The Role of Mediated Oxidation on the Electro-irradiated Treatment of Amoxicillin and Ampicillin Polluted Wastewater
Abstract
:1. Introduction
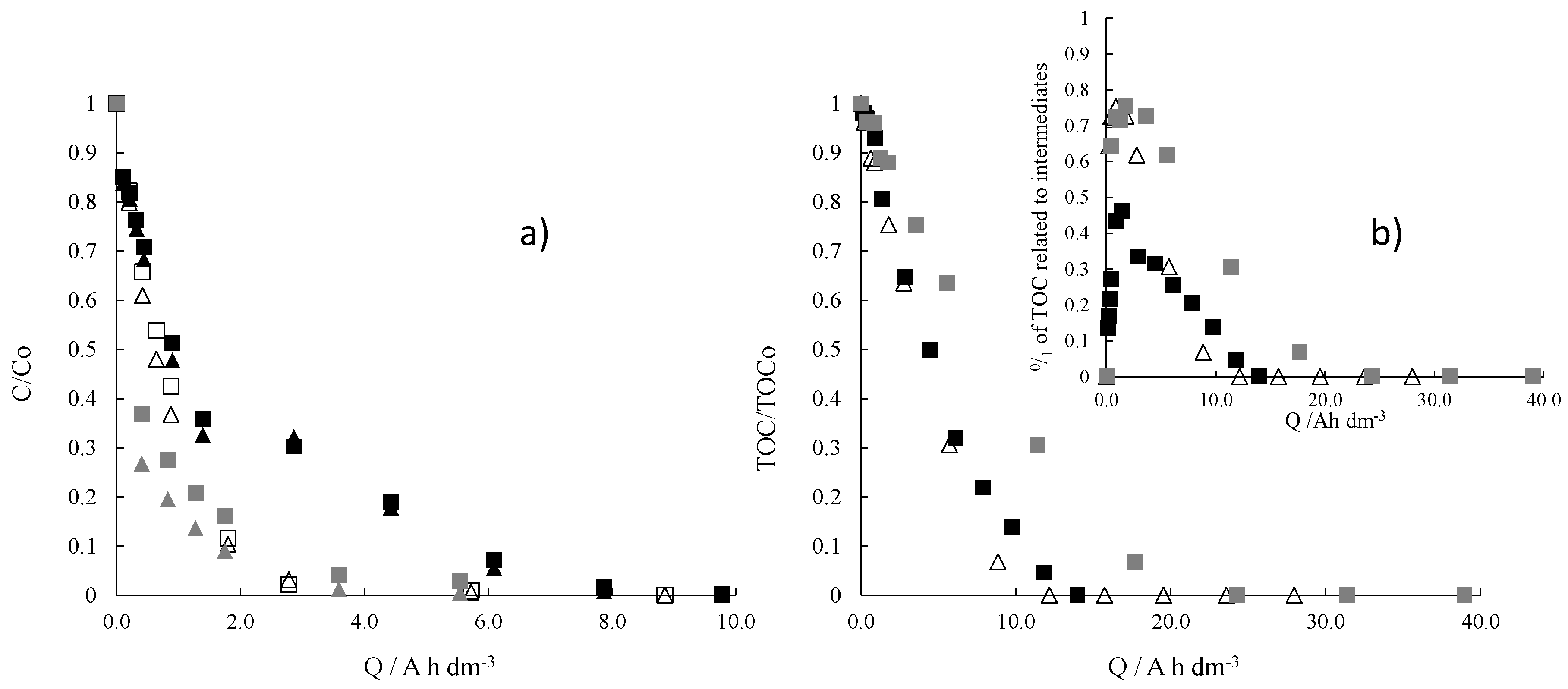
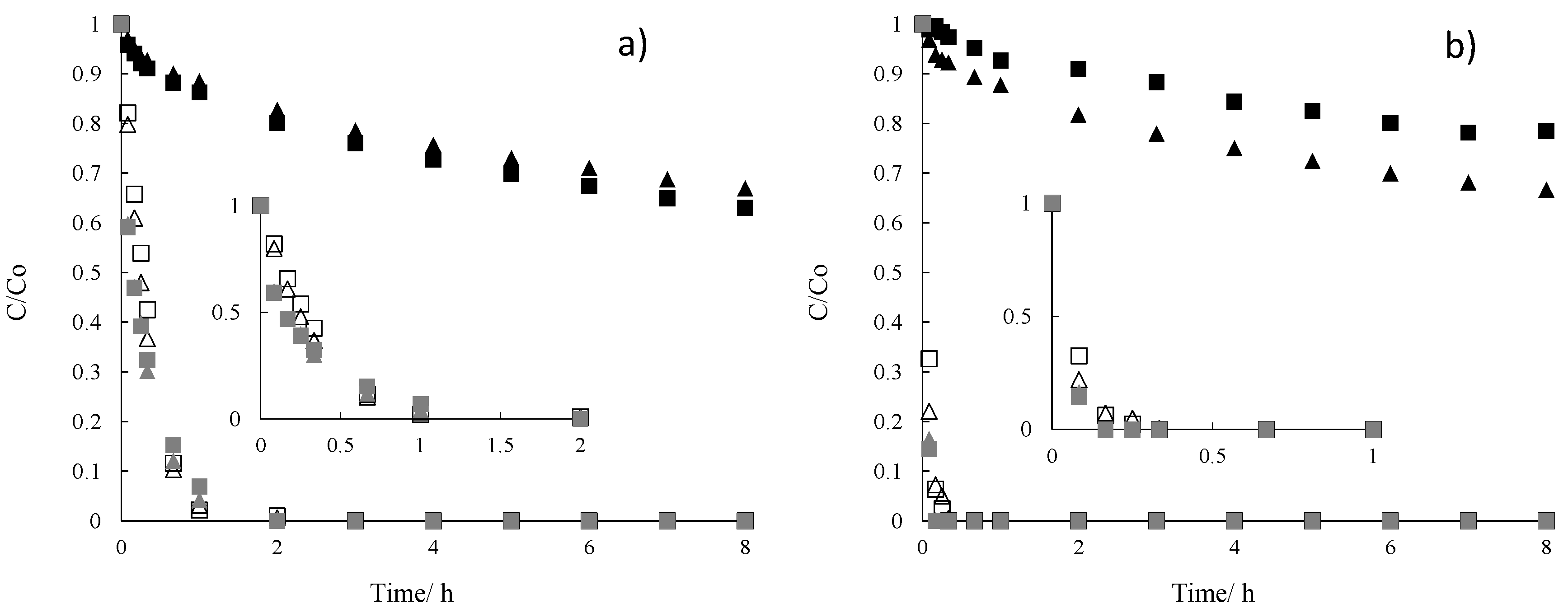
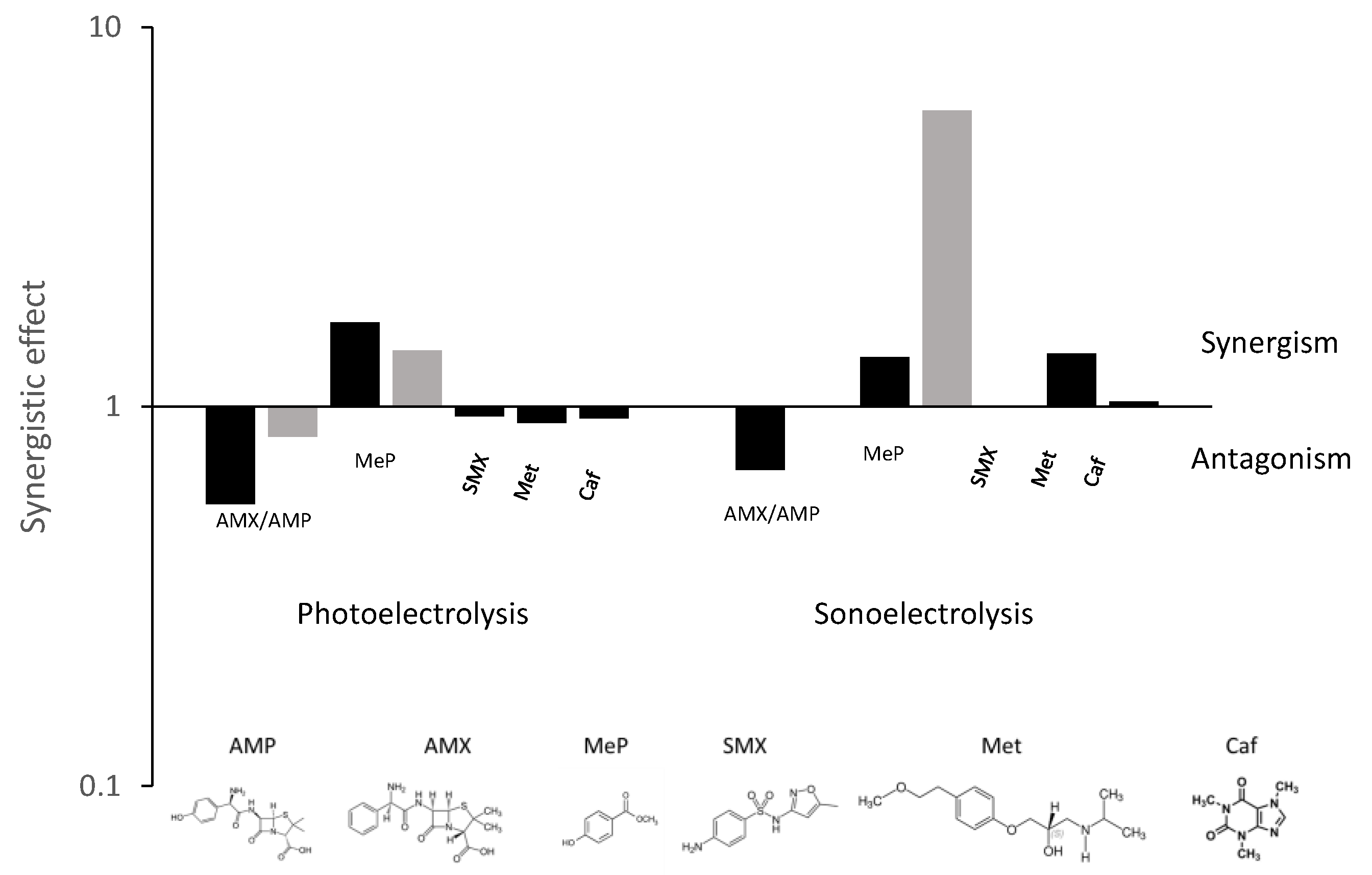
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Electrochemical Cell
3.3. Analysis Procedures
4. Conclusions
- The conductive diamond electrolysis is able to attain the complete mineralization of amoxicillin and ampicillin solutions. The removal efficiency depends on the current density and supporting media, with the antibiotic degradation rate and mineralization favored in the presence of chloride.
- The mineralization rate of AMX and AMP solutions during electrolysis, photoelectrolysis and sonoelectrolysis fits well to pseudo-first order kinetics, although two or three reaction zones are distinguished in sulphate and chloride media, respectively. This may indicate the existence of complex mechanisms in which indirect oxidation is very important.
- The effect of irradiating UV light and/or US waves during the electrolysis of AMX and AMP is not very relevant. This may be explained in terms of the high contribution of mediated oxidation in the single electrolysis process or of the massive formation of radicals during electro-irradiated processes, which recombine to form stable oxidants.
Author Contributions
Funding
Conflicts of Interest
References
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Oturan, N.; Oturan, M.A. Electrochemically Assisted Remediation of Pesticides in Soils and Water: A Review. Chem. Rev. 2014, 114, 8720–8745. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Radjenovic, J.; Jelic, A.; Petrovic, M.; Barcelo, D. Determination of pharmaceuticals in sewage sludge by pressurized liquid extraction (PLE) coupled to liquid chromatography-tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2009, 393, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Urtiaga, A.M.; Perez, G.; Ibanez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Al Aukidy, M.; Verlicchi, P.; Voulvoulis, N. A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci. Total Environ. 2014, 493, 54–64. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. Electro-Fenton, UVA Photoelectro-Fenton and Solar Photoelectro-Fenton Treatments of Organics in Waters Using a Boron-Doped Diamond Anode: A Review. J. Mex. Chem. Soc. 2014, 58, 239–255. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Canizares, P.; Saez, C.; Sanchez-Carretero, A.; Rodrigo, M.A. Synthesis of novel oxidants by electrochemical technology. J. Appl. Electrochem. 2009, 39, 2143–2149. [Google Scholar] [CrossRef]
- De Araujo, D.M.; Cotillas, S.; Saez, C.; Canizares, P.; Martinez-Huitle, C.A.; Andres Rodrigo, M. Activation by light irradiation of oxidants electrochemically generated during Rhodamine B elimination. J. Electroanal. Chem. 2015, 757, 144–149. [Google Scholar] [CrossRef]
- Yoon, Y.; Cho, E.; Jung, Y.; Kwon, M.; Yoon, J.; Kang, J.-W. Evaluation of the formation of oxidants and by-products using Pt/Ti, RuO2/Ti, and IrO2/Ti electrodes in the electrochemical process. Environ. Technol. 2015, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sires, I.; Oturan, N.; Oturan, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Cavalcanti, E.B.; Brillas, E. Mineralization of the antibiotic chloramphenicol by solar photoelectro-Fenton. From stirred tank reactor to solar pre-pilot plant. Appl. Catal. B Environ. 2014, 144, 588–598. [Google Scholar] [CrossRef]
- Haidar, M.; Dirany, A.; Sires, I.; Oturan, N.; Oturan, M.A. Electrochemical degradation of the antibiotic sulfachloropyridazine by hydroxyl radicals generated at a BDD anode. Chemosphere 2013, 91, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2-IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Song, Y.; Tian, Z.; Tu, X.; Hu, X.; Liu, R. Enhanced mineralization of antibiotic berberine by the photoelectrochemical process in presence of chlorides and its optimization by response surface methodology. Environ. Earth Sci. 2015, 73, 4947–4955. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Saez, C.; Canizares, P.; Rodrigo, M.A. Electrolytic and electro-irradiated technologies for the removal of chloramphenicol in synthetic urine with diamond anodes. Water Res. 2018, 128, 383–392. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Saez, C.; Canizares, P.; Rodrigo, M.A. Removal of pharmaceuticals from the urine of polymedicated patients: A first approach. Chem. Eng. J. 2018, 331, 606–614. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-watertreatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.R.; Gonzalez, T.; Palo, P.; Sanchez-Martin, J.; Rodrigo, M.A.; Saez, C. Electrochemical Degradation of a Real Pharmaceutical Effluent. Water Air Soil Pollut. 2012, 223, 2685–2694. [Google Scholar] [CrossRef]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Perez, J.F.; Llanos, J.; Saez, C.; Lopez, C.; Canizares, P.; Rodrigo, M.A. Treatment of real effluents from the pharmaceutical industry: A comparison between Fenton oxidation and conductive-diamond electro-oxidation. J. Environ. Manag. 2017, 195, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, D.; Motheo, A.J.; Sáez, C.; Rodrigo, M.A. Effect of the electrolyte on the electrolysis and photoelectrolysis of synthetic methyl paraben polluted wastewater. Sep. Purif. Technol. 2019, 208, 201–207. [Google Scholar] [CrossRef]
- Serrano, K.; Michaud, P.A.; Comninellis, C.; Savall, A. Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim. Acta 2002, 48, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Cotillas, S.; de Vidales, M.J.M.; Llanos, J.; Saez, C.; Canizares, P.; Rodrigo, M.A. Electrolytic and electro-irradiated processes with diamond anodes for the oxidation of persistent pollutants and disinfection of urban treated wastewater. J. Hazard. Mater. 2016, 319, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Martin de Vidales, M.J.; Cotillas, S.; Perez-Serrano, J.F.; Llanos, J.; Saez, C.; Canizares, P.; Rodrigo, M.A. Scale-up of electrolytic and photoelectrolytic processes for water reclaiming: A preliminary study. Environ. Sci. Pollut. Res. Int. 2016, 23, 19713–19722. [Google Scholar] [CrossRef] [PubMed]
- Boye, B.; Dieng, M.M.; Brillas, E. Electrochemical degradation of 2,4,5-trichlorophenoxyacetic acid in aqueous medium by peroxi-coagulation. Effect of pH and UV light. Electrochim. Acta 2003, 48, 781–790. [Google Scholar] [CrossRef]
- Diagne, M.; Oturan, N.; Oturan, M.A.; Sires, I. UV-C light-enhanced photo-Fenton oxidation of methyl parathion. Environ. Chem. Lett. 2009, 7, 261–265. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chen, K.H.; Huang, Y.H. Mineralization of organic acids by the photo-electrochemical process in the presence of chloride ions. J. Taiwan Inst. Chem. Eng. 2014, 45, 962–966. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Souza, F.L.; Sáez, C.; Cañizares, P.; Motheo, A.J.; Rodrigo, M.A. Coupling photo and sono technologies to improve efficiencies in conductive diamond electrochemical oxidation. Appl. Catal. B Environ. 2013, 144, 121–128. [Google Scholar] [CrossRef]
- Rubi-Juarez, H.; Cotillas, S.; Saez, C.; Canizares, P.; Barrera-Diaz, C.; Rodrigo, M.A. Use of conductive diamond photo-electrochemical oxidation for the removal of pesticide glyphosate. Sep. Purif. Technol. 2016, 167, 127–135. [Google Scholar] [CrossRef]
- Aquino, J.M.; Miwa, D.W.; Rodrigo, M.A.; Motheo, A.J. Treatment of actual effluents produced in the manufacturing of atrazine by a photo-electrolytic process. Chemosphere 2017, 172, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vieira dos Santos, E.; Saez, C.; Canizares, P.; Rodrigo, M.A.; Martinez-Huitle, C.A. Coupling Photo and Sono Technologies with BDD Anodic Oxidation for Treating Soil-Washing Effluent Polluted with Atrazine. J. Electrochem. Soc. 2018, 165, E262–E267. [Google Scholar] [CrossRef]
- Comninellis, C.; Nerini, A. Anodic-oxidation of phenol in the presence of nacl for waste-water treatment. J. Appl. Electrochem. 1995, 25, 23–28. [Google Scholar] [CrossRef]
- Garbellini, G.S.; Salazar-Banda, G.R.; Avaca, L.A. Effects of ultrasound on the degradation of pentachlorophenol by Boron-Doped diamond electrodes. Port. Electrochim. Acta 2010, 28, 405–415. [Google Scholar] [CrossRef]
- Ma, Y.S.; Sung, C.F.; Lin, J.G. Degradation of carbofuran in aqueous solution by ultrasound and Fenton processes: Effect of system parameters and kinetic study. J. Hazard. Mater. 2010, 178, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Bringas, E.; Saiz, J.; Ortiz, I. Kinetics of ultrasound-enhanced electrochemical oxidation of diuron on boron-doped diamond electrodes. Chem. Eng. J. 2011, 172, 1016–1022. [Google Scholar] [CrossRef]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Effect of resonance frequency, power input, and saturation gas type on the oxidation efficiency of an ultrasound horn. Ultrason. Sonochem. 2011, 18, 209–215. [Google Scholar] [CrossRef]
- Martin de Vidales, M.J.; Barba, S.; Saez, C.; Canizares, P.; Rodrigo, M.A. Coupling ultraviolet light and ultrasound irradiation with Conductive-Diamond Electrochemical Oxidation for the removal of progesterone. Electrochim. Acta 2014, 140, 20–26. [Google Scholar] [CrossRef]
- Steter, J.R.; Dionisio, D.; Lanza, M.R.V.; Motheo, A.J. Electrochemical and sonoelectrochemical processes applied to the degradation of the endocrine disruptor methyl paraben. J. Appl. Electrochem. 2014, 44, 1317–1325. [Google Scholar] [CrossRef]
- Martin de Vidales, M.J.; Saez, C.; Perez, J.F.; Cotillas, S.; Llanos, J.; Canizares, P.; Rodrigo, M.A. Irradiation-assisted electrochemical processes for the removal of persistent organic pollutants from wastewater. J. Appl. Electrochem. 2015, 45, 799–808. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Saez, C.; Canizares, P.; Rodrigo, M.A. Disinfection of urine by conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2018, 229, 63–70. [Google Scholar] [CrossRef]



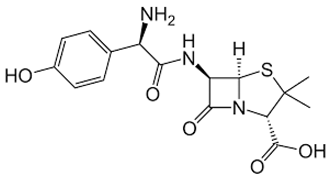
| Amoxicillin | Ampicillin |
|---|---|
 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.L.; Sáez, C.; Lanza, M.R.V.; Cañizares, P.; Rodrigo, M.A. The Role of Mediated Oxidation on the Electro-irradiated Treatment of Amoxicillin and Ampicillin Polluted Wastewater. Catalysts 2019, 9, 9. https://doi.org/10.3390/catal9010009
Silva FL, Sáez C, Lanza MRV, Cañizares P, Rodrigo MA. The Role of Mediated Oxidation on the Electro-irradiated Treatment of Amoxicillin and Ampicillin Polluted Wastewater. Catalysts. 2019; 9(1):9. https://doi.org/10.3390/catal9010009
Chicago/Turabian StyleSilva, Fernando L., Cristina Sáez, Marcos R.V. Lanza, Pablo Cañizares, and Manuel A. Rodrigo. 2019. "The Role of Mediated Oxidation on the Electro-irradiated Treatment of Amoxicillin and Ampicillin Polluted Wastewater" Catalysts 9, no. 1: 9. https://doi.org/10.3390/catal9010009





