Heterogeneous Catalytic Synthesis of 2-Methylbenzimidazole from 2-Nitroaniline and Ethanol Over Mg Modified Cu-Pd/γ-Al2O3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Benzimidazoles Over Pd-Cu/γ-Al2O3 Based Catalysts
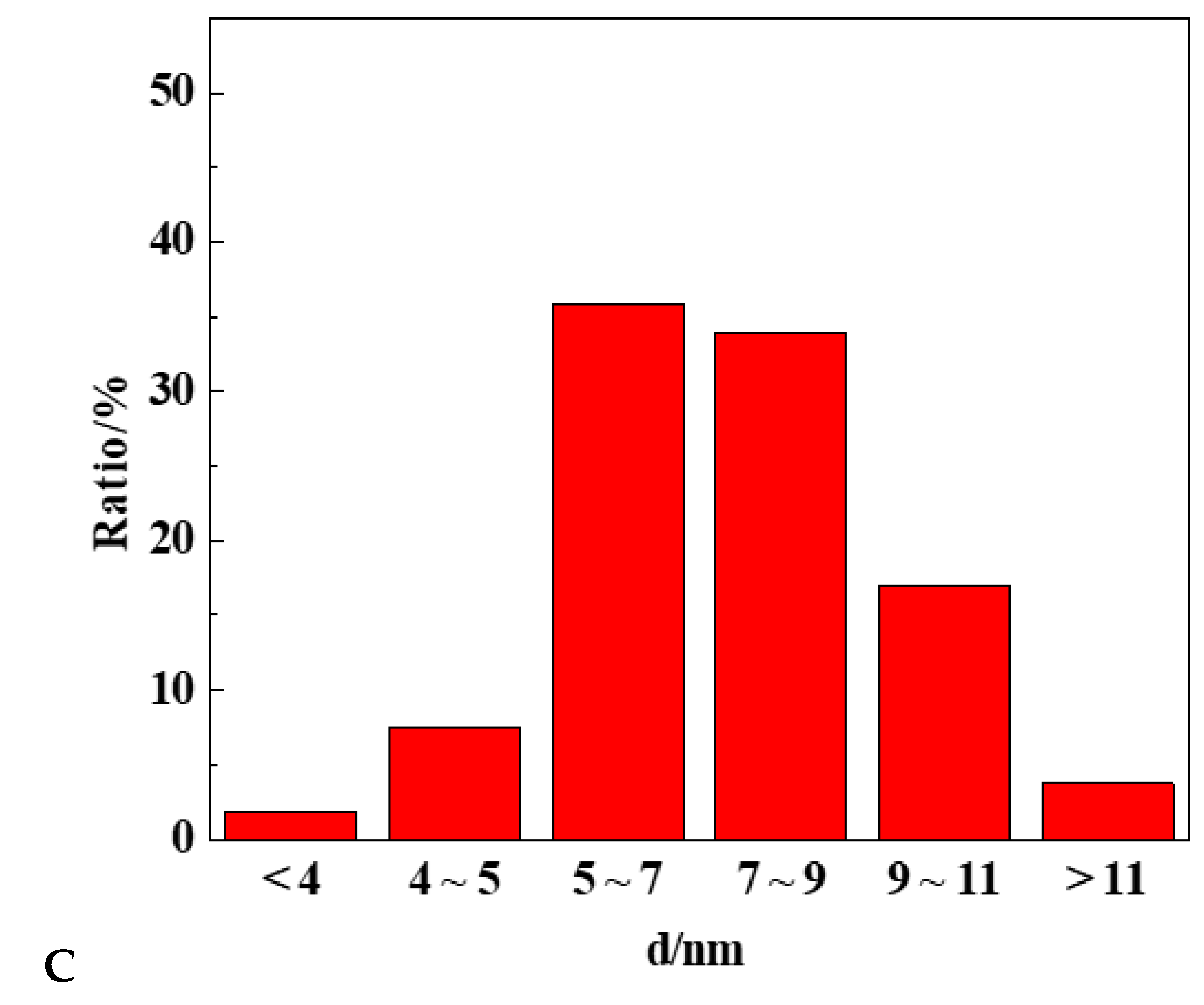
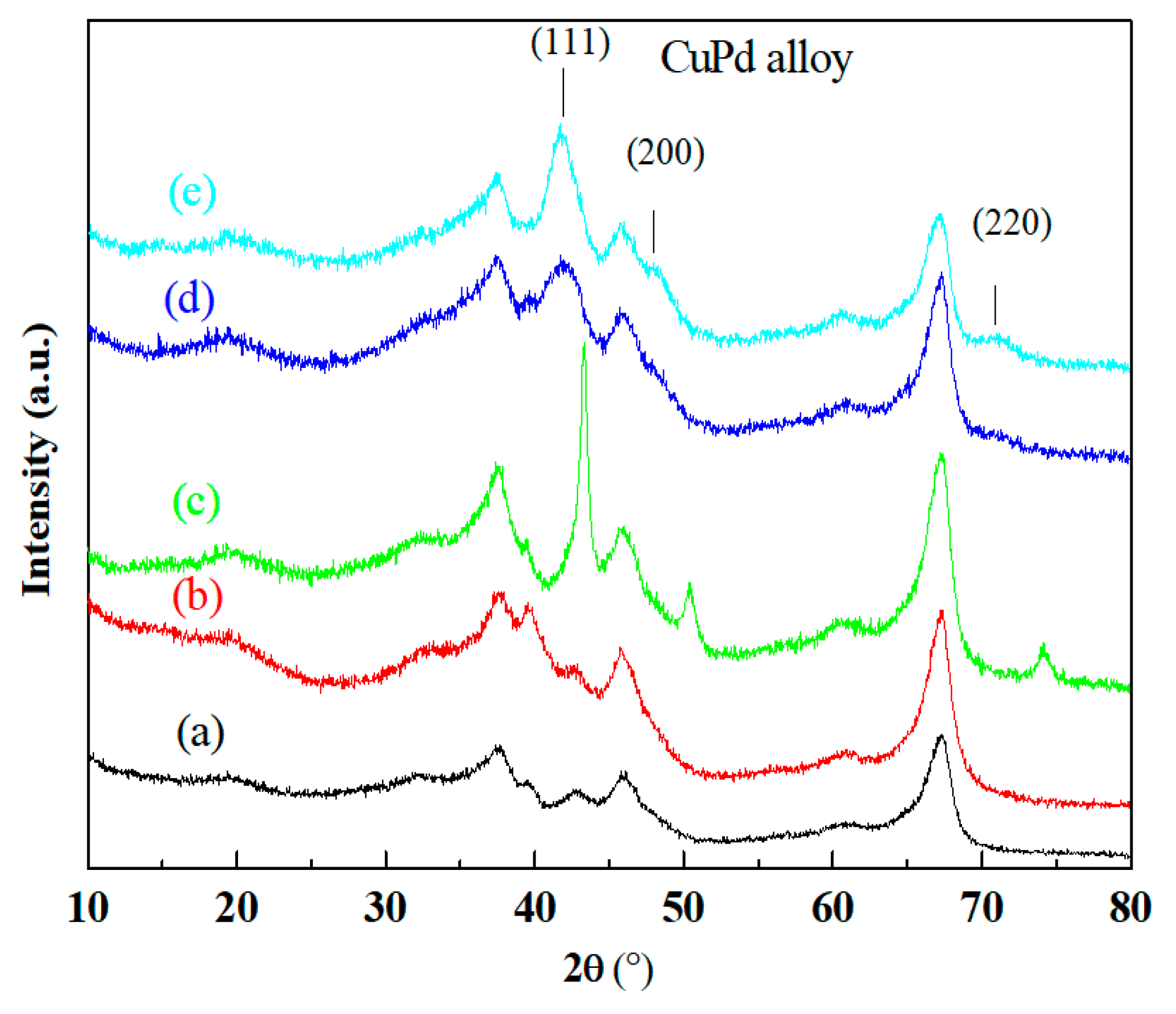
2.2. Effect of Mg Modification on the Formation of CuPd Alloy
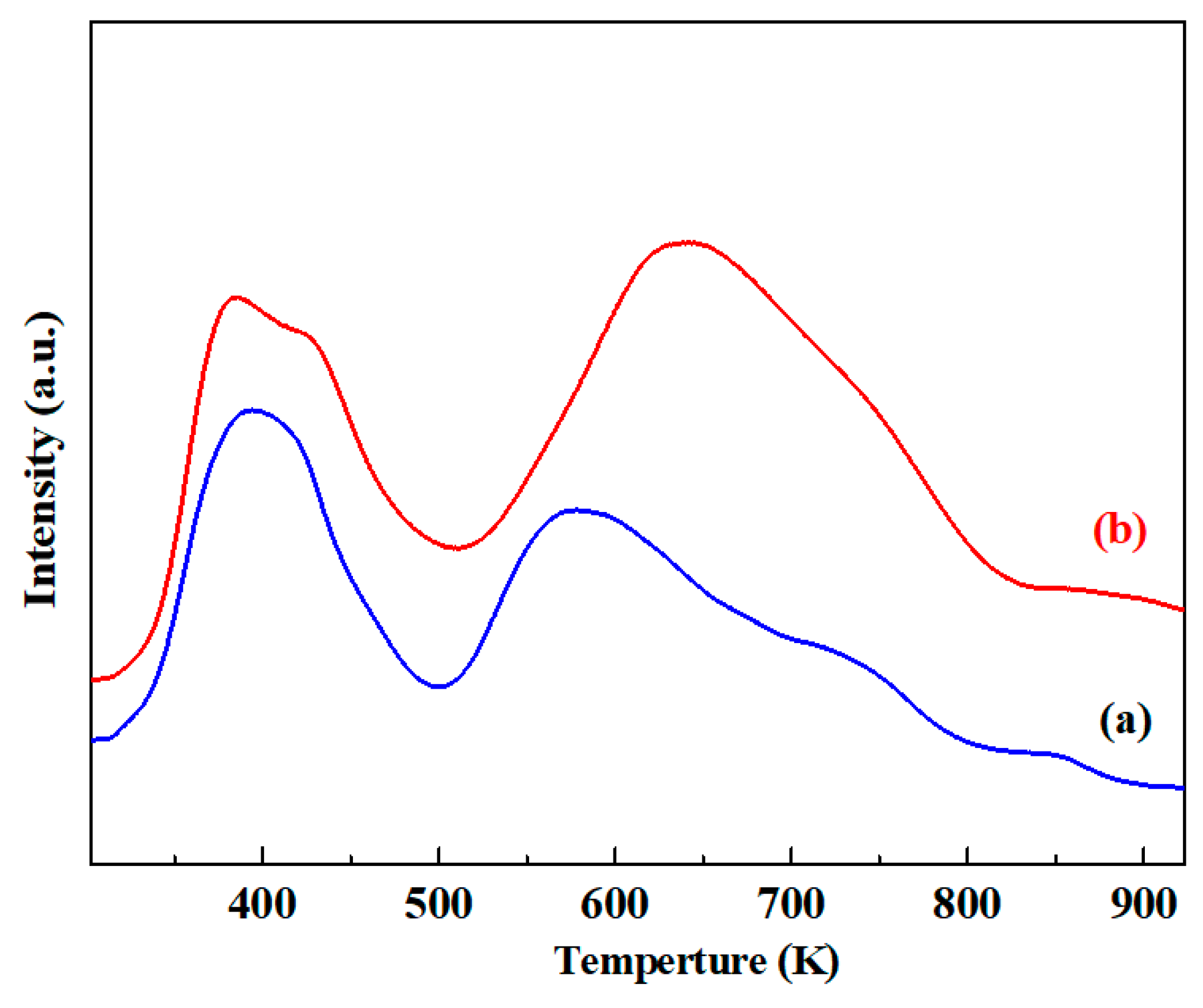
2.3. Effect of Mg Modification on the Surface Acidity and Basicity of Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Gangal, S.; Rauf, A. Convinient one-pot synthesis of novel 2-substituted benzimidazoles, tetrahydrobenzimidazoles and imidazoles and evaluation of their in vitro antibacterial and antifungal activities. Eur. J. Med. Chem. 2009, 44, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009, 44, 4244–4248. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.F.; Lal, C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem. 2009, 44, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Mungra, D.C.; Patel, M.P.; Patel, R.G. Microwave-assisted synthesis of some new tetrazolo[1,5-a]quinoline-based benzimidazoles catalyzed by p-TsOH and investigation of their antimicrobial activity. Med. Chem. Res. 2011, 20, 782–789. [Google Scholar] [CrossRef]
- Jain, R.; Agarwal, D.D.; Sahu, P.K.; Selvam, D.T.; Sharma, Y.; Gupta, R.; Prakash, A. Mild and highly efficient copper(II) sulfate catalyzed one pot synthesis of 2-aryl benzimidazole using atmospheric air as an oxidant and its antibacterial study. Med. Chem. Res. 2013, 22, 1788–1794. [Google Scholar] [CrossRef]
- Chen, G.Z.; Liu, Z.G.; Zhang, Y.L.; Shan, X.O.; Jiang, L.L.; Zhao, Y.J.; He, W.F.; Feng, Z.G.; Yang, S.L.; Liang, G. Synthesis and anti-inflammatory evaluation of novel benzimidazole and imidazopyridine derivatives. ACS Med. Chem. Lett. 2013, 4, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, X.X.; Yu, X.Q.; Shi, L.; Sun, Y. Acid-catalyzed solvent-free synthesis of 2-arylbenzimidazoles under microwave irradiation. J. Mol. Catal. A Chem. 2013, 22, 1788–1794. [Google Scholar] [CrossRef]
- Lu, J.; Ge, H.G.; Bai, Y.J. Solvent-free synthesis of 2-substituted benzimidazoles under microwave-irradiation using PPA as a catalyst. Chin. J. Org. Chem. 2002, 22, 782–784. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Moreno-Diaz, H.; Aguirre-Crespo, F.; León-Rivera, I.; Villalobos-Molina, R.; Muñoz-Muñiz, O.; Estrada-Soto, S. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg. Med. Chem. Lett. 2006, 16, 4169–4173. [Google Scholar] [CrossRef]
- Sun, P.P.; Hu, Z.X. The convenient synthesis of benzimidazole derivatives catalyzed by I2 in aqueous media. Heterocycl. Chem. 2006, 43, 773–775. [Google Scholar] [CrossRef]
- Lin, S.N.; Yang, L.H. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett. 2005, 46, 4315–4319. [Google Scholar] [CrossRef]
- Duan, L.P.; Li, Q.; Wu, N.B.; Xu, D.F.; Zhang, H.B. Synthesis of 2,5-disubstitued benzimidazole using SnCl2-catalyzed reduction system at room temperature. Chin. Chem. Lett. 2014, 25, 155–158. [Google Scholar] [CrossRef]
- Trivedi, R.; De, S.K.; Gibbs, R.A. A convenient one-pot synthesis of 2-substituted benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11. [Google Scholar] [CrossRef]
- Shen, M.G.; Cai, C. Ytterbium perfluorooctanesulfonates catalyzed synthesis of benzimidazole derivatives in fluorous solvents. J. Fluor. Chem. 2007, 128, 232–235. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Shinde, D.B. BF3.OEt2 promoted solvent-free synthesis of benzimidazole derivatives. Chin. Chem. Lett. 2006, 17, 453–456. [Google Scholar]
- Li, X.T.; Hu, R.H.; Tong, Y.; Pan, Q.; Miao, D.Z.; Han, S.Q. An efficient route for the synthesis of benzimidazoles via a hydrogentransfer strategy between o-nitroanilines and alcohols. Tetrahedron Lett. 2016, 57, 4645–4649. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H. A Heterogeneous catalyst for efficient and green synthesis of 2-arylbenzothiazoles and 2-arylbenzimidazoles. Appl. Catal. A 2009, 353, 74–79. [Google Scholar] [CrossRef]
- Gadekar, L.S.; Arbad, B.R.; Lande, M.K. Eco-friendly synthesis of benzimidazole derivatives using solid acid scolecite catalyst. Chin. Chem. Lett. 2010, 21, 1053–1056. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M. An Efficient and eco-friendly tungstate promoted zirconia (WOx/ZrO2) solid acid catalyst for the synthesis of 2-aryl benzimidazoles. Catal. Lett. 2010, 137, 63–68. [Google Scholar] [CrossRef]
- Rathod, S.B.; Lande, M.K.; Arbad, B.R. Synthesis, characterization and catalytic application of MoO3/CeO2-ZrO2 solid heterogeneous catalyst for the synthesis of benzimidazole derivatives. Bull. Korean Chem. Soc. 2010, 31, 2835–2840. [Google Scholar] [CrossRef]
- Tateyama, K.; Wada, K.; Miura, H.; Hosokawa, S.; Abe, R.; Inoue, M. Dehydrogenative synthesis of benzimidazoles under mild conditions with supported iridium catalysts. Catal. Sci. Technol. 2016, 6, 1677–1684. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Corma, A.; Sabater, M.J. New route for the synthesis of benzimidazoles by a one-pot multistep process with mono and bifunctional solid catalysts. Tetrahedron 2010, 66, 730–735. [Google Scholar] [CrossRef]
- Chaudhari, C.; Siddiki, S.; Shimizu, K. Acceptorless dehydrogenative synthesis of benzothiazoles and benzimidazoles from alcohols or aldehydes by heterogeneous Pt catalysts under neutral conditions. Tetrahedron Lett. 2015, 56, 4885–4888. [Google Scholar] [CrossRef]
- Sun, Z.H.; Bottari, B.; Barta, K. Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles. Green Chem. 2015, 17, 5172–5181. [Google Scholar] [CrossRef]
- Selvam, K.; Swaminathan, M. An easy one-step photocatalytic synthesis of 1-aryl-2-alkylbenzimidazoles by platinum loaded TiO2 nanoparticles under UV and solar light. Tetrahedron Lett. 2011, 52, 3386–3392. [Google Scholar] [CrossRef]
- Feng, F.; Ye, J.; Cheng, Z.; Xu, X.X.; Zhang, Q.F.; Ma, L.; Lu, C.S.; Li, X.N. Cu–Pd/γ-Al2O3 catalyzed the coupling of multistep reactions: Direct synthesis of benzimidazole derivatives. RSC Adv. 2016, 6, 72750–72755. [Google Scholar] [CrossRef]
- Fang, W.H.; Zhang, Q.H.; Chen, J.; Deng, W.P.; Wang, Y. Gold nanoparticles on hydrotalcites as efficient catalysts for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2010, 46, 1547–1549. [Google Scholar] [CrossRef]
- Shimizu, K.; Kon, K.; Shimura, K.; Hakim, S.S. Acceptor-free dehydrogenation of secondary alcohols by heterogeneous cooperative catalysis between Ni nanoparticles and acid–base sites of alumina supports. J. Catal. 2013, 300, 242–250. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquot, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Sá, J.; Gross, S.; Vinek, H. Effect of the reducing step on the properties of Pd-Cu bimetallic catalysts used for denitration. Appl. Catal. A 2005, 294, 226–234. [Google Scholar] [CrossRef]
- Mierczynskia, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P. Highly selective Pd–Cu/ZnAl2O4 catalyst for hydrogen production. Appl. Catal. A 2014, 479, 26–34. [Google Scholar] [CrossRef]
- Das, N.N.; Das, R. Synthesis, characterization and activation of quaternary layered double hydroxides for the one-pot synthesis of methyl isobutyl ketone. React. Kinet. Mech. Catal. 2010, 99, 397–408. [Google Scholar] [CrossRef]
- Marínez-Ortiz, M.J.; Tichit, D.; Gonzalez, P.; Coq, B. The “one-pot” synthesis of 4-methyl-2-pentanone (methyl isobutyl ketone) from acetone over PdCu catalysts prepared from layered double hydroxides. J. Mol. Catal. A Chem. 2003, 201, 199–210. [Google Scholar] [CrossRef]
- Batista, J.; Pintar, A.; Mandrino, D.; Jenko, M.; Martin, V. XPS and TPR examinations of γ-alumina-supported Pd-Cu catalysts. Appl. Catal. A 2001, 206, 113–124. [Google Scholar] [CrossRef]
- Sá, J.; Vinek, H. Catalytic hydrogenation of nitrates in water over a bimetallic catalyst. Appl. Catal. B 2005, 57, 247–256. [Google Scholar] [CrossRef]
- Guy, K.A.; Xu, H.P.; Yang, J.C.; Werth, C.J.; Shapley, J.R. Catalytic nitrate and nitrite reduction with Pd-Cu/PVP colloids in water: composition, structure, and reactivity correlations. J. Phys. Chem. C 2009, 113, 8177–8185. [Google Scholar] [CrossRef]
- Cosimo, J.I.D.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Cosimo, J.I.D. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions. J. Catal. 2003, 215, 220–233. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Devassy, B.M.; Lefebvre, F.; Halligudi, S.B. Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes. J. Catal. 2005, 231, 1–10. [Google Scholar] [CrossRef]




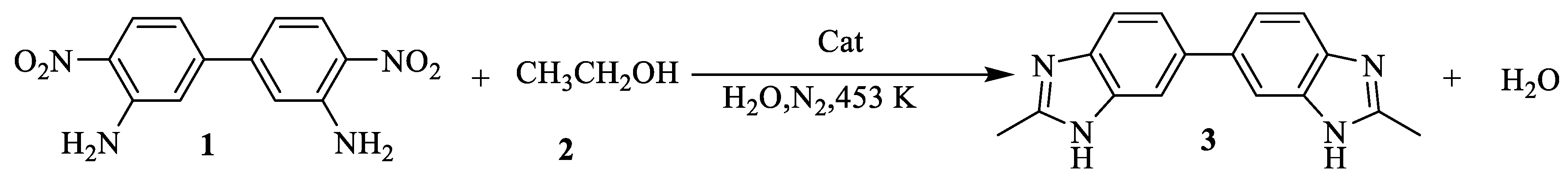
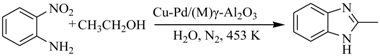 | ||||
|---|---|---|---|---|
| Entry | Catalyst a | Reaction Time (h) | Conv. (%) | Yield (%) b |
| 1 | Cu/γ-Al2O3 | 12 | 0 | 0 |
| 2 | Pd/γ-Al2O3 | 12 | 6.5 | 6.5 |
| 3 | Cu/Al2O3+Pd/γ-Al2O3 | 12 | 67.3 | 64.7 |
| 4 | Cu-Pd/γ-Al2O3 | 6 | 89.5 | 89.2 |
| 5 | Cu-Pd/(Mg)γ-Al2O3 | 6 | 100 | 98.8 |
| 6 | Cu-Pd/(Ca)γ-Al2O3 | 6 | 83.5 | 82.9 |
| 7 | Cu-Pd/(Sr)γ-Al2O3 | 6 | 99.1 | 98.2 |
| 8 | Cu-Pd/(Ba)γ-Al2O3 | 6 | 95.7 | 95.0 |
| 9 | Cu-Pd/(Na)γ-Al2O3 | 6 | 95.5 | 96.6 |
| 10 | Cu-Pd/(K)γ-Al2O3 | 6 | 100 | 98.3 |
| 11 | Cu-Pd/(Cs)γ-Al2O3 | 6 | 98.4 | 97.8 |
| 12 | Cu-Pd/(La)γ-Al2O3 | 6 | 96.8 | 94.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, F.; Deng, Y.; Cheng, Z.; Xu, X.; Zhang, Q.; Lu, C.; Ma, L.; Li, X. Heterogeneous Catalytic Synthesis of 2-Methylbenzimidazole from 2-Nitroaniline and Ethanol Over Mg Modified Cu-Pd/γ-Al2O3. Catalysts 2019, 9, 8. https://doi.org/10.3390/catal9010008
Feng F, Deng Y, Cheng Z, Xu X, Zhang Q, Lu C, Ma L, Li X. Heterogeneous Catalytic Synthesis of 2-Methylbenzimidazole from 2-Nitroaniline and Ethanol Over Mg Modified Cu-Pd/γ-Al2O3. Catalysts. 2019; 9(1):8. https://doi.org/10.3390/catal9010008
Chicago/Turabian StyleFeng, Feng, Yaqin Deng, Zheng Cheng, Xiaoliang Xu, Qunfeng Zhang, Chunshan Lu, Lei Ma, and Xiaonian Li. 2019. "Heterogeneous Catalytic Synthesis of 2-Methylbenzimidazole from 2-Nitroaniline and Ethanol Over Mg Modified Cu-Pd/γ-Al2O3" Catalysts 9, no. 1: 8. https://doi.org/10.3390/catal9010008




