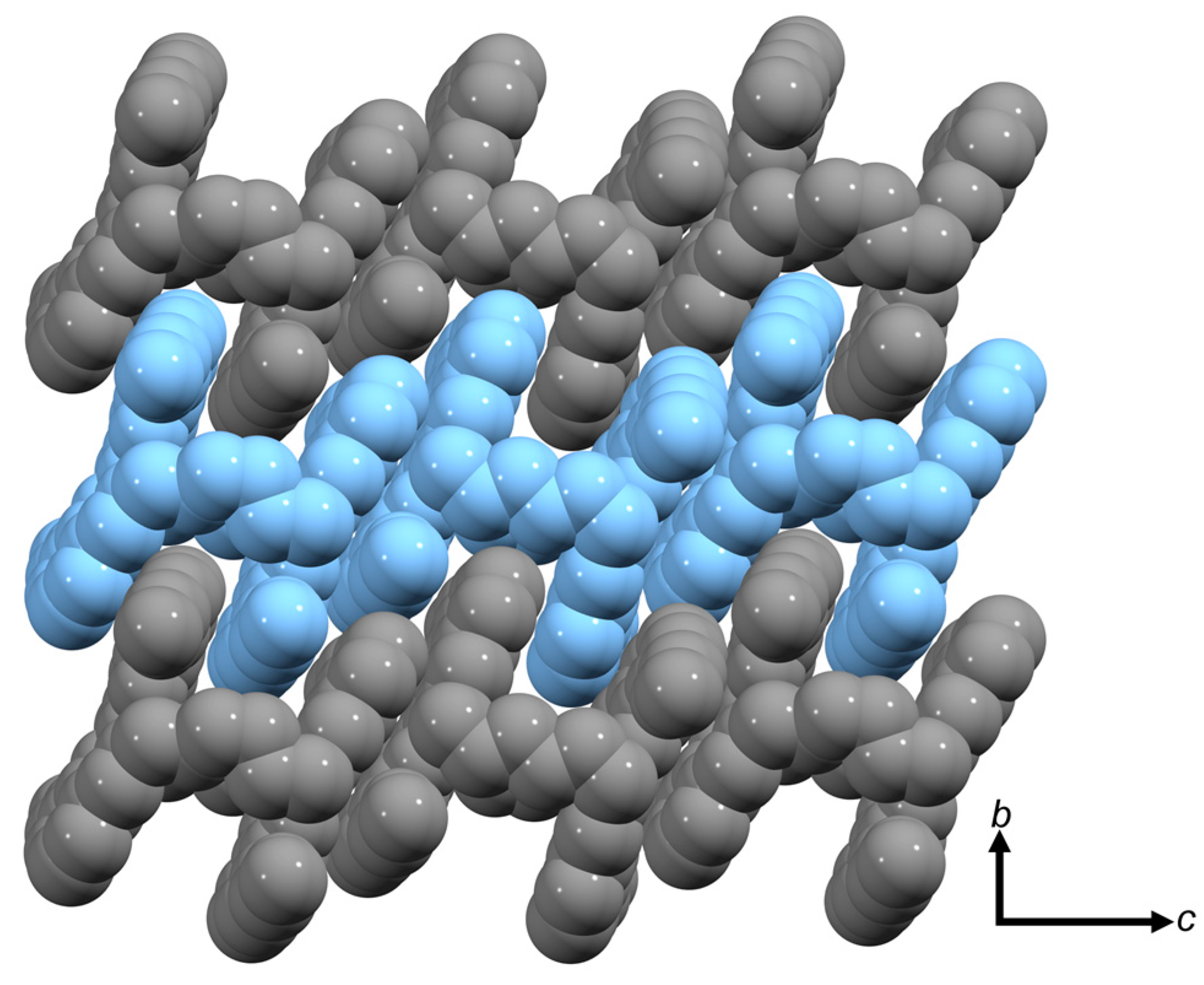
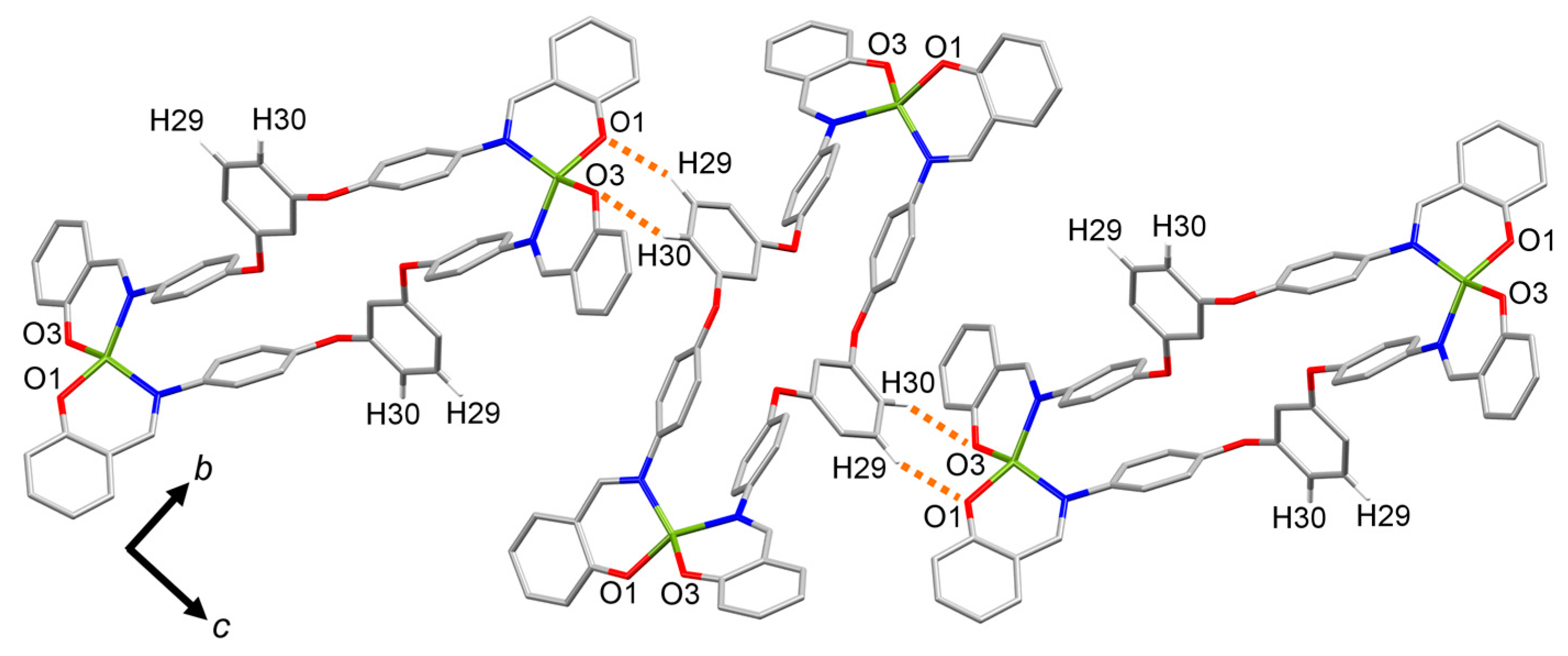
2.4. Molecular Structure of the Cu(II) Metallomacrocycle with H2L4
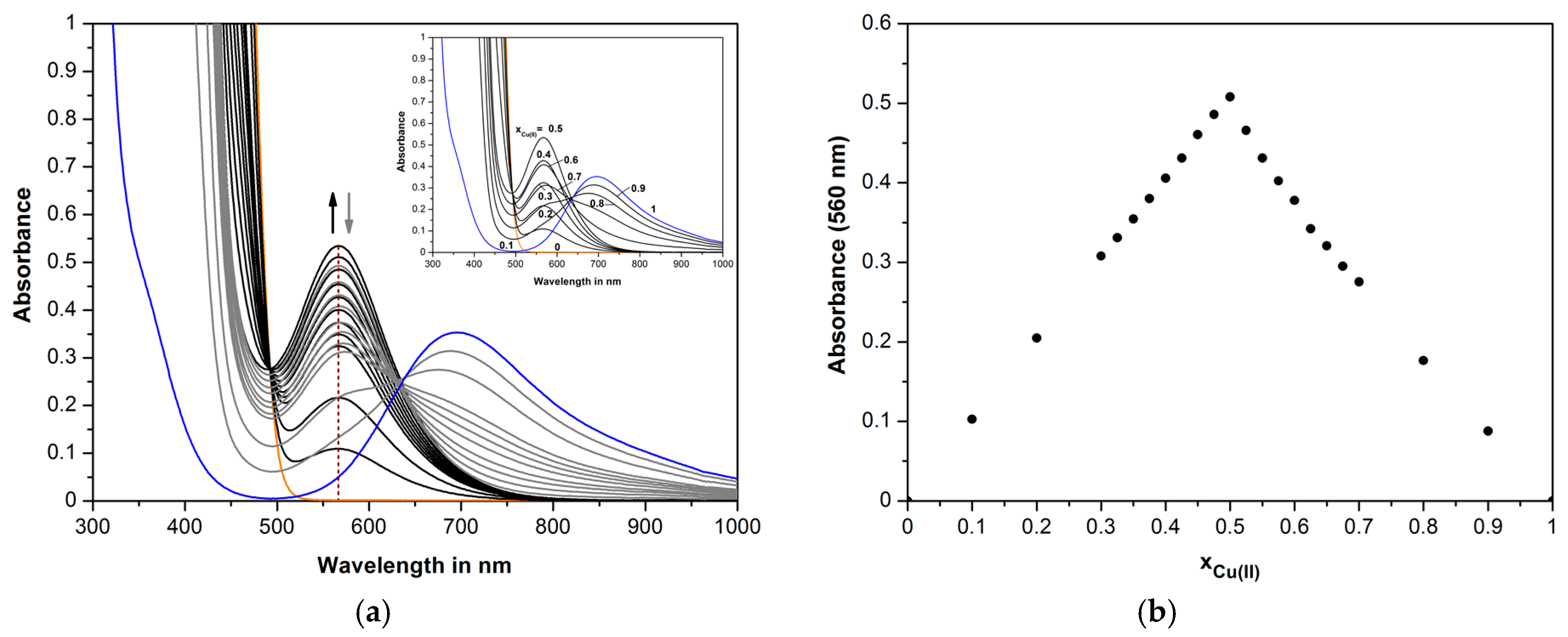
2.5. UV/Vis Studies of Cu(II) with the Diimines H2L1-H2L4
3. Experimental Section
3.1. Reagents and Methodology
3.2. X-ray Structure Determinations
3.3. Ligand Syntheses
3.3.1. (±)-trans-N,N′-Bis(3-ethoxysalicylidene)-1,2-diaminocyclohexane (H2L1)
3.3.2. 2-{[(5-(2-Hydroxybenzylideneamino)-1,3,3-trimethylcyclohexyl)methylimino]methyl}phenol (H2L2)
3.3.3. N,N′-Bis(3-ethoxysalicylidene)-1,6-diaminohexane (H2L3)
3.3.4. 1,3-Bis[4-(salicylideneamino)phenyloxy]benzol (H2L4)
3.4. Complex Syntheses
3.4.1. [Cu2(L1)(NO3)2(H2O)]·MeOH
3.4.2. [Cu2(L2)2]
3.4.3. [Cu2(L3)2]
3.4.4. [Cu2(L4)2]·4H2O
3.5. Liquid-Liquid Extraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshida, N.; Oshio, H.; Ito, T. Self-assembling tetranuclear copper(II) complex of a bis-bidentate Schiff base: Double-helical structure induced by aromatic π···π and CH···π interactions. Chem. Commun. 1998, 63–64. [Google Scholar] [CrossRef]
- Yoshida, N.; Oshio, H.; Ito, T. Copper(II)-assisted self-assembly of bis-N,O-bidentate Schiff bases: New building blocks for a double-helical supramolecular motif. J. Chem. Soc. Perkin Trans. 2 1999, 975–983. [Google Scholar] [CrossRef]
- Lacroix, P.G. Second-order optical nonlinearities in coordination chemistry: The case of bis(salicylaldiminato)metal Schiff base complexes. Eur. J. Inorg. Chem. 2001, 2001, 339–348. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-M.; Huang, J.-S. Metal complexes of chiral binaphthyl Schiff-base ligands and their application in stereoselective organic transformations. Coord. Chem. Rev. 2003, 242, 97–113. [Google Scholar] [CrossRef]
- Sakamoto, M.; Manseki, K.; Okawa, H. d–f Heteronuclear complexes: Synthesis, structures and physicochemical aspects. Coord. Chem. Rev. 2001, 219–221, 379–414. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassaa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, P.G.; Malfant, I.; Real, J.-A.; Rodriguez, V. From magnetic to nonlinear optical switches in spin-crossover complexes. Eur. J. Inorg. Chem. 2013, 2013, 615–627. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S.; Bertolo, L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Coord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- Biswas, S.; Ghosh, A. Use of Cu(II)-di-Schiff bases as metalloligands in the formation of complexes with Cu(II), Ni(II) and Zn(II) perchlorate. Polyhedron 2013, 65, 322–331. [Google Scholar] [CrossRef]
- Nathan, L.C.; Koehne, J.E.; Gilmore, J.M.; Hannibal, K.A.; Dewhirst, W.E.; Mai, T.D. The X-ray structures of a series of copper(II) complexes with tetradentate Schiff base ligands derived from salicylaldehyde and polymethylenediamines of varying chain length. Polyhedron 2003, 22, 887–894. [Google Scholar] [CrossRef]
- Kelly, N.; Schulz, J.; Gloe, K.; Doert, T.; Gloe, K.; Wenzel, M.; Acker, M.; Weigand, J.J. Self-assembly of double-stranded copper(II) helicates with 2-ethoxy-2-hydroxyphenyl substituted diimines. Synthesis, molecular structure, and host-guest recognition of H2O. Z. Anorg. Allg. Chem. 2015, 641, 2215–2221. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Tozzo, E.; Romera, S.; dos Santos, M.P.; Muraro, M.; de A. Santos, R.H.; Liao, L.M.; Vizotto, L.; Dockal, E.R. Synthesis, spectral studies and X-ray crystal structure of N,N′-(±)-trans-1,2-cyclohexylenebis(3-ethoxysalicylideneamine). J. Mol. Struct. 2008, 876, 210–220. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Hierarchical multicomponent assembly utilizing sequential metal-ligand and hydrogen-bonding interactions. CrystEngComm 2009, 11, 657–662. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Host-guest chemistry of a chiral Schiff base copper(II) complex: Can chiral information be transferred to the guest cation. CrystEngComm 2010, 12, 1764–1773. [Google Scholar] [CrossRef]
- Koner, R.; Lee, G.-H.; Wang, Y.; Wei, H.-H.; Mohanta, S. Two new diphenoxo-bridged discrete dinuclear CuIIGdIII compounds with cyclic diimino moieties: Syntheses, structures, and magnetic properties. Eur. J. Inorg. Chem. 2005, 2005, 1500–1505. [Google Scholar] [CrossRef]
- Hazra, S.; Koner, R.; Nayak, M.; Sparkes, H.A.; Howard, J.A.K.; Mohanta, S. Cocrystallized dinuclear-mononuclear CuII3NaI and double-decker-triple-decker CuII5KI3 complexes derived from N,N′-ethylenebis(3-ethoxysalicylaldimine). Cryst. Growth Des. 2009, 9, 3603–3608. [Google Scholar] [CrossRef]
- Berkessel, A.; Roland, K.; Schröder, M.; Neudörfl, J.M.; Lex, J. Enantiomerically pure isophorone diamine [3-(aminomethyl)-3,5,5-trimethylcyclohexylamine]: A chiral 1,4-diamine building block made available on large scale. J. Org. Chem. 2006, 71, 9312–9318. [Google Scholar] [CrossRef] [PubMed]
- Ha, K. 6,6′-Diethoxy-2,2′-[hexane-1,6-diylbis(nitrilomethanylylidene)]diphenol. Acta Cryst. 2011, E67. [Google Scholar] [CrossRef] [PubMed]
- Wester, D.; Palenik, G.J. Pentagonal bipyramidal complexes of nickel(II) and copper(II). The relative importance of ligand geometry vs. crystal field effects. J. Am. Chem. Soc. 1974, 96, 7565–7566. [Google Scholar] [CrossRef]
- Nelson, S.M. Developments in the synthesis and coordination chemistry of macrocyclic Schiff base ligands. Pure Appl. Chem. 1980, 52, 2461–2476. [Google Scholar] [CrossRef]
- Drew, M.G.B.; Nelson, J.; Nelson, S.M. Synthesis of some pentagonal-bipyramidal complexes of manganese(II), iron(II), cobalt(II), nickel(II), copper(II), and zinc(II) with a heptadentate Schiff-base ligand and the crystal and molecular structures of a copper(II) complex. Dalton Trans. 1981, 1685–1690. [Google Scholar] [CrossRef]
- Sakurai, T.; Kobayashi, K.; Tsuboyama, S.; Konyo, Y.; Nagao, A.; Ishizu, K. Structure of dichloro(5,6,8,9,11,12,14,15-octahydro-2,3-benzo-1,4,7,10,13-pentaoxacyclopentadec-2-ene) copper(II) chloroform solvate, a Cu(II) crown ether complex with pentagonaI-bipyramidal geometry. Acta Cryst. 1983, C39, 206–208. [Google Scholar]
- Diouf, O.; Gaye, M.; Sail, A.S.; Tamboura, F.; Barry, A.H.; Jouini, T. Crystal structure of diaqua-2,6-diacetylpyridine-bis(acetylhydrazone)copper(II) complex dinitrate hydrate. Z. Kristallogr. NCS 2001, 216, 421–422. [Google Scholar]
- Zhang, Y.; Feng, W.; Liu, H.; Zhang, Z.; Lü, X.; Song, J.; Fan, D.; Wong, W.-K.; Jones, R.A. Photo-luminescent hetero-trinuclear Zn2Ln (Ln = Nd, Yb, Er or Gd) complexes based on the binuclear Zn2L precursor. Inorg. Chem. Commun. 2012, 24, 148–152. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Oshio, H.; Ito, T. Supramolecular motifs in metal complexes of Schiff bases. Part 6.1 Topology of two types of self-assembly of bis-N,O-bidentate Schiff base ligands by copper(II) ions. J. Chem. Soc. Perkin Trans. 2001, 1674–1678. [Google Scholar] [CrossRef]
- Bruker. Apex Suite (V. 2012.4); Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Bruker AXS Area Detector Scaling and Absorption Correction; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565–566. [Google Scholar] [CrossRef]


| Bond Lengths | |||
|---|---|---|---|
| Cu1-N1 | 1.926(3) | Cu2-O3 | 1.987(2) |
| Cu1-N2 | 1.951(3) | Cu2-O4 | 2.341(3) |
| Cu1-O1 | 1.893(2) | Cu2-O5 | 1.955(2) |
| Cu1-O3 | 1.951(2) | Cu2-O7 | 2.723(3) |
| Cu1-O7 | 2.658(3) | Cu2-O8 | 2.084(4) |
| Cu2-O10 | 2.522(7) | ||
| Cu2-O1W | 1.951(3) | ||
| Bond Lengths | |
|---|---|
| Cu1-N1 | 2.0065(17) |
| Cu1-N4 | 1.9875(19) |
| Cu1-O1 | 1.8947(15) |
| Cu1-O4 | 1.8828(15) |
| Cu2-N2 | 1.9903(19) |
| Cu2-N3 | 1.9963(17) |
| Cu2-O2 | 1.9007(16) |
| Cu2-O3 | 1.8958(17) |
| Bond Lengths | |
|---|---|
| Cu1-N1 | 2.0022(17) |
| Cu1-N3 | 1.9991(17) |
| Cu1-O2 | 1.8791(14) |
| Cu1-O6 | 1.8722(15) |
| Cu2-N2 | 1.9897(19) |
| Cu2-N4 | 1.989(2) |
| Cu2-O3 | 1.8900(16) |
| Cu2-O7 | 1.8838(15) |
| Bond Lengths | |
|---|---|
| Cu1-N1 | 1.976(3) |
| Cu1-N2 | 1.982(3) |
| Cu1-O1 | 1.900(2) |
| Cu1-O3 | 1.899(2) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, N.; Taube, F.; Gloe, K.; Doert, T.; Seichter, W.; Heine, A.; Weigand, J.J.; Gloe, K.
Spacer-Controlled Supramolecular Assemblies of Cu(II) with Bis(2-Hydroxyphenylimine) Ligands. from Monoligand Complexes to Double-Stranded Helicates and Metallomacrocycles
. Crystals 2016, 6, 120.
https://doi.org/10.3390/cryst6090120
Kelly N, Taube F, Gloe K, Doert T, Seichter W, Heine A, Weigand JJ, Gloe K.
Spacer-Controlled Supramolecular Assemblies of Cu(II) with Bis(2-Hydroxyphenylimine) Ligands. from Monoligand Complexes to Double-Stranded Helicates and Metallomacrocycles
. Crystals. 2016; 6(9):120.
https://doi.org/10.3390/cryst6090120
Kelly, Norman, Franziska Taube, Kerstin Gloe, Thomas Doert, Wilhelm Seichter, Axel Heine, Jan J. Weigand, and Karsten Gloe.
2016. "Spacer-Controlled Supramolecular Assemblies of Cu(II) with Bis(2-Hydroxyphenylimine) Ligands. from Monoligand Complexes to Double-Stranded Helicates and Metallomacrocycles
" Crystals 6, no. 9: 120.
https://doi.org/10.3390/cryst6090120