A Heterobimetallic 2-D Coordination Polymer [Na2(Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture
Abstract
:1. Introduction
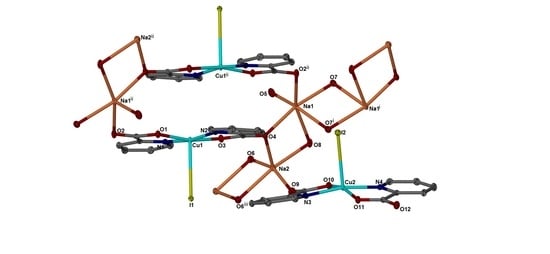
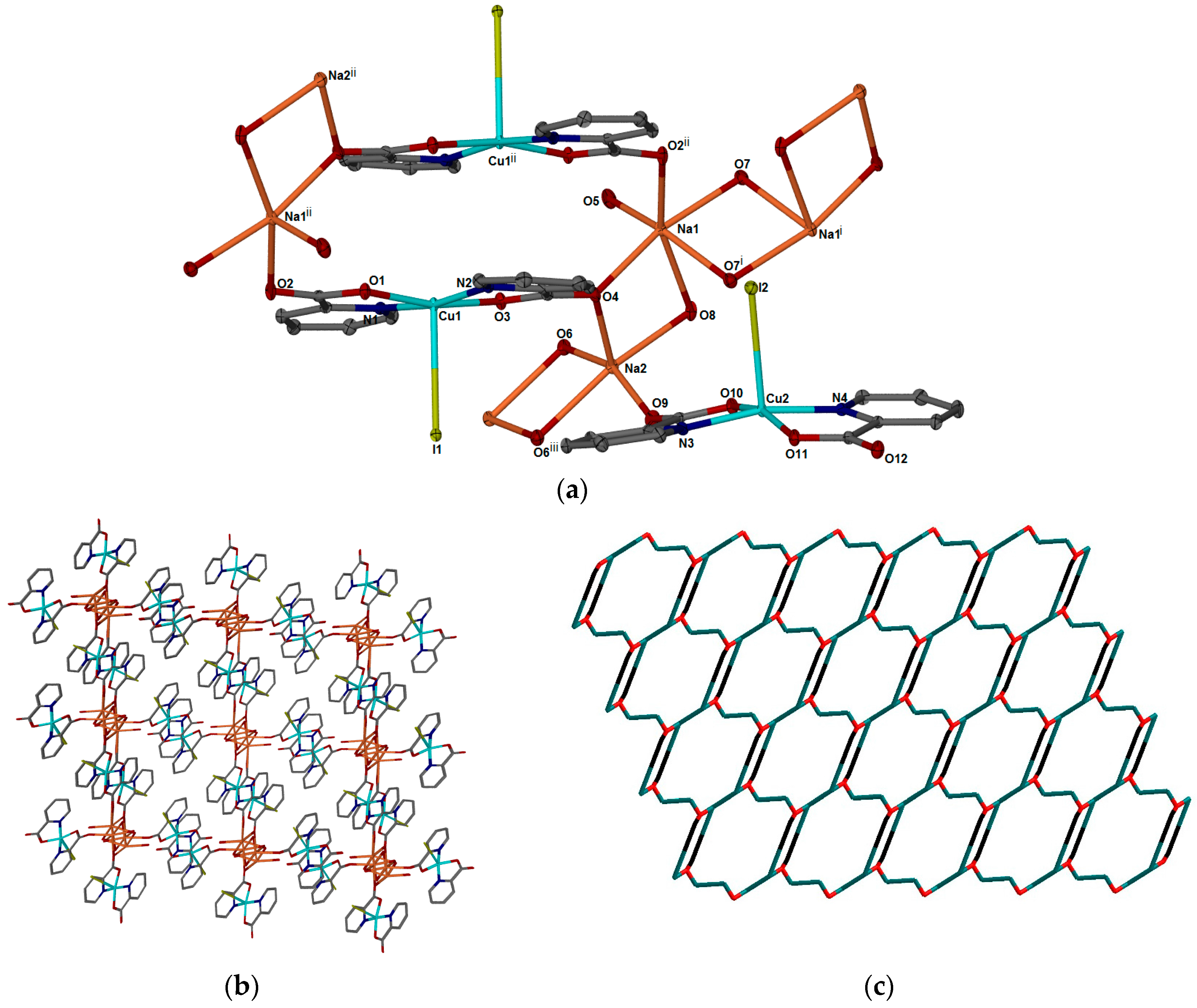
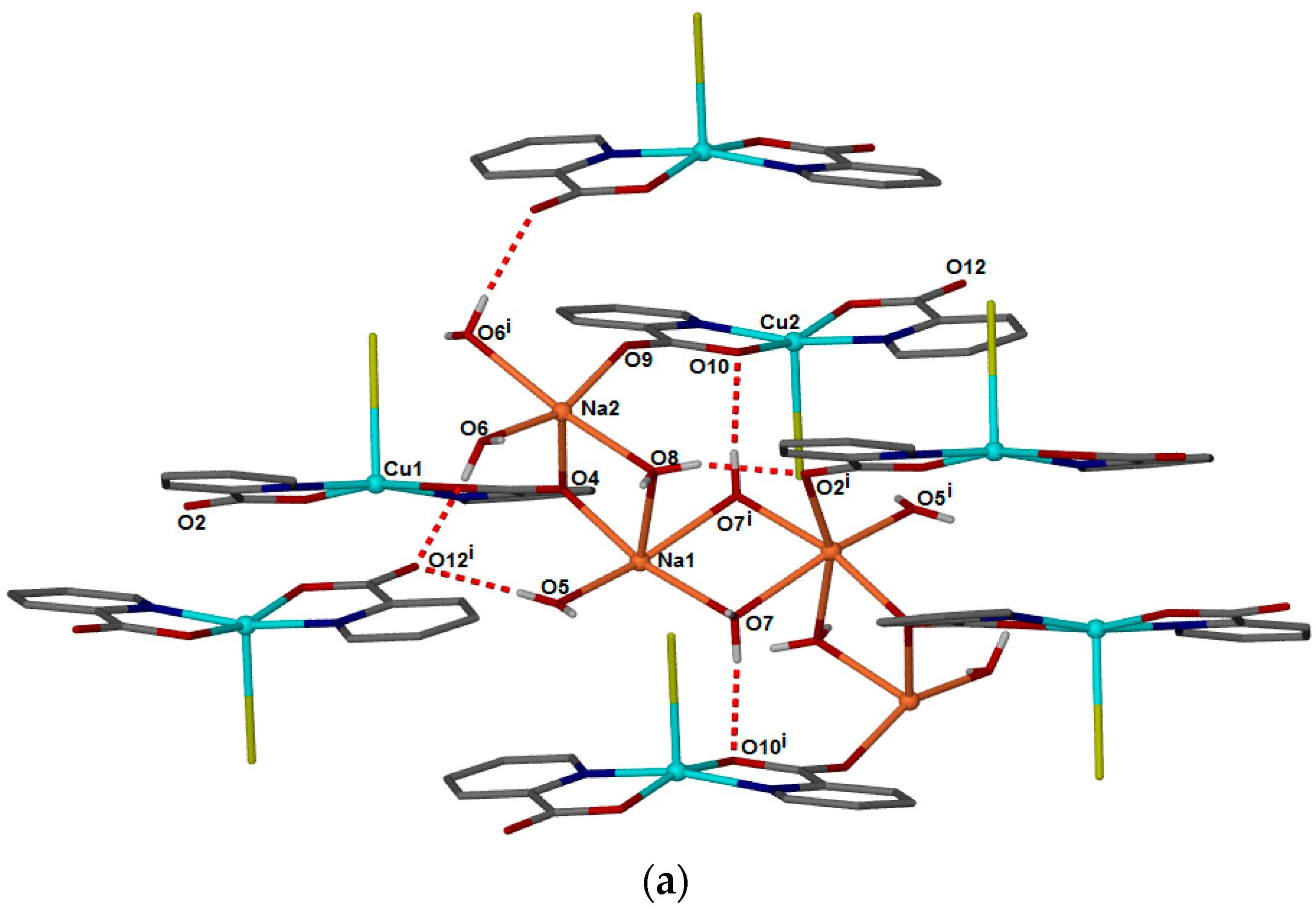
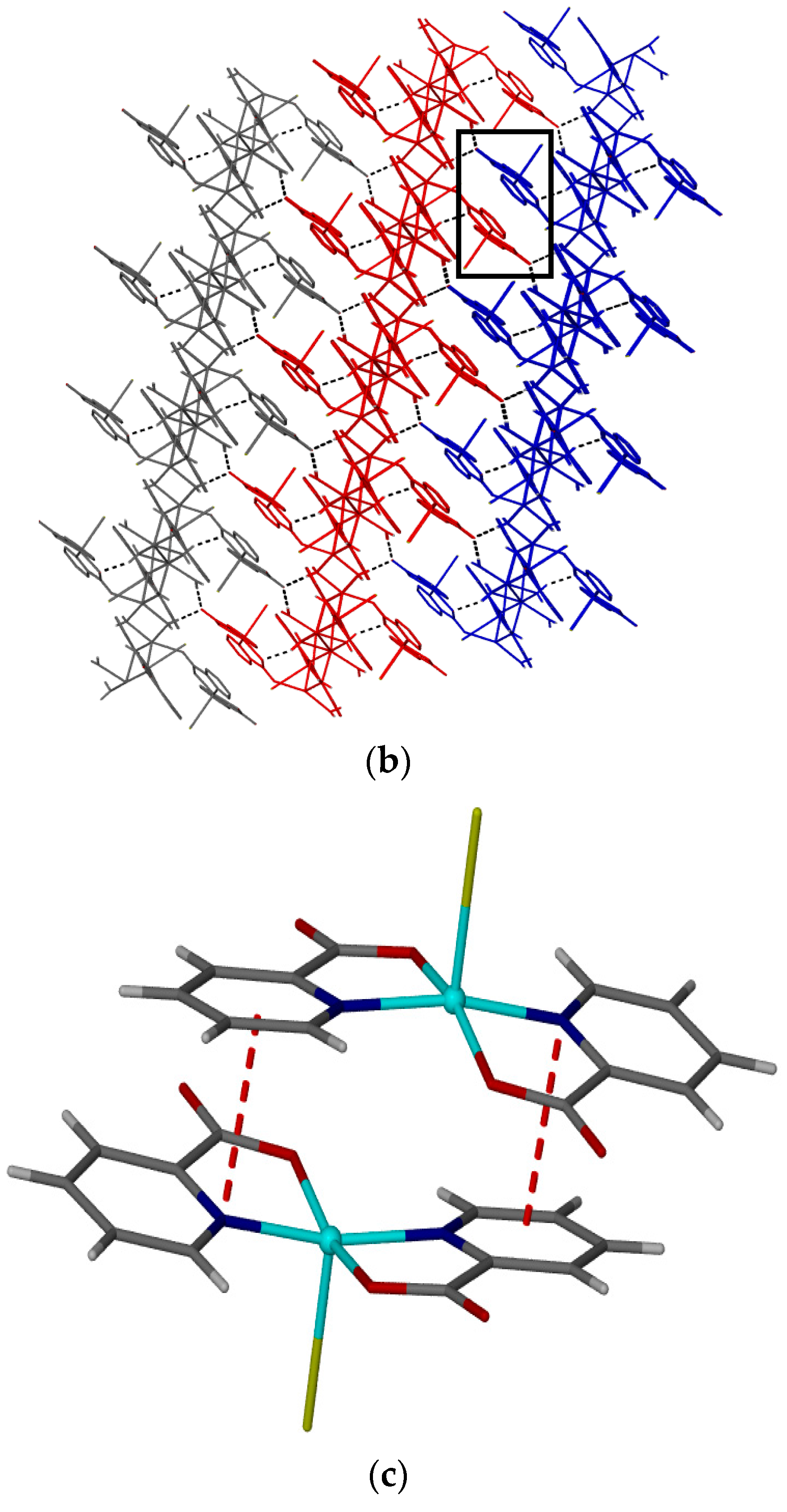
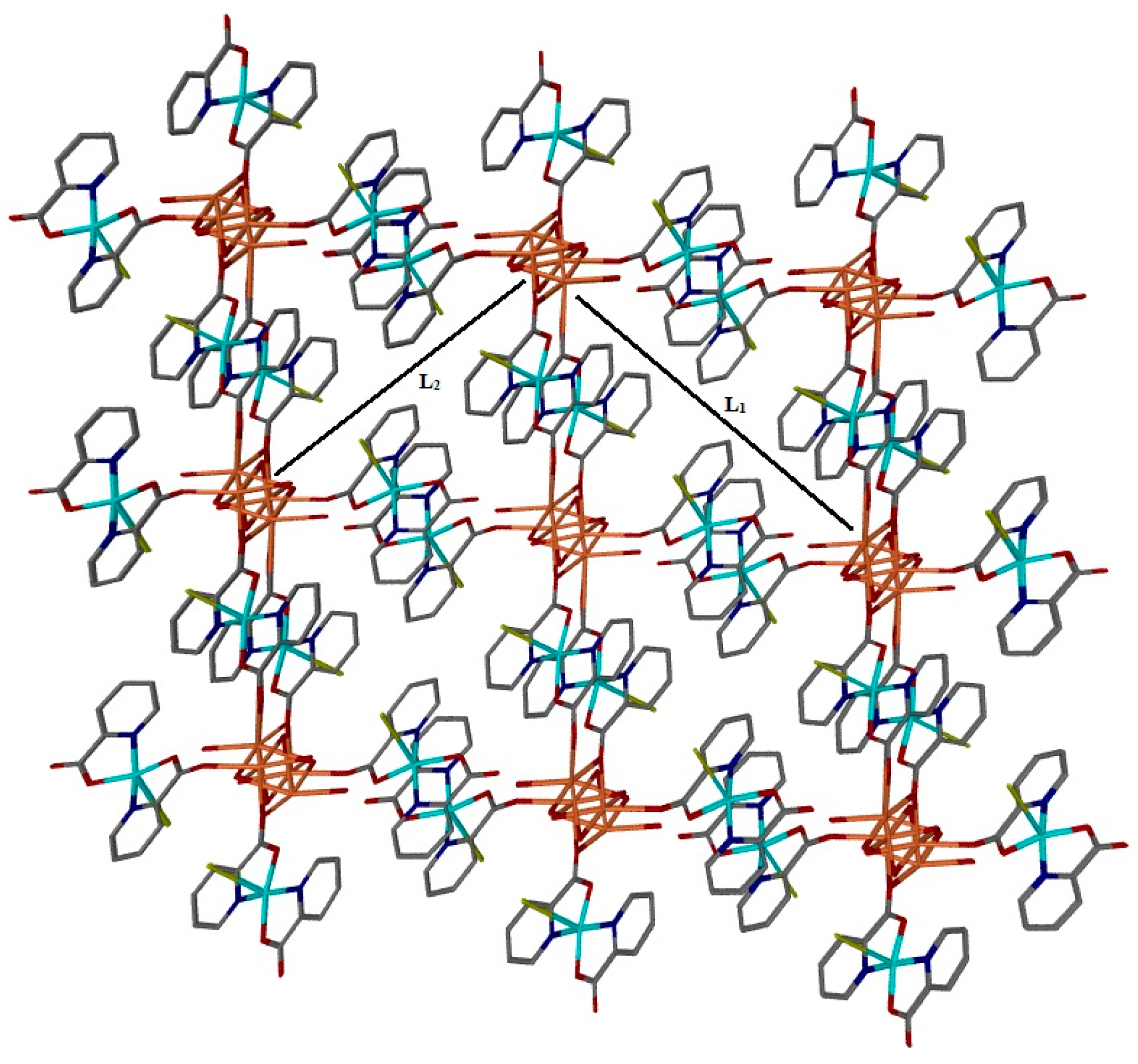
2. Results and Discussion
3. Materials and Methods
Single Crystal X-Ray Structure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separation. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [PubMed]
- Ricco, R.; Malfatti, L.; Takahashi, M.; Hill, A.J.; Falcaro, P. Applications of magnetic metal-organic framework composites. J. Mater. Chem. A. 2013, 1, 13033–13045. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. Gas storage in porous metal-organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gandara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminium metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-H.; Hou, L.; Liu, B.; Cui, L.; Wang, Y.-Y.; Shi, Q.-Z. Two novel interpenetrating MOFs constructed from a derivative of phenanthroline and a V-shaped flexible dicarboxylate ligand contains unique chiral structure. Inorg. Chim. Acta 2012, 382, 13–18. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.H.; Tang, L.F.; Wang, X.G.; Zhao, X.J.; Batten, S.R. Molecular tectonics of metal-organic frameworks (MOFs): A rational design strategy for unusual mixed-connected network topologies. Chem. Eur. J. 2007, 13, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Biswas, R.; Drew, M.G.B.; Frontera, A.; Ghosh, A. Host-guest supramolecular interactions in the coordination of 4,4′-azobis(pyridine) with MnX2 (X=NCS−, NCNCN−, and PF6−): Structure analyses and theoretical study. Inorg. Chem. 2012, 51, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Ribas, J.; Bharadwaj, P.K. Characterization of 3-D metal-organic frameworks formed through hydrogen bonding interactions of 2-D networks with rectangular voids by CoII- and NiII-pyridine-2,6-dicarboxylate and 4,4′-bipyridine or 1,2-di(pyridyl)ethylene. Cryst. Growth Des. 2005, 5, 623–629. [Google Scholar] [CrossRef]
- Biswas, C.; Drew, M.G.B.; Escudero, D.; Frontera, A.; Ghosh, A. Anion-π, lone-pair-π, π-π, and hydrogen-bonding interactions in CuII complex of 2-picolinate and protonated 4,4’-bipyridine: Crystal structure and theoretical studies. Eur. J. Inorg. Chem. 2009, 15, 2238–2246. [Google Scholar] [CrossRef]
- Deshpande, M.S.; Kumbhar, A.S.; Puranik, V.G.; Selvaraj, K. Supramolecular self-assembled ruthenium-polypyridyl framework encapsulating discrete water cluster. Cryst. Growth Des. 2006, 6, 743–748. [Google Scholar]
- Li, Z.Q.; Qiu, L.G.; Wang, W.; Xu, T.; Wu, Y.; Jiang, X. Fabrication of nanosheets of a fluorescent metal-organic framework [Zn(bdc)(H2O)]n (bdc=1,4-benzenedicarboxylate): Ultrasonic synthesis and sensing of ethylamine. Inorg. Chem. Commun. 2008, 11, 1375–1377. [Google Scholar] [CrossRef]
- Li, Q.W.; Zhang, W.Y.; Miljani, O.S.; Sue, C.-H.; Zhao, Y.-L.; Liu, L.H.; Knobler, C.B.; Staoddart, J.F.; Yaghi, O.M. Docking in metal-organic frameworks. Science 2009, 325, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Rahazat, N.R.A.; Haque, R.A.; Ng, S.W.; Razali, M.R. Mononuclear and coordination polymer of silver(I) complexes: Design, synthesis and crystal structure analysis. J. Coord. Chem. 2015, 8, 1317–1331. [Google Scholar] [CrossRef]
- Sulaiman, N.; Salimin, N.R.; Haque, R.A.; Iqbal, M.A.; Ng, S.W.; Razali, M.R. Synthesis, spectroscopic characterization, single crystal X-ray determination and cytotoxicity activity against human breast cancer (MCF-7) and colon cancer (HCT 116) cell lines of silver(I) coordination polymer. Polyhedron 2015, 97, 188–196. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Janiak, G. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligand. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Tahli, A.; Köc, Ü.; Elshaarawy, R.F.M.; Kautz, A.C.; Janiak, C. A cadmium anionic 1-D coordination polymer {[Cd(H2O)6][Cd2(atr)2(µ2-btc)2(H2O)4]2H2O}n within a 3-D supramolecular charge-assisted hydrogen-bonded and π-stacking network. Crystals 2016, 6, 23. [Google Scholar] [CrossRef]
- Wang, C.-C.; Sheu, G.-B.; Ke, S.-Y.; Shin, C.-Y.; Cheng, Y.-J.; Chen, Y.-T.; Cho, C.-H.; Ho, M.-L.; Chen, W.-T.; Liao, R.-S.; et al. A 3D porous supramolecular architecture via π-π assembly of 2D metal-organic frameworks (MOFs): Structure-versus-luminescence reversibility and gas adsorption properties. CrystEngComm. 2015, 17, 1264–1272. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Barbour, L.J. X-Seed—A software tool for supramolecular chemistry. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
| Cu1-I1 | 2.9588(3) | Cu1-N2 | 1.9844(15) |
| Cu1-I2 | 2.9288(3) | Cu2-O10 | 1.9636(12) |
| Cu1-O1 | 1.9500(13) | Cu2-O11 | 1.9564(12) |
| Cu1-O3 | 1.9453(13) | Cu2-N3 | 1.9741(15) |
| Cu1-N1 | 1.9704(15) | Cu2-N4 | 1.9693(15) |
| Na1···Na2 | 3.4098(10) | Na1-O8 | 2.5508(16) |
| Na1···Na1i | 3.5958(14) | Na2-O4 | 2.2881(15) |
| Na1-O2ii | 2.4111(15) | Na2-O6 | 2.3186(15) |
| Na1-O4 | 2.2958(14) | Na2-O6i | 2.4114(16) |
| Na1-O5 | 2.3347(16) | Na2-O9iii | 2.2638(15) |
| Na1-O7 | 2.3656(15) | Na2-O8 | 2.3921(16) |
| Na1-O7i | 2.4351(15) |
| D-H···A | D-H | H···A | D···A | D-H···A |
| O5-HW···O12i | 0.865(10) | 1.986(10) | 2.8504(19) | 177(3) |
| O6-HW···O12i | 0.871(10) | 1.952(11) | 2.8169(19) | 172(3) |
| O7-HW···O10i | 0.875(10) | 2.112(17) | 2.9317(18) | 156(3) |
| O8-HW···O2i | 0.873(10) | 0.873(10) | 2.8286(18) | 161(3) |
| Empirical formula | C24H24Cu2I2N4Na2O12 |
| Formula weight | 987.33 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 11.5781(6) |
| b/Å | 12.1044(7) |
| c/Å | 13.4651(7) |
| α/deg | 65.4000(10) |
| β/deg | 71.7270(10) |
| γ/deg | 68.1360(10) |
| V/Å3 | 1564.03(15) |
| Z | 2 |
| Density calcd/(g/cm3) | 2.097 |
| µ/mm−1 | 3.428 |
| F(000) | 956 |
| T/K | 173(2) |
| Θ range (deg) | 2.25–30.35 |
| reflns collected | 60617 |
| reflns unique | 9316 |
| reflns obs [I > 2σ(I)] | 8842 |
| Rint | 0.0417 |
| R1 (obs, all) | 0.0211 |
| wR2 (obs, all) | 0.0508 |
| GooF | 1.112 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamari, A.A.; Haque, R.A.; Razali, M.R. A Heterobimetallic 2-D Coordination Polymer [Na2(Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture. Crystals 2016, 6, 96. https://doi.org/10.3390/cryst6100096
Kamari AA, Haque RA, Razali MR. A Heterobimetallic 2-D Coordination Polymer [Na2(Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture. Crystals. 2016; 6(10):96. https://doi.org/10.3390/cryst6100096
Chicago/Turabian StyleKamari, Anis A., Rosenani A. Haque, and Mohd R. Razali. 2016. "A Heterobimetallic 2-D Coordination Polymer [Na2(Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture" Crystals 6, no. 10: 96. https://doi.org/10.3390/cryst6100096