Statistically Guided Synthesis of MoV-Based Mixed-Oxide Catalysts for Ethane Partial Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Doped Mo8V2Nb1Ox Catalysts
2.2. Design of Experiment-Parameter Space
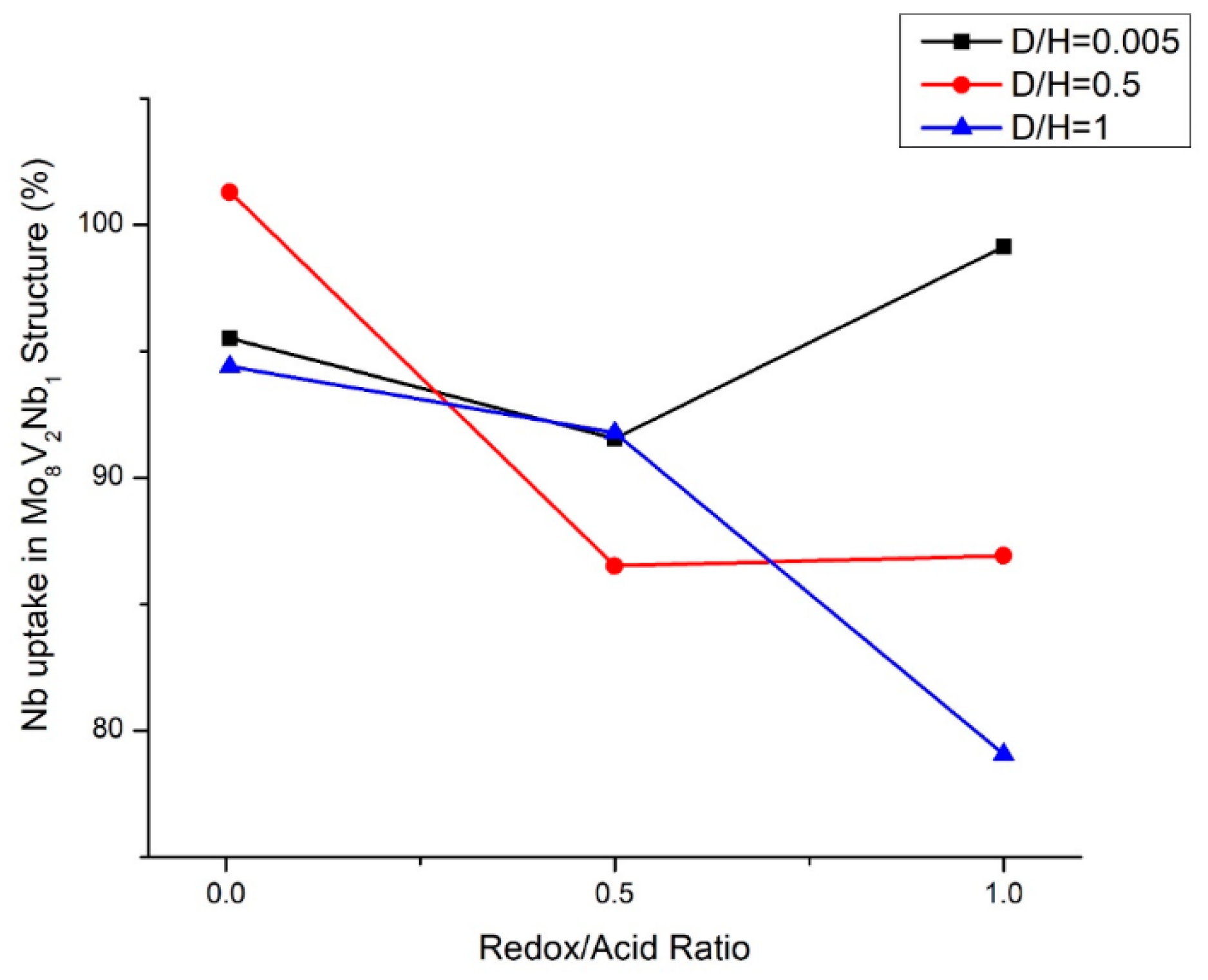
2.3. Energy Dispersive X-ray Spectroscopy (EDS) Elemental Uptake Analysis
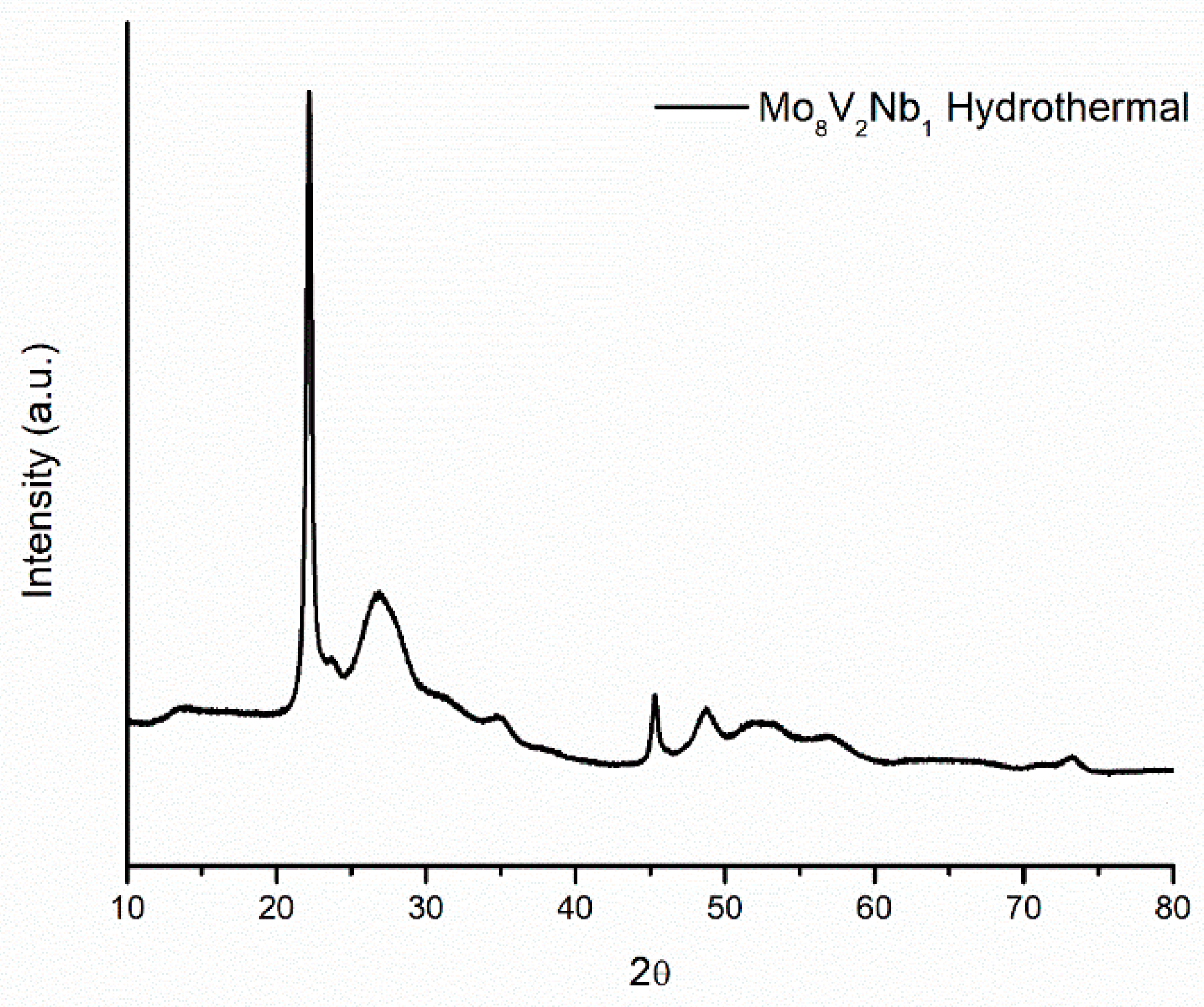
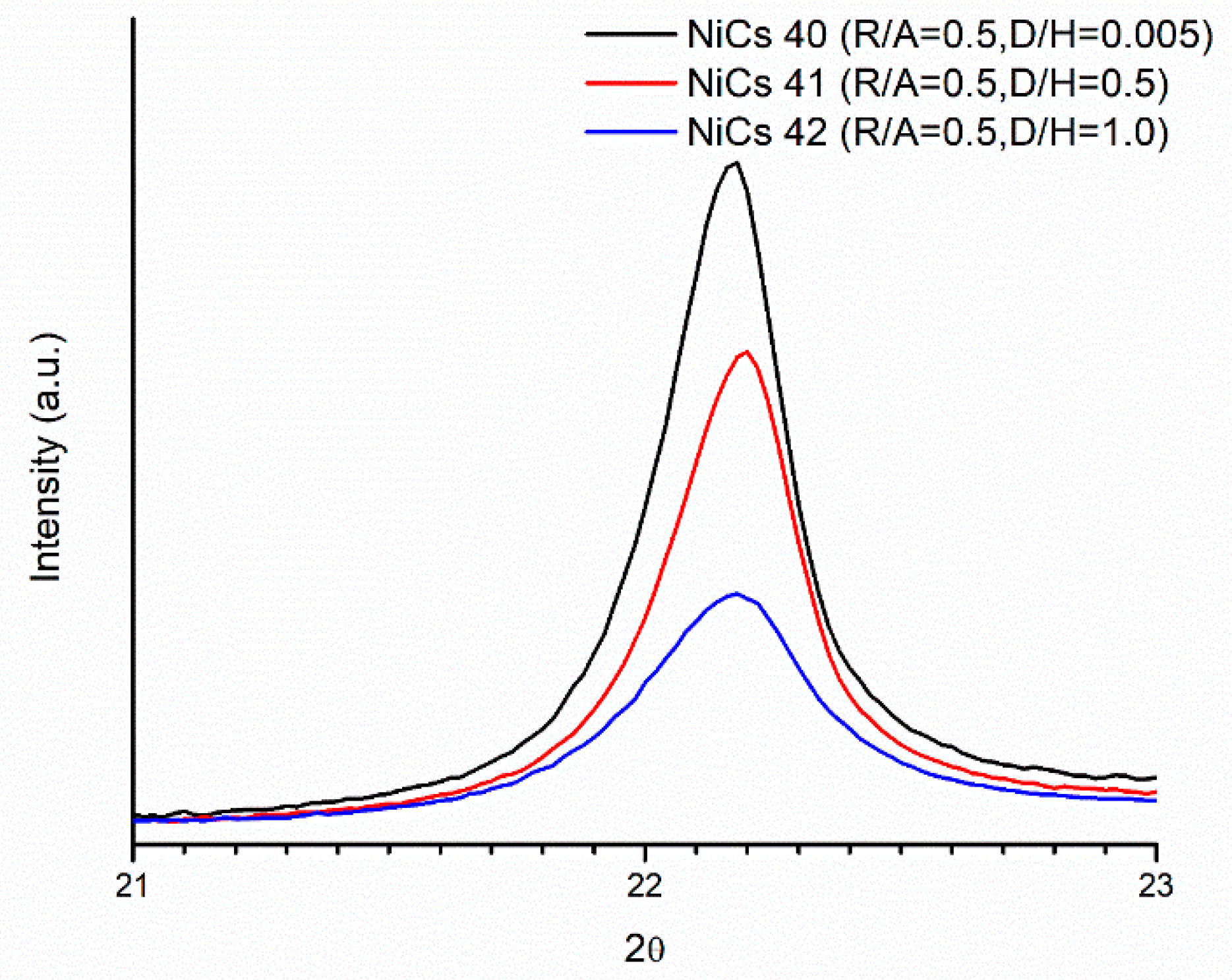
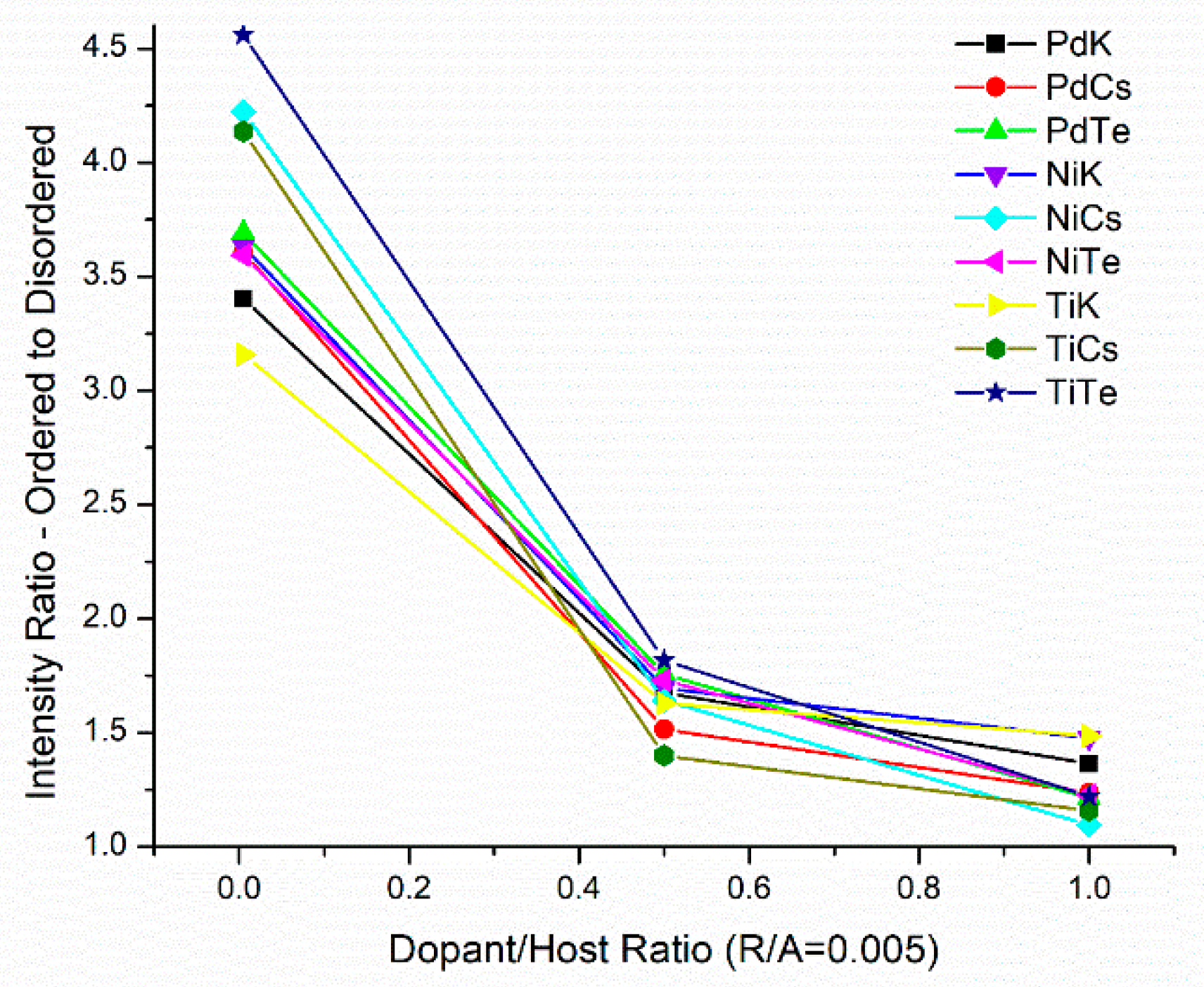
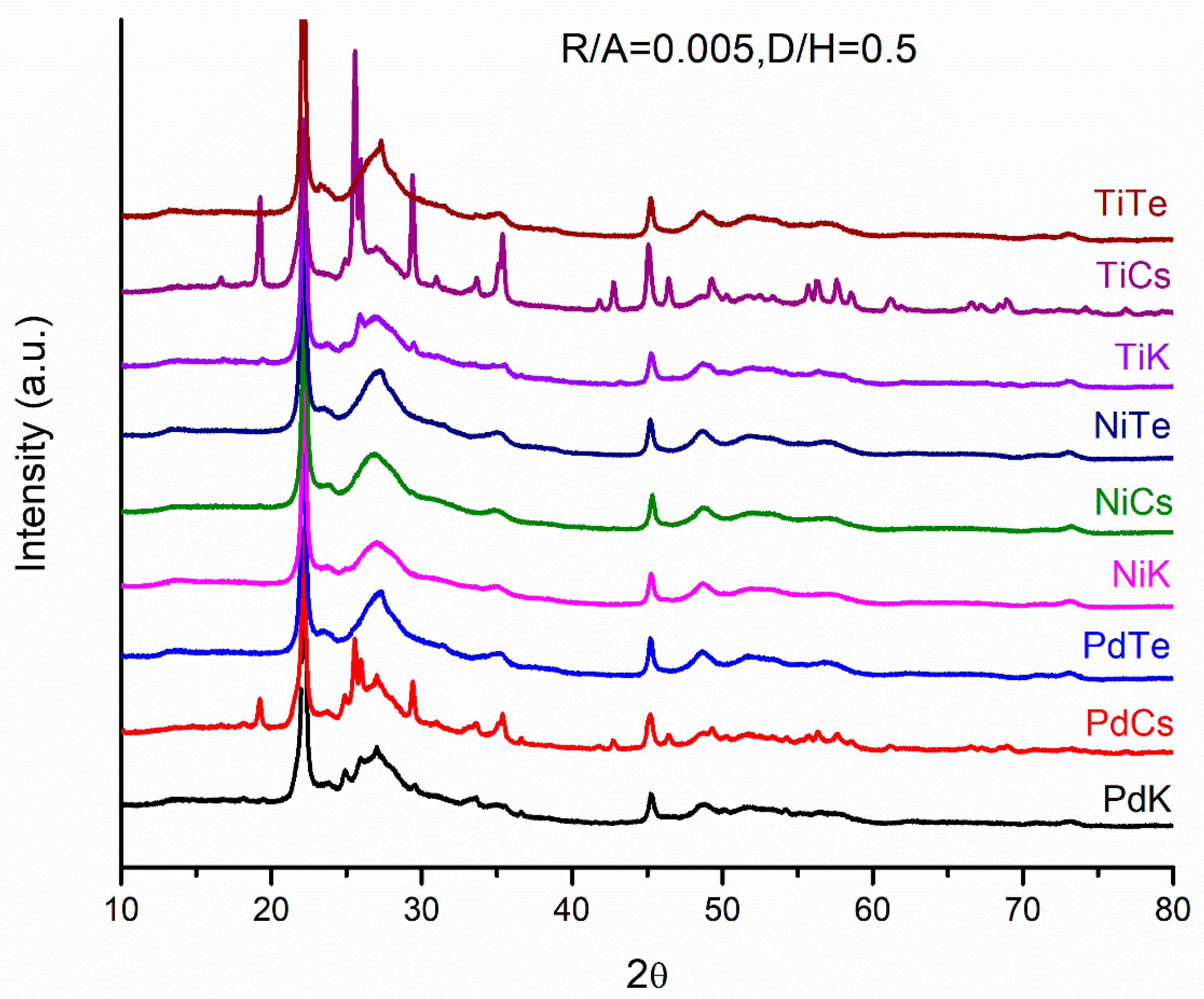
2.4. Crystalline Structure of Doped Mo8V2Nb1Ox Catalysts
2.5. Ethane Partial Oxidation
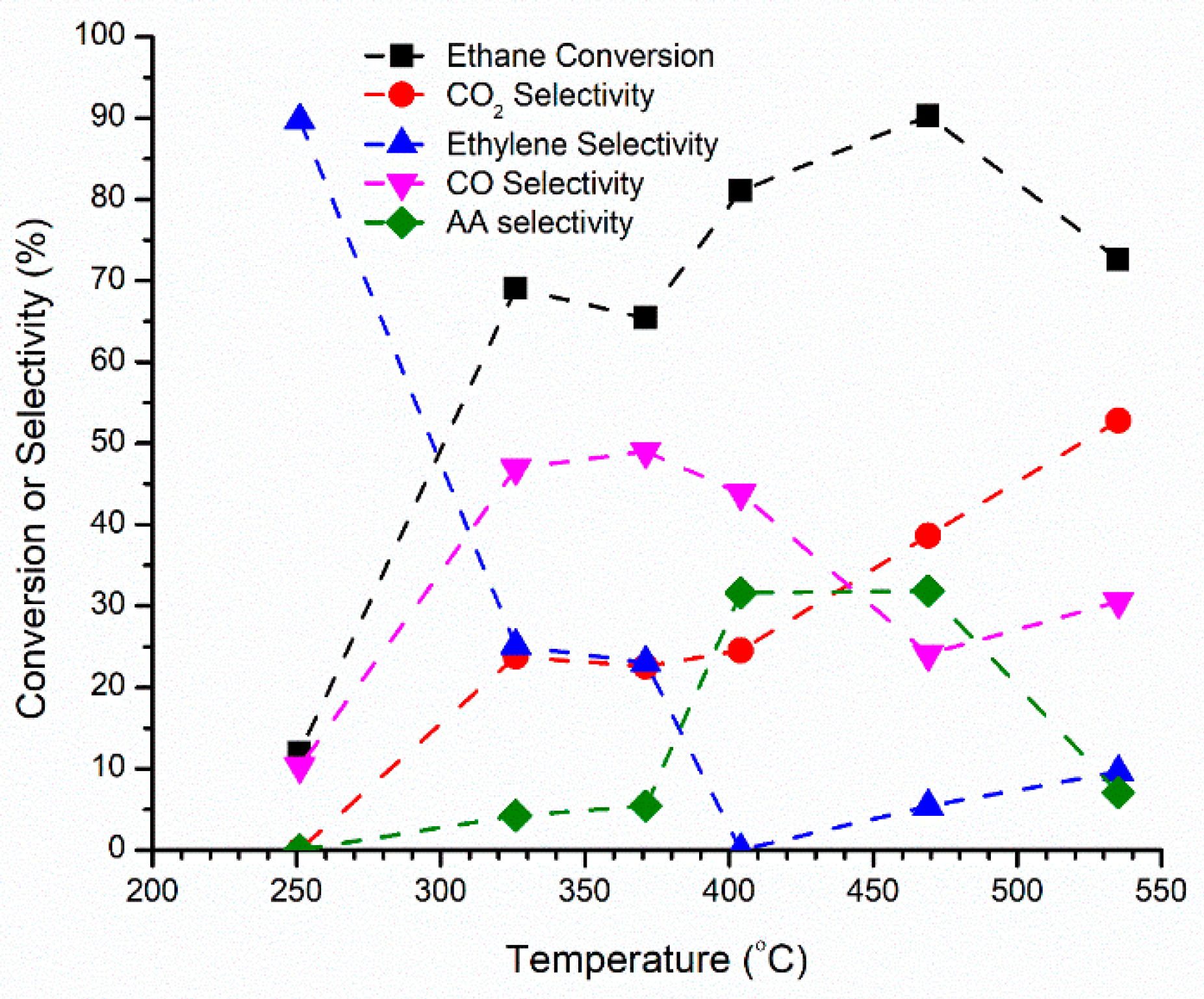
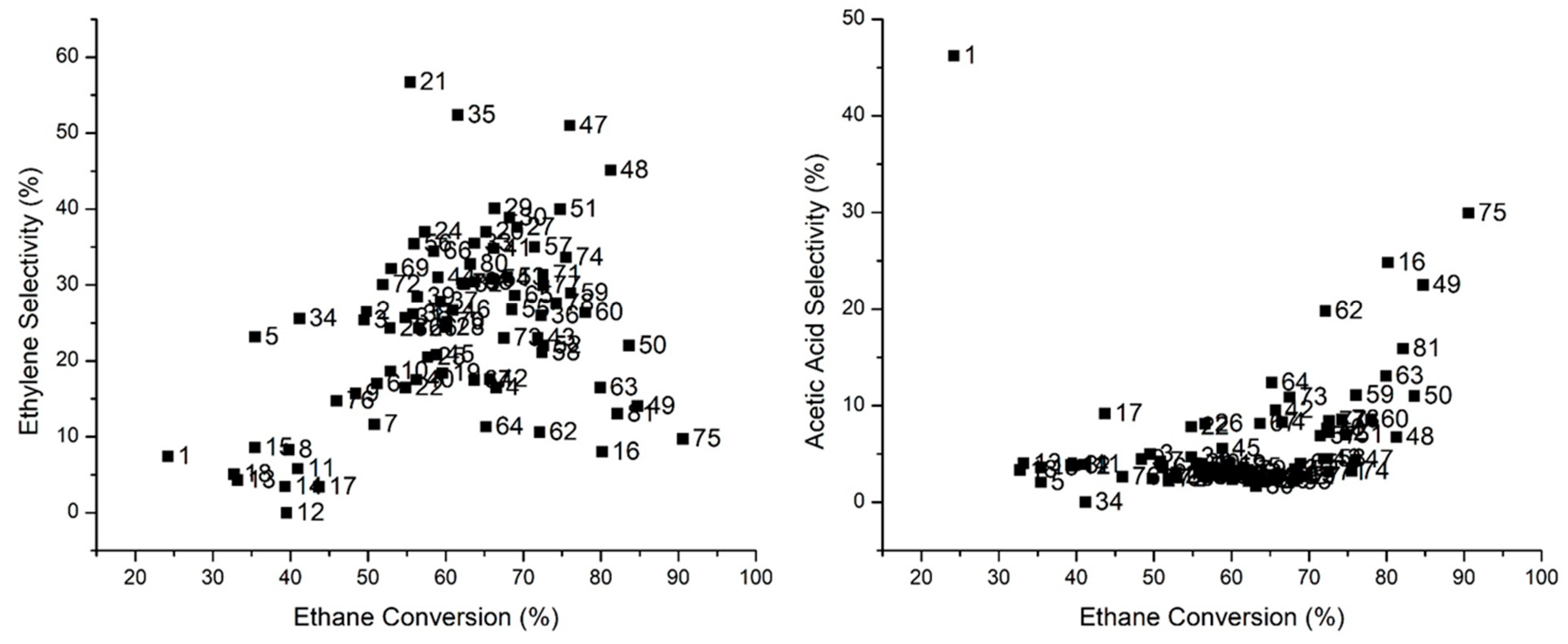
2.5.1. Ethane Partial Oxidation on Doped Mo8V2Nb1 Catalysts
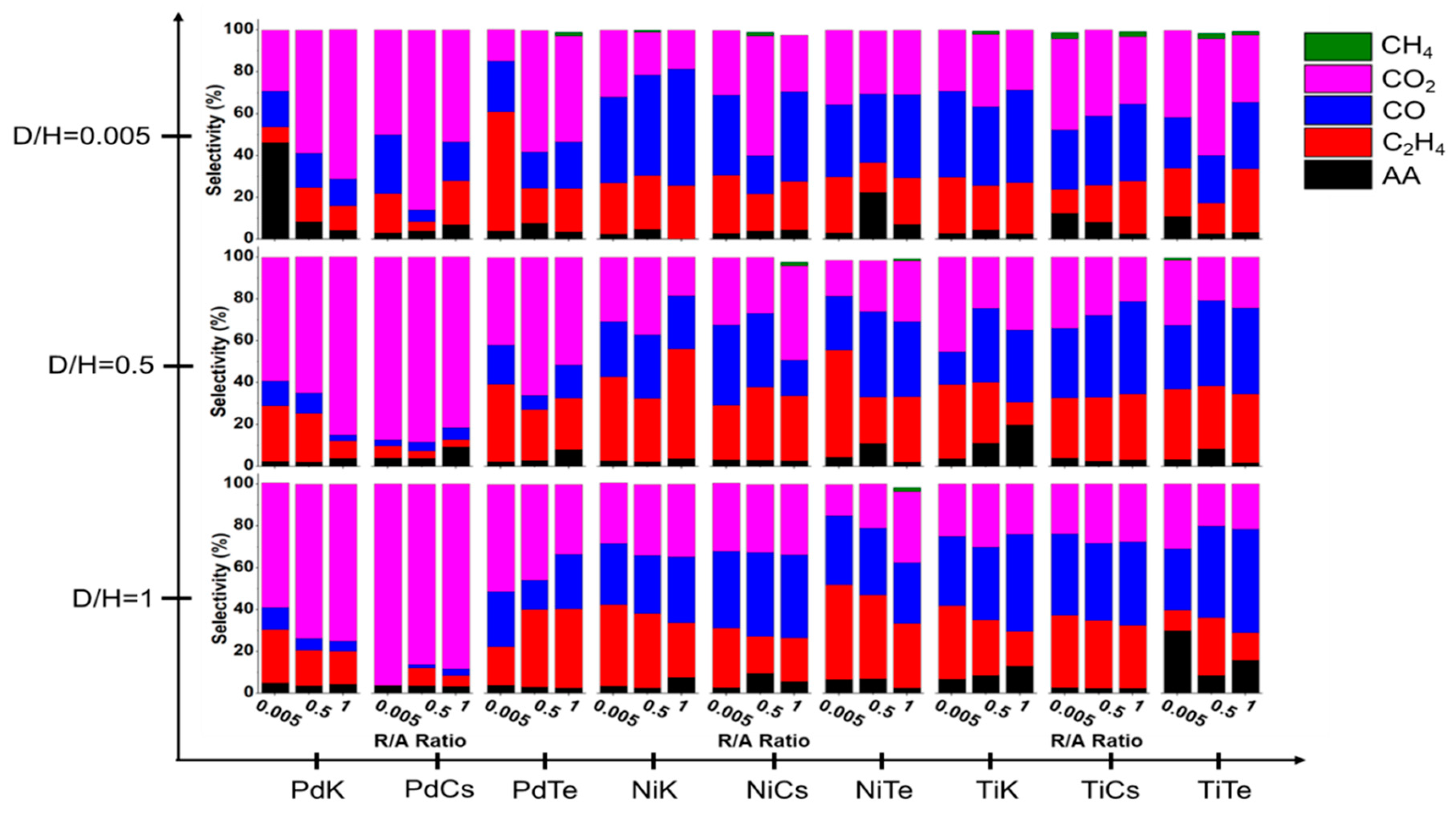
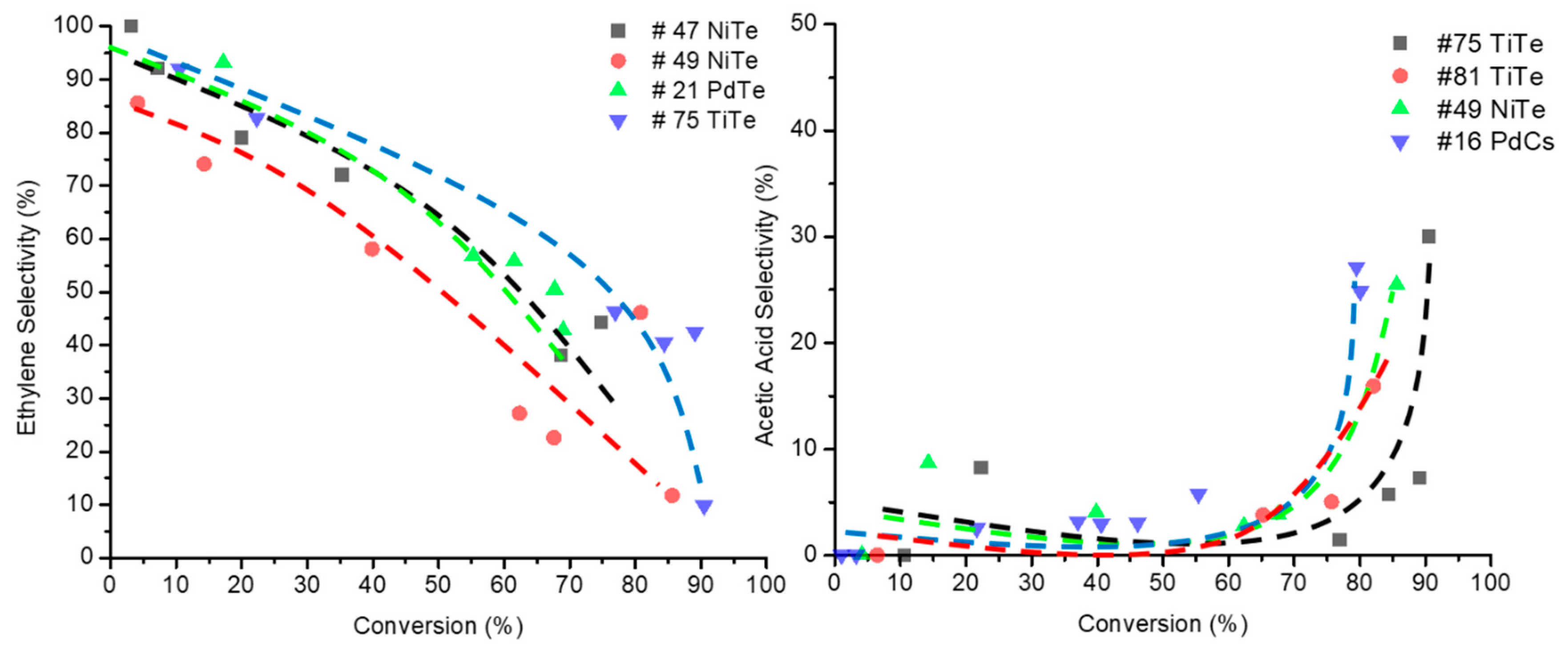
2.5.2. Effect of Doping Mo8V2Nb1Ox on EPO Product Distribution
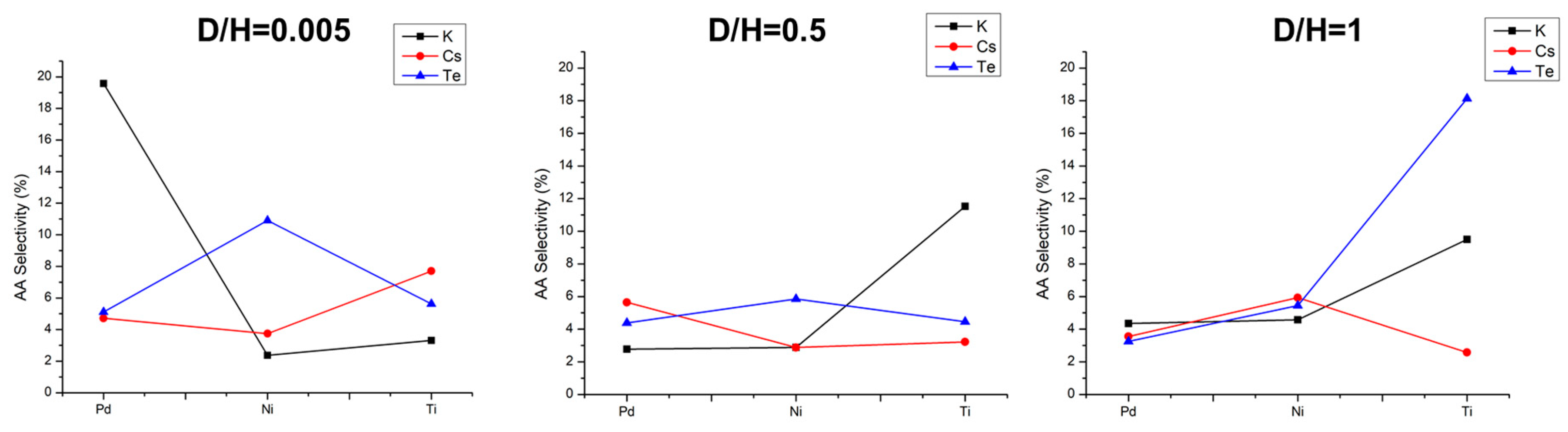
2.5.3. Effect of Doped Mo8V2Nb1 Structure on Catalytic Activity
3. Experimental Methods
3.1. Catalyst Synthesis
3.2. XRD
3.3. Scanning Electron Microscopy/EDS (SEM/EDS)
3.4. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Banares, M.A. Supported metal oxide and other catalysts for ethane conversion: A review. Catal. Today 1999, 51, 319–348. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Brik, Y.; Kacimi, M.; Ziyad, M.; Bozon-Verduraz, F. Titania-supported cobalt and cobalt–phosphorus catalysts: Characterization and performances in ethane oxidative dehydrogenation. J. Catal. 2001, 202, 118–128. [Google Scholar] [CrossRef]
- Cimino, S.; Donsì, F.; Russo, G.; Sanfilippo, D. Olefins production by catalytic partial oxidation of ethane and propane over Pt/LaMnO3 catalyst. Catal. Today 2010, 157, 310–314. [Google Scholar] [CrossRef]
- Le Bars, J.; Auroux, A.; Forissier, M.; Vedrine, J. Active sites of V2O5/γ-Al2O3 catalysts in the oxidative dehydrogenation of ethane. J. Catal. 1996, 162, 250–259. [Google Scholar] [CrossRef]
- Bodke, A.; Olschki, D.; Schmidt, L.; Ranzi, E. High selectivities to ethylene by partial oxidation of ethane. Science 1999, 285, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.C.; Nare, D.N.; Schmidt, L.D. Catalytic partial oxidation of ethane to ethylene and syngas over rh and pt coated monoliths: Spatial profiles of temperature and composition. Chem. Eng. Sci. 2010, 65, 3893–3902. [Google Scholar] [CrossRef]
- Bergh, S.; Guan, S.; Hagemeyer, A.; Lugmair, C.; Turner, H.; Volpe, A.F.; Weinberg, W.H.; Mott, G. Gas phase oxidation of ethane to acetic acid using high-throughput screening in a massively parallel microfluidic reactor system. Appl. Catal. A 2003, 254, 67–76. [Google Scholar] [CrossRef]
- Rahman, F.; Loughlin, K.F.; Al-Saleh, M.A.; Saeed, M.R.; Tukur, N.M.; Hossain, M.M.; Karim, K.; Mamedov, A. Kinetics and mechanism of partial oxidation of ethane to ethylene and acetic acid over mov type catalysts. Appl. Catal. A 2010, 375, 17–25. [Google Scholar] [CrossRef]
- Roussel, M.; Barama, S.; Löfberg, A.; Al-Sayari, S.; Karim, K.; Bordes-Richard, E. Mov-based catalysts in ethane oxidation to acetic acid: Influence of additives on redox chemistry. Catal. Today 2009, 141, 288–293. [Google Scholar] [CrossRef]
- Tessier, L.; Bordes, E.; Gubelmann-Bonneau, M. Active specie on vanadium-containing catalysts for the selective oxidation of ethane to acetic acid. Catal. Today 1995, 24, 335–340. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Kinetics and mechanism of ethane oxidation to acetic acid on catalysts based on Mo-V-Nb oxides. J. Phys. Chem. C 2008, 112, 15001–15008. [Google Scholar] [CrossRef]
- Botella, P.; Dejoz, A.; Nieto, J.L.; Concepcion, P.; Vázquez, M. Selective oxidative dehydrogenation of ethane over movsbo mixed oxide catalysts. Appl. Catal. A 2006, 298, 16–23. [Google Scholar] [CrossRef]
- Botella, P.; Garcıa-González, E.; Dejoz, A.; Nieto, J.L.; Vázquez, M.; González-Calbet, J. Selective oxidative dehydrogenation of ethane on movtenbo mixed metal oxide catalysts. J. Catal. 2004, 225, 428–438. [Google Scholar] [CrossRef]
- Botella, P.; Nieto, J.L.; Solsona, B.; Mifsud, A.; Márquez, F. The preparation, characterization, and catalytic behavior of movtenbo catalysts prepared by hydrothermal synthesis. J. Catal. 2002, 209, 445–455. [Google Scholar] [CrossRef]
- Ruth, K.; Kieffer, R.; Burch, R. Mo-V-Nb oxide catalysts for the partial oxidation of ethane: I. Preparation and structural characterisation. J. Catal. 1998, 175, 16–26. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Support and promoter effects in the selective oxidation of ethane to acetic acid catalyzed by Mo-V-Nb oxides. Appl. Catal. A 2008, 334, 339–347. [Google Scholar] [CrossRef]
- Chen, N.F.; Ueda, W.; Oshihara, K. Hydrothermal synthesis of Mo-V-M-O complex metal oxide catalysts active for partial oxidation of ethane. Chem. Commun. 1999, 517–518. [Google Scholar] [CrossRef]
- Chen, N.F.; Oshihara, K.; Ueda, W. Selective oxidation of ethane over hydrothermally synthesized Mo–V–Al–Ti oxide catalyst. Catal. Today 2001, 64, 121–128. [Google Scholar] [CrossRef]
- Thorsteinson, E.; Wilson, T.; Young, F.; Kasai, P. The oxidative dehydrogenation of ethane over catalysts containing mixed oxides of molybdenum and vanadium. J. Catal. 1978, 52, 116–132. [Google Scholar] [CrossRef]
- Oshihara, K.; Nakamura, Y.; Sakuma, M.; Ueda, W. Hydrothermal synthesis of novel crystalline Mo-V-M-O (M = Al, Ga, Fe) mixed oxide in the presence of triethylammonium chloride and their catalytic performance for selective ethane oxidation. Catal. Today 2001, 71, 153–159. [Google Scholar] [CrossRef]
- Linke, D.; Wolf, D.; Baerns, M.; Timpe, O.; Schlögl, R.; Zeyβ, S.; Dingerdissen, U. Catalytic partial oxidation of ethane to acetic acid over Mo1V0.25Nb0.12Pd0.0005Ox: I. Catalyst performance and reaction mechanism. J. Catal. 2002, 205, 16–31. [Google Scholar] [CrossRef]
- Valente, J.S.; Quintana-Solórzano, R.; Armendáriz-Herrera, H.; Barragán-Rodríguez, G.; López-Nieto, J. Kinetic study of oxidative dehydrogenation of ethane over movtenb mixed-oxide catalyst. Ind. Eng. Chem. Res. 2013, 53, 1775–1786. [Google Scholar] [CrossRef]
- Ishchenko, E.; Kardash, T.Y.; Gulyaev, R.; Ishchenko, A.; Sobolev, V.; Bondareva, V. Effect of k and Bi doping on the M1 phase in MoVTeNbO catalysts for ethane oxidative conversion to ethylene. Appl. Catal. A 2016, 514, 1–13. [Google Scholar] [CrossRef]
- Ueda, W.; Oshihara, K. Selective oxidation of light alkanes over hydrothermally synthesized Mo-V-M-O (M = Al, Ga, Bi, Sb, and Te) oxide catalysts. Appl. Catal. A 2000, 200, 135–143. [Google Scholar] [CrossRef]
- Nguyen, T.; Aouine, M.; Millet, J. Optimizing the efficiency of movtenbo catalysts for ethane oxidative dehydrogenation to ethylene. Catal. Commun. 2012, 21, 22–26. [Google Scholar] [CrossRef]
- Valente, J.S.; Armendáriz-Herrera, H.C.; Quintana-Solórzano, R.; Del Angel, P.; Nava, N.; Massó, A.; López Nieto, J.M. Chemical, structural, and morphological changes of a MoVTeNb catalyst during oxidative dehydrogenation of ethane. ACS Catal. 2014, 4, 1292–1301. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; Burrington, J.D.; Andersson, A.; Holmberg, J.; Ueda, W.; Kubo, J.; Lugmair, C.G.; Volpe, A.F. Active centers, catalytic behavior, symbiosis and redox properties of MoV(Nb,Ta)Teo ammoxidation catalysts. Top. Catal. 2006, 38, 7–16. [Google Scholar] [CrossRef]
- DeSanto, P.; Buttrey, D.J.; Grasselli, R.K.; Lugmair, C.G.; Volpe, A.F.; Toby, B.H.; Vogt, T. Structural aspects of the M1 and M2 phases in MoVNbTeO propane ammoxidation catalysts. Z. Krist-Cryst. Mater. 2004, 219, 152–165. [Google Scholar] [CrossRef]
- Guliants, V.V.; Bhandari, R.; Swaminathan, B.; Vasudevan, V.K.; Brongersma, H.H.; Knoester, A.; Gaffney, A.M.; Han, S. Roles of surface Te, Nb, and Sb oxides in propane oxidation to acrylic acid over bulk orthorhombic Mo−V−O phase. J. Phys. Chem. B 2005, 109, 24046–24055. [Google Scholar] [CrossRef] [PubMed]
- Heracleous, E.; Lemonidou, A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part I: Characterization and catalytic performance. J. Catal. 2006, 237, 162–174. [Google Scholar] [CrossRef]
- Sadakane, M.; Yamagata, K.; Kodato, K.; Endo, K.; Toriumi, K.; Ozawa, Y.; Ozeki, T.; Nagai, T.; Matsui, Y.; Sakaguchi, N.; et al. Synthesis of orthorhombic Mo-V-Sb oxide species by assembly of pentagonal Mo6O21 polyoxometalate building blocks. Angew. Chem. 2009, 121, 3840–3844. [Google Scholar] [CrossRef]
- Hibst, H.; Rosowski, F.; Cox, G. New cs-containing Mo–V4+ based oxides with the structure of the m1 phase—base for new catalysts for the direct alkane activation. Catal. Today 2006, 117, 234–241. [Google Scholar] [CrossRef]
- Weirich, T.E.; Portillo, J.; Cox, G.; Hibst, H.; Nicolopoulos, S. Ab initio determination of the framework structure of the heavy-metal oxide CsxNb2.54W2.46O14 from 100 kv precession electron diffraction data. Ultramicroscopy 2006, 106, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Solsona, B.; Vázquez, M.; Ivars, F.; Dejoz, A.; Concepción, P.; Nieto, J.L. Selective oxidation of propane and ethane on diluted Mo–V–Nb–Te mixed-oxide catalysts. J. Catal. 2007, 252, 271–280. [Google Scholar] [CrossRef]
- Wagner, J.B.; Timpe, O.; Hamid, F.A.; Trunschke, A.; Wild, U.; Su, D.S.; Widi, R.K.; Hamid, S.B.A.; Schlögl, R. Surface texturing of Mo–V–Te–Nb–Ox selective oxidation catalysts. Top. Catal. 2006, 38, 51–58. [Google Scholar] [CrossRef]
- Nieto, J.L.; Botella, P.; Vázquez, M.; Dejoz, A. The selective oxidative dehydrogenation of ethane over hydrothermally synthesised movtenb catalysts. Chem. Commun. 2002, 1906–1907. [Google Scholar] [CrossRef]
- López-Medina, R.; Sobczak, I.; Golinska-Mazwa, H.; Ziolek, M.; Bañares, M.A.; Guerrero-Pérez, M.O. Spectroscopic surface characterization of movnbte nanostructured catalysts for the partial oxidation of propane. Catal. Today 2012, 187, 195–200. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Synergistic effects of TiO2 and palladium-based Cocatalysts on the selective oxidation of ethene to acetic acid on Mo–V–Nb oxide domains. Angew. Chem. Int. Ed. 2007, 46, 8649–8652. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Wolf, D.; Baerns, M.; Zeyβ, S.; Dingerdissen, U. Catalytic partial oxidation of ethane to acetic acid over Mo1V0.25Nb0.12Pd0.0005Ox: II. Kinetic modelling. J. Catal. 2002, 205, 32–43. [Google Scholar] [CrossRef]
- Schuurman, Y.; Ducarme, V.; Chen, T.; Li, W.; Mirodatos, C.; Martin, G. Low temperature oxidative dehydrogenation of ethane over catalysts based on group viii metals. Appl. Catal. A 1997, 163, 227–235. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Jing, Y.; Xie, Y. Support effects on the catalytic behavior of NiO/Al2O3 for oxidative dehydrogenation of ethane to ethylene. Appl. Catal. A 2003, 240, 143–150. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part II: Mechanistic aspects and kinetic modeling. J. Catal. 2006, 237, 175–189. [Google Scholar] [CrossRef]
- Amakawa, K.; Kolen’ko, Y.V.; Villa, A.; Schuster, M.E.; Csepei, L.-I.; Weinberg, G.; Wrabetz, S.; Naumann d’Alnoncourt, R.; Girgsdies, F.; Prati, L. Multifunctionality of crystalline MoV (TeNb) M1 oxide catalysts in selective oxidation of propane and benzyl alcohol. ACS Catal. 2013, 3, 1103–1113. [Google Scholar] [CrossRef]
- Sopa, M.; Wącław-Held, A.; Grossy, M.; Pijanka, J.; Nowińska, K. Ethane to acetic acid oxidation over supported heteropoly acids. Appl. Catal. A 2005, 285, 119–125. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; DeSanto, P., Jr.; Burrington, J.D.; Lugmair, C.G.; Volpe, A.F., Jr.; Weingand, T. Active centers in Mo–V–Nb–Te–Ox (amm) oxidation catalysts. Catal. Today 2004, 91, 251–258. [Google Scholar] [CrossRef]
- Holmberg, J.; Grasselli, R.K.; Andersson, A. Catalytic behaviour of M1, M2, and M1/M2 physical mixtures of the Mo–V–Nb–Te–oxide system in propane and propene ammoxidation. Appl. Catal. A 2004, 270, 121–134. [Google Scholar] [CrossRef]
- Azaroff, L.V.; Buerger, M.J. Analytical methods for indexing powder photographs. In The powder method in x-ray crystallography; The Maple Press Company: York, PA, USA, 1958; Volume 1, pp. 79–90. [Google Scholar]
- Henry, N.F.; Wooster, W.A.; Lipson, H. Measurements of crystal grain size in polycrystalline aggregates. In The interpretation of x-ray diffraction photographs; Macmillan: London, UK, 1951; Volume 1, pp. 212–230. [Google Scholar]
- Karim, K.; Mamedov, A.; Al-Hazmi, M.H.; Al-Andis, N. Oxidative dehydrogenation of ethane over MoVMnW oxide catalysts. React. Kinet. Catal. Lett. 2003, 80, 3–11. [Google Scholar] [CrossRef]
- Oyama, S.T. Adsorbate bonding and the selection of partial and total oxidation pathways. J. Catal. 1991, 128, 210–217. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, X.; Wachs, I.E. Comparative study of bulk and supported V−Mo−Te−Nb−O mixed metal oxide catalysts for oxidative dehydrogenation of propane to propylene. J. Phys. Chem. B 2003, 107, 6333–6342. [Google Scholar] [CrossRef]
- Hävecker, M.; Wrabetz, S.; Kröhnert, J.; Csepei, L.-I.; d’Alnoncourt, R.N.; Kolen’ko, Y.V.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid. J. Catal. 2012, 285, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Kosuke, N.; Murayama, T.; Ueda, W. Single-crystalline-phase mo3vox: An efficient catalyst for the partial oxidation of acrolein to acrylic acid. ChemCatChem 2013, 5, 2869–2873. [Google Scholar] [CrossRef]
- Wagner, J.B.; Hamid, S.A.; Othman, D.; Timpe, O.; Knobl, S.; Niemeyer, D.; Su, D.S.; Schlögl, R. Nanostructuring of binary molybdenum oxide catalysts for propene oxidation. J. Catal. 2004, 225, 78–85. [Google Scholar] [CrossRef]
| Factor | Variable Type | Low | Center Point | High |
|---|---|---|---|---|
| A: Redox element (R) | Categorical | Pd | Ni | Ti |
| B: Acid/base element (A) | Categorical | K | Cs | Te |
| C: Dopant to Host ratio (D/H) | Numerical | 0.005 | 0.5 | 1.0 |
| D: Redox to acid ratio (R/A) | Numerical | 0.005 | 0.5 | 1.0 |
| Factor | Ethane Conversion | Ethene Selectivity | Acetic Acid Selectivity | Secondary-Phase Abundance | Grain Size |
|---|---|---|---|---|---|
| Redox element | Ti > Ni >> Pd | Ni > Ti >> Pd | Ti > Pd > Ni | No effect | Ni > Pd > Ti |
| Acid/base element | Te > K > Cs | Te > K > Cs | K = Te > Cs | Cs > Te > K | No effect |
| Redox/acid ratio | No effect | 0.005 optimal | 0.005 optimal | Decreases | No effect |
| Dopant/host ratio | 1 optimal | 0.5 optimal (0.005 for Pd) | 0.005 optimal | Increases | Decreases |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, J.D.; Mingle, K.; Bureerug, T.; Wen, C.; Lauterbach, J. Statistically Guided Synthesis of MoV-Based Mixed-Oxide Catalysts for Ethane Partial Oxidation. Catalysts 2018, 8, 370. https://doi.org/10.3390/catal8090370
Jimenez JD, Mingle K, Bureerug T, Wen C, Lauterbach J. Statistically Guided Synthesis of MoV-Based Mixed-Oxide Catalysts for Ethane Partial Oxidation. Catalysts. 2018; 8(9):370. https://doi.org/10.3390/catal8090370
Chicago/Turabian StyleJimenez, Juan D., Kathleen Mingle, Teeraya Bureerug, Cun Wen, and Jochen Lauterbach. 2018. "Statistically Guided Synthesis of MoV-Based Mixed-Oxide Catalysts for Ethane Partial Oxidation" Catalysts 8, no. 9: 370. https://doi.org/10.3390/catal8090370




