Abstract
The synthesis and crystal structure of the one-dimensional coordination polymer, [Cd(spar)2]n·n(H2O), are described, where spar− is the sparfloxacinate anion, C19H21N4O3F2−. The Cd2+ ion is bonded to four spar− ligands: Two O,O-chelate with their β-keto carboxylate groupings and two are monodentate-bound through a carboxylate O atom, to result in a distorted CdO6 octahedral coordination geometry. The bridging ligands lead to [100] polymeric chains in the crystal and N–H···O hydrogen bonds and possible weak aromatic p–p stacking interactions help to consolidate the structure. Crystal data: C38H44CdF4N8O7, Mr = 913.21, triclinic,  (No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, R(F) = 0.036, wR(F2) = 0.082.
(No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, R(F) = 0.036, wR(F2) = 0.082.
 (No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, R(F) = 0.036, wR(F2) = 0.082.
(No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, R(F) = 0.036, wR(F2) = 0.082.1. Introduction
Sparfloxacin (C19H22N4O3F2; Hspar; systematic name: 5-amino-1-cyclopropyl-7-[(3R*,5S*)(3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxo-quinoline-3-carboxylic acid), is a quinolone derivative (Figure 1) [] with significant antibiotic properties [,], although ironically, the effectiveness of Hspar and related compounds in treating infections appear to promote the subsequent colonization by “super bugs” such as MRSA []. Our own interest in this class of compounds, along with that of others, is focused on their potential as multi-dentate and bridging ligands in the construction of new complexes [] and coordination polymers [,].
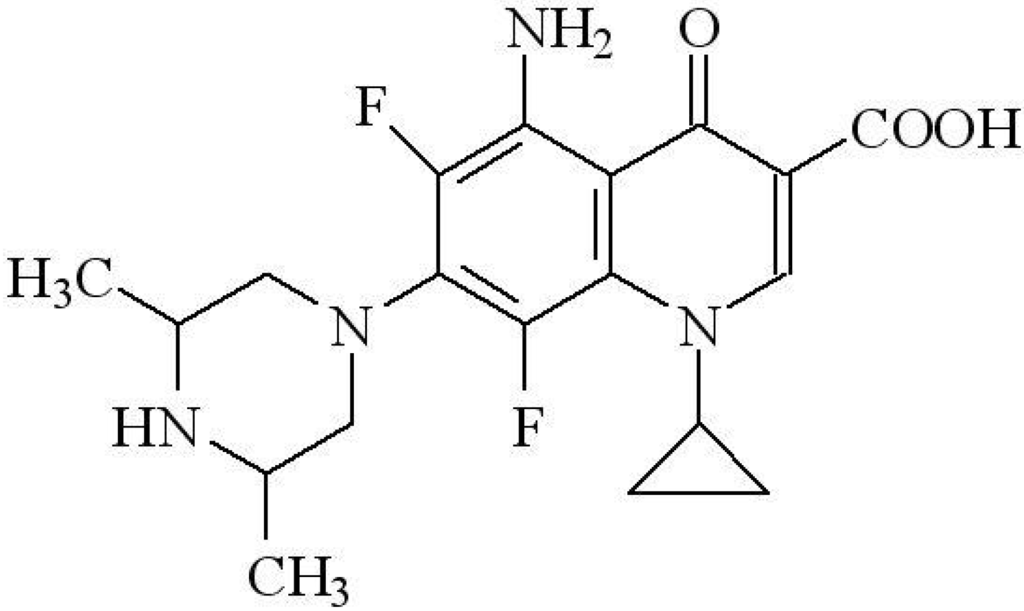
Figure 1.
Chemical scheme for sparfloxacin (C19H22N4O3F2).
The crystal structure of the hydrated, zwitterionic molecule of Hspar has been reported [] and its hydrate polymorphism demonstrated, which may have important pharmacological consequences []. A molecular salt of the H2spar+ cation with sulfate counter-ions is known []. The crystal structures of its anionic complexes with nickel [], copper [,] and zinc [] have been reported. As an extension of these studies, we now describe the hydrothermal synthesis and crystal structure of the polymeric title compound, [Cd(spar)2]n·n(H2O), (1).
2. Results and Discussion
2.1. Crystal Structure of [Cd(spar)2]n·nH2O (1)
Compound 1 is a one-dimensional coordination polymer: The asymmetric unit contains a Cd2+ cation, two mono-anionic spar− ligands and a disordered water molecule of crystallisation (Figure 2).
The metal ion in 1 is coordinated by two bidentate spar− anions, with the ketone O-atom and its syn-carboxylate O atom (O3 and O2, respectively, in the C1-containing ion and O6 and O5, respectively, in the C20-ion) serving as the donors, which generates a six-membered chelate ring in each case. The metal coordination sphere is completed by two monodentate-O bonded spar− species: In each case the carboxylate O-atom anti to the ketone O-atom is involved. Together, these lead to a moderately distorted octahedral geometry for the CdO6 polyhedron (Table 1, Figure 3), with the monodentate O-atoms is a cis disposition. The mean Cd–O separation is 2.293 Å, the angular variance [] for the O–Cd–O bond angles is 105.2° and the bond-valence-sum (BVS) for the metal ion, calculated by the Brown–Altermatt formalism [], is 2.11 (expected value = 2.00). The –O2–C1–C2–C3–O3–Cd1– six-membered chelate ring approximates to a distorted half-chair, with O2 and Cd1 displaced by 0.320(5) Å and −0.702(7) Å, respectively, from the plane of the other four atoms (r.m.s. deviation = 0.026 Å). The –O5–C20–C21–C22–O6–Cd1– ring can be described in the same way, with O5 and Cd1 displaced by 0.190(5) Å and −1.013(7) Å, respectively, from the other atoms (r.m.s. deviation = 0.008 Å). The dihedral angle between the near-planar segments of the chelate rings is 32.0(3)°.
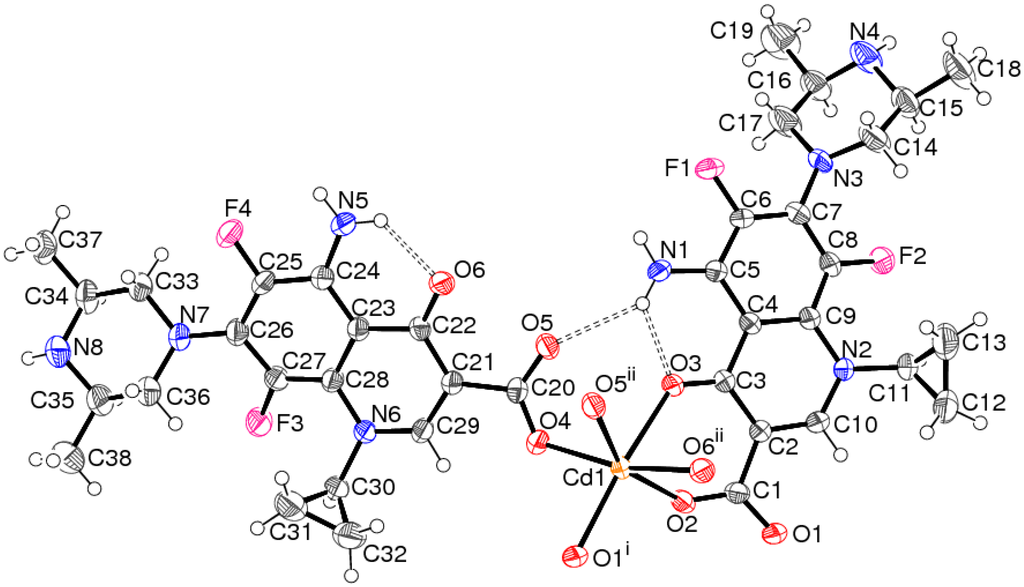
Figure 2.
The asymmetric unit of 1 (50% displacement ellipsoids), expanded to show the complete Cd2+ coordination sphere. Hydrogen bonds are shown as double-dashed lines and the minor disorder components of the piperazine rings of the ligands and the disordered, uncoordinated water molecule are omitted for clarity. See Table 1 for symmetry codes.
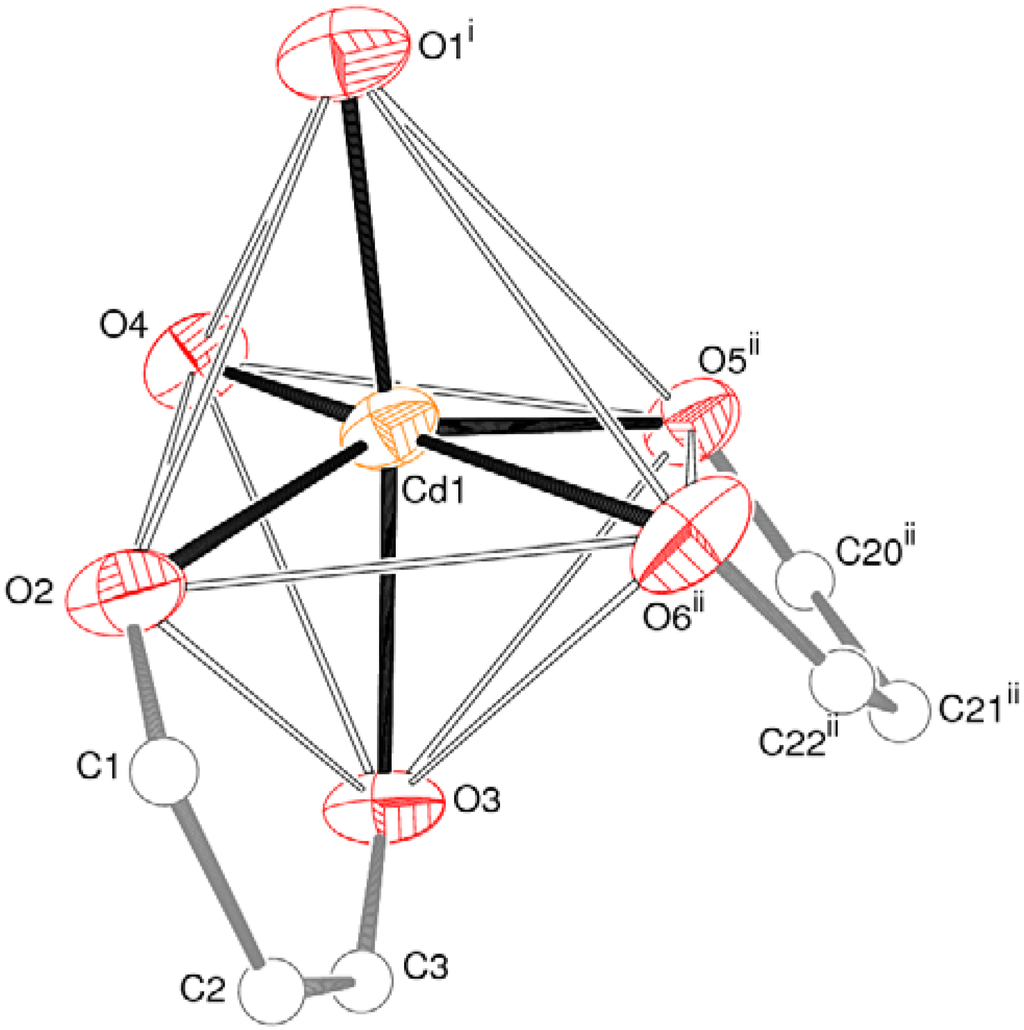
Figure 3.
Detail of 1 showing the coordination geometry of the Cd2+ ion (50% displacement ellipsoids for Cd and O). The octahedral edges are shown as open lines and the C atoms of the chelate rings are shown as spheres. See Table 1 for symmetry codes.

Table 1.
Selected geometrical data (Å,°) for 1.
| Cd1–O4 | 2.264(2) | Cd1–O1 i | 2.269(2) |
| Cd1–O5 ii | 2.280(2) | Cd1–O2 | 2.292(2) |
| Cd1–O6 ii | 2.304(2) | Cd1–O3 | 2.3466(19) |
| O4–Cd1–O1 i | 87.58(8) | O4–Cd1–O5 ii | 90.75(8) |
| O1 i–Cd1–O5 ii | 102.81(8) | O4–Cd1–O2 | 104.12(8) |
| O1 i–Cd1–O2 | 98.16(7) | O5 ii–Cd1–O2 | 154.76(7) |
| O4–Cd1–O6 ii | 164.30(7) | O1 i–Cd1–O6 ii | 100.15(8) |
| O5 ii–Cd1–O6 ii | 74.28(8) | O2–Cd1–O6 ii | 88.40(8) |
| O4–Cd1–O3 | 92.27(7) | O1 i–Cd1–O3 | 170.82(8) |
| O5 ii–Cd1–O3 | 86.37(7) | O2–Cd1–O3 | 72.97(7) |
| O6 ii–Cd1–O3 | 82.26(8) |
Symmetry codes: i 2 − x, 1 − y, 1 − z; ii 1 − x, 1 − y, 1 − z.
The important geometrical features of the first spar− anion (containing C1) are as follows: The C1–O1 and C1–O2 bond lengths of 1.266(4) Å and 1.250(3) Å, respectively, are typical for a delocalised carboxylate group and the dihedral angle between C1/O1/O2 and the adjacent N2-containing ring (r.m.s. deviation = 0.051 Å) is 11.5(5)°. The dihedral angle between the cyclopropane ring and the N2 ring is 66.4(2)°. The dihedral angle between the N2 ring and the C5 ring (r.m.s. deviation = 0.021 Å), which are fused at the C4–C9 bond, is 7.50(15)°, indicating a significant puckering to the quinolone system. The piperazine ring adopts a typical chair conformation with the N–Cq (q = quinolone) bond in an equatorial orientation. Its geometry is complicated by disorder of the C atoms bearing the terminal methyl groups over two orientations, in a 0.766(10):0.234(10) ratio, but both of these maintain the (3R*,5S*) relative configurations of these stereogenic atoms.
The second spar− anion (containing C20) has a broadly similar geometry: The C20–O4 and C20–O5 bond lengths are 1.273(3) Å and 1.250(3) Å, respectively, and the dihedral angle between C20/O4/O5 and the N6 ring (r.m.s. deviation = 0.037°) is 4.0(4)°. The dihedral angle between the N6 ring and the pendent three-membered ring is 73.5(2)°. The N6 and C24 rings (r.m.s. deviation for the latter = 0.041 Å), fused at the C23–C28 bond, are tilted by 4.38(16)°. The piperazine ring in the second anion shows the same type of positional disorder as the first, in a 0.908(8):0.092(8) ratio for the two orientations.
The extended structure of 1 features polymeric chains in the [100] direction (Figure 4), such that each spar− anion links two cadmium metal ions. Adjacent metal ions are fused via eight-membered loops, generated by crystallographic inversion symmetry.
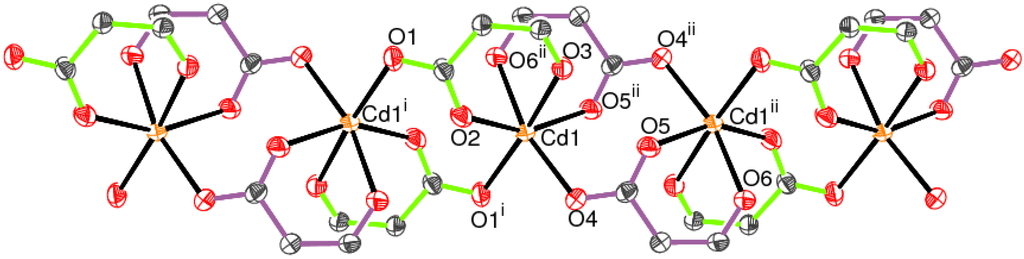
Figure 4.
Fragment of a [100] polymeric chain in 1 showing only the O atoms and linking C atoms of the ligands (50% displacement ellipsoids; symmetry codes as in Table 1). The bonds of the C1 and C20 spar− anions are colored mint and plum, respectively.
To complete the structure of 1, several N–H···O hydrogen bonds occur of varying strengths, including a bifurcated N–H···(O,O) link (Table 2). All of these bonds are intra-chain interactions. It is notable that neither of the piperazine H atoms (attached to N4 and N8) participates in a hydrogen bond, perhaps due in part to the steric crowding of the two adjacent methyl groups. Weak aromatic p–p stacking (centroid–centroid separation = 3.7164(17) Å between the N6 and C24 rings) might also play some role in consolidating the structure of 1.

Table 2.
Hydrogen-Bond geometries for 1.
| N1–H1A···O1 iii | 0.86 | 2.33 | 3.021(3) | 138 |
| N1–H1B···O3 | 0.86 | 1.96 | 2.598(3) | 130 |
| N1–H1B···O5 | 0.86 | 2.35 | 3.061(3) | 141 |
| N5–H5A···O4 iii | 0.86 | 2.59 | 3.221(3) | 131 |
| N5–H5B···O6 | 0.86 | 1.98 | 2.609(3) | 129 |
The four columns specify the D–H, H···A and D···A separations (Å) and the D–H···A angle (°), respectively. Symmetry code: iii x − 1, y, z.
2.2. Spectroscopy
By analogy with data from related compounds [], the 1629 cm−1 band is assigned as a C=O(pyridone) stretch and the 1570 cm−1 and 1364 cm−1 signals correspond to the carboxylate –CO2 asymmetric and symmetric stretches, respectively.
3. Experimental Section
3.1. Synthesis and Characterization
A mixture of cadmium acetate trihydrate (0.25 mmol), sparfloxacin (0.5 mmol), 1,4-benzenedicarboxylic acid (0.25 mmol), sodium hydroxide (1 mmol), and water (15 mL) was stirred for 30 min at room temperature. The mixture was then transferred to a 25-mL Teflon-lined reactor and heated to 423 K for 72 h under autogenous pressure. Upon cooling, colorless prisms of 1 were recovered from the reaction mixture by vacuum filtration. The role of the 1,4-benzenedicarboxylic acid in the synthesis is unknown, but it has not proved possible to prepare 1 if it is not present.
Elemental analysis: calc (%) for C38H44CdF4N8O7: C 49.98, H 4.86, N 12.27; found (%): C 49.76, H 4.49, N 12.04. IR (cm−1, KBr): 3450 (br, m), 1629 (s), 1570 (s), 1449 (s), 1364 (w), 1290 (s).
3.2. Single-Crystal Data Collection and Analysis
The single-crystal data for 1 (colorless prism 0.20 × 0.20 × 0.18 mm) were collected using a Bruker APEX II CCD diffractometer (graphite monochromated MoKα radiation, λ = 0.71073 Å) at room temperature. Data reduction with SAINT [] then proceeded and the structure was solved by direct methods with SHELXS-97 []. The resulting atomic model was developed and refined against |F|2 with SHELXL-97 [] and the “observed data” threshold for calculating the R(F) residuals was set as I > 2σ(I). The C-bound H atoms were placed in idealised locations (C–H = 0.93–0.98 Å) and refined as riding atoms. The N-bound H atoms were located in difference maps: Those attached to N1 and N5 were relocated to idealised locations (N–H = 0.86 Å) and refined as riding and those attached to N4 and N8 were refined as riding in their as-found relative locations. Due to the disorder of the piperazine rings, the location of the N4 and N8 H atoms are perhaps less certain, although they appeared reasonably distinctly in difference maps. The constraint Uiso(H) = 1.2Ueq(carrier) or 1.5Ueq(methyl carrier) was applied as appropriate. The H atoms associated with the disordered water molecule could not be located; based on geometrical considerations, one of the water molecules may form a hydrogen bond to N4. The structural model was analysed and validated with PLATON [] and full refinement details are given in the deposited cif.
Crystal data for 1: C38H44CdF4N8O7, Mr = 913.21, triclinic, 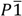 (No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, F(000) = 936, T = 296(2) K, ρcalc = 1.558 g·cm−3, μ = 0.640 mm−1, 27884 reflections recorded (3.4° ≤ 2θ ≤ 50.0°; −10 ≤ h ≤ 10, −15 ≤ k ≤ 15, −20 ≤ l ≤ 20), RInt = 0.039, 6848 merged reflections, 6225 with I > 2σ(I), 541 variable parameters, R(F) = 0.036, wR(F2) = 0.082, min./max. ∆ρ = −0.58/0.46 e Å−3. Cambridge Structural Database deposition number: CCDC-888200.
(No. 2), Z = 2, a = 9.2256(4) Å, b = 12.8767(5) Å, c = 17.4297(7) Å, α = 89.505(2)°, β = 85.062(2)°, γ = 70.757(2)°, V = 1947.20(14) Å3, F(000) = 936, T = 296(2) K, ρcalc = 1.558 g·cm−3, μ = 0.640 mm−1, 27884 reflections recorded (3.4° ≤ 2θ ≤ 50.0°; −10 ≤ h ≤ 10, −15 ≤ k ≤ 15, −20 ≤ l ≤ 20), RInt = 0.039, 6848 merged reflections, 6225 with I > 2σ(I), 541 variable parameters, R(F) = 0.036, wR(F2) = 0.082, min./max. ∆ρ = −0.58/0.46 e Å−3. Cambridge Structural Database deposition number: CCDC-888200.
4. Conclusions
The synthesis and crystal structure of the title one-dimensional coordination polymer have been described, in which the metal atom adopts a distorted octahedral geometry arising from its coordination by two O,O-bidentate and two O-monodentate spar− anions. The linkages in the polymeric chain are centrosymmetric eight-membered rings. The structure of 1 is completely different to that of Cd2(enro)4(H2O)2·4H2O (Henro = enroflaxacin; C19H22N3O3F), in which isolated binuclear complexes occur and the metal coordination geometry is a CdO6 trigonal prism []. In [Cd(cipro)2]n·2.5nH2O (Hcipro = ciprofloxacin; C17H18FN3O4) [], the Cd2+ ion is coordinated by O,O-bidentate and Np-bonded (p = piperazine) cipro− ions to generate a coordination network containing trans-CdN2O4 octahedra. Conversely, Cd(Hcipro)2Cl2·4H2O is a mononuclear complex containing O,O-bidentate neutral, zwitterionic Hcipro molecules and two charge-balancing chloride ions to generate trans-CdCl2O4 octahedra [].
References
- Andersson, M.I.; MacGowan, A.P. Development of the quniolones. J. Antimicrob. Chemother. 2003, 51, 1–11. [Google Scholar] [CrossRef]
- Miyamoto, T.; Matsumoto, J.-I.; Chiba, K.; Egawa, H.; Shibamori, K.; Minamida, A.; Nishimura, Y.; Okada, H.; Kataoka, M.; Fujita, M.; Hirose, T.; Nakano, J. Pyridonecarboxylic acids as antibacterial agents. 14: Synthesis and structure-activity relationship of 5-substituted 6,8-difluoroquinolones, including sparfloxacin, a new quinoline antibacterial agent with improved potency. J. Med. Chem. 1990, 33, 1645–1656. [Google Scholar] [CrossRef]
- Qadri, S.M.; Ueno, Y.; Burns, J.J.; Almodovar, E.; Rabea, N. In vitro activity of sparfloxacin (CI-978), a new broad-spectrum fluoroquinolone. Chemotherapy 1992, 38, 99–106. [Google Scholar] [CrossRef]
- Tacconelli, E.; De Angelis, G.; Cataldo, M.A.; Pozzi, E.; Cauda, R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 61, 26–38. [Google Scholar] [CrossRef]
- An, Z.; Gao, J.; Harrison, W.T.A. Two binuclear complexes containing the enrofloxacinate anion: Cd2(C19H21N3O3F)4(H2O)2·4H2O and Pb2(C19H21N3O3F)4·4H2O. J. Coord. Chem. 2010, 63, 3871–3879. [Google Scholar] [CrossRef]
- Xiao, D.-R.; Wang, E.-B.; An, H.-Y.; Su, Z.-M.; Li, Y.-G.; Gao, L.; Sun, C.-Y.; Xu, L. Rationally designed, polymeric, extended metal-ciprofloxacin complexes. Chem. Eur. J. 2005, 11, 6673–6686. [Google Scholar] [CrossRef]
- An, Z.; Liu, L.-R. Poly[bis[μ-1-cyclopropyl-6-fluoro-4-oxido-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylato]nickel(II)]. Acta Cryst. 2008, E64, m176. [Google Scholar]
- Sivalakshmidevi, A.; Vyas, K.; Om Reddy, G. Sparfloxacin, an antibacterial drug. Acta Cryst. 2000, C56, e115–e116. [Google Scholar]
- Llinas, A.; Burley, J.C.; Prior, T.J.; Glen, R.C.; Goodman, J.M. Concomitant hydrate polymorphism in the precipitation of sparfloxacin from aqueous solution. Cryst. Growth Des. 2008, 8, 114–118. [Google Scholar] [CrossRef]
- Li, T.; Yang, L.; Wang, Y.C.; Lian, Q. Bis[(2R,6S)-4-(5-amino-3-carboxy-1-cyclopropyl-6,8-difluoro-4-oxo-1,4-dihydroquinolin-7-yl)-2,6-dimethylpiperazin-1-ium] sulfate pentahydrate. Acta Cryst. 2011, E67, o3366. [Google Scholar]
- Skyrianou, K.C.; Raptopoulou, C.P.; Psycharis, V.; Kessissoglou, D.P.; Psomas, G. Structure, cyclic voltammetry and DNA-binding properties of the bis(pyridine)bis(sparfloxacinato)nickel(II) complex. Polyhedron 2009, 28, 3265–3271. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Karaliota, K.; Katsaros, N.; Psomas, G. Crystal structure, spectroscopic, and biological study of the copper(II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg. Med. Chem. Lett. 2006, 16, 3864–3867. [Google Scholar] [CrossRef]
- Shingnapurkar, D.; Butcher, R.; Afrasiabi, Z.; Sinn, E.; Ahmed, F.; Sarkar, F.; Padhye, S. Neutral dimeric copper-sparfloxacin conjugate having butterfly motif with antiproliferative effects against hormone independent BT20 breast cancer cell line. Inorg. Chem. Commun. 2007, 10, 459–462. [Google Scholar] [CrossRef]
- Tarushi, A.; Polatoglou, E.; Kljun, J.; Turel, I.; Psomas, G.; Kessissoglou, D.P. Interaction of Zn(II) with quinolone drugs: Structure and biological evaluation. Dalton Trans. 2011, 40, 9461–9473. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation–quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar]
- APEX2 and SAINT Diffractometer Control Software; Bruker AXS Inc.: Madison, WI, USA, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar]
- Lopez-Gresa, M.P.; Ortiz, R.; Perello, L.; Latorre, J.; Liu-Gonzalez, M.; Garcia-Granda, S.; Perez-Priede, M.; Canton, E. Interactions of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin)—Spectroscopic and X-ray structural characterization. Antibacterial studies. J. Inorg. Biochem. 2002, 92, 65–74. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).