Efficient Physisorption of Candida Antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis
Abstract
:1. Introduction
2. Results and Discussion
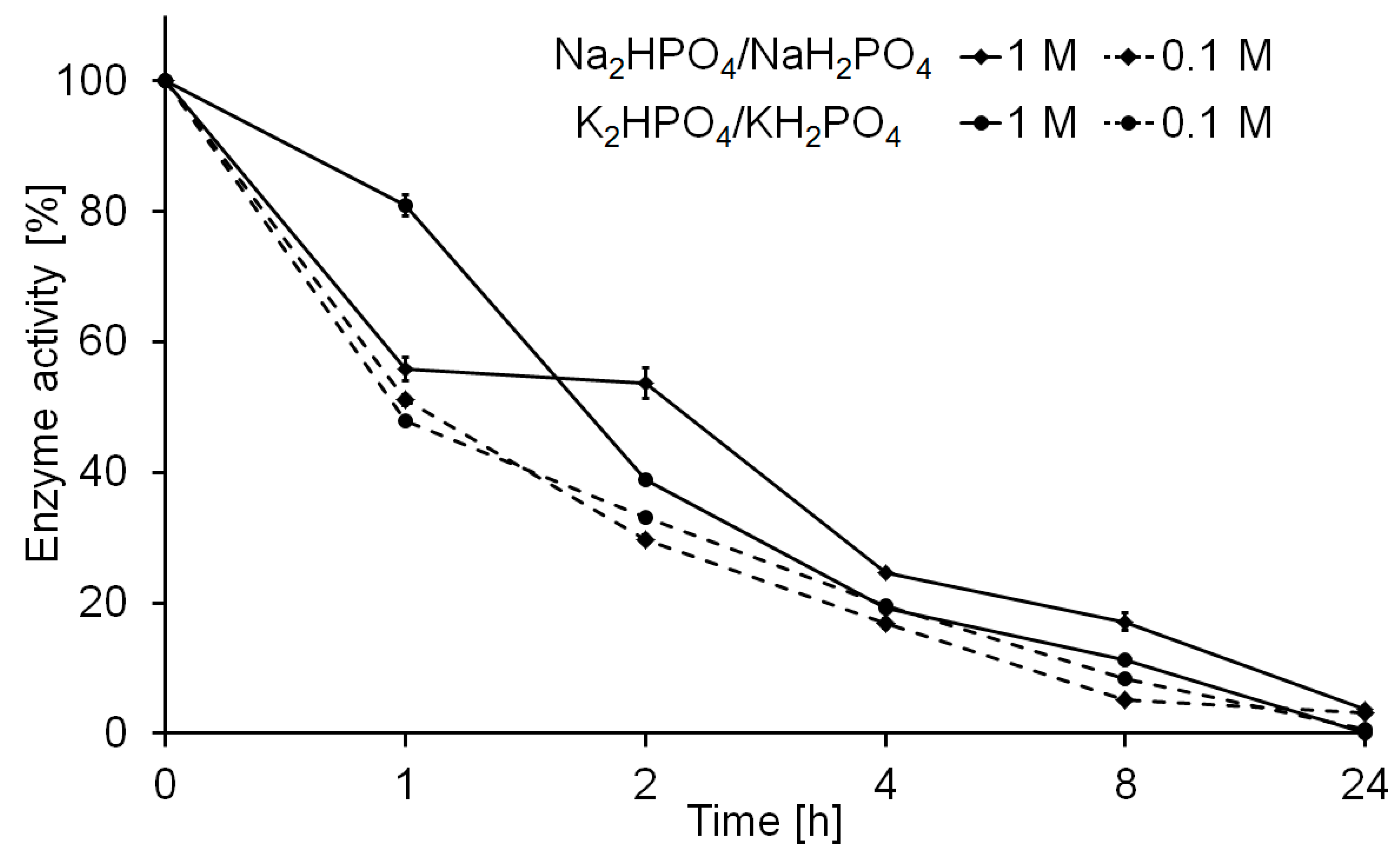
2.1. Lipase Immobilization
2.2. Polymerization of Bio-Based Polymers
2.3. Biocatalyst Preparation Stability in a Continous Polymerization Process
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Enzymes
3.3. Immobilization of CaLB on Accurel Beads
3.4. Activity Assays for Immobilized Enzymes
3.5. Enzymatic Polycondensation Using a Thin-Film Reaction System
3.6. Gel Permeation Chromatography (GPC)
3.7. 1H-NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.S.; Am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key green engineering research areas for sustainable manufacturing: A perspective from pharmaceutical and fine chemicals manufacturers. Org. Process. Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- European Association of Plastics Recycling and Recovery Organisations. Plastics—The Facts 2016. Available online: https://www.plasticseurope.org/application/files/4315/1310/4805/plastic-the-fact-2016.pdf (accessed on 30 August 2018).
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2018, 40, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Recent developments in lipase-catalyzed synthesis of polyesters. Macromol. Rapid Commun. 2009, 30, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Paggiola, G.; Hunt, A.J.; McElroy, C.R.; Sherwood, J.; Clark, J.H. Biocatalysis in bio-derived solvents: An improved approach for medium optimisation. Green Chem. 2014, 16, 2107–2110. [Google Scholar] [CrossRef]
- Cooper, G.M. The central role of enzymes as biological catalysts. In The Cell: A Molecular Approach, 2nd ed.; Cooper, G.M., Ed.; Sinauer Associates: Sunderland, MA, USA, 2000; pp. 145–146. [Google Scholar]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Tąta, A.; Sokołowska, K.; Swider, J.; Konieczna-Molenda, A.; Proniewicz, E.; Witek, E. Study of cellulolytic enzyme immobilization on copolymers of N-vinylformamide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, A.; Telefoncu, A. Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B Enzym. 2007, 45, 10–14. [Google Scholar] [CrossRef]
- Safari Sinegani, A.A.; Emtiazi, G.; Shariatmadari, H. Sorption and immobilization of cellulase on silicate clay minerals. J. Colloid Interface Sci. 2005, 290, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Tümtürk, H.; Karaca, N.; Demirel, G.; Şahin, F. Preparation and application of poly(N,N-dimethylacrylamide-co-acrylamide) and poly(N-isopropylacrylamide-co-acrylamide)/κ-Carrageenan hydrogels for immobilization of lipase. Int. J. Biol. Macromol. 2007, 40, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Bryjak, J.; Aniulyte, J.; Liesiene, J. Evaluation of man-tailored cellulose-based carriers in glucoamylase immobilization. Carbohydr. Res. 2007, 342, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Raafat, A.I.; Araby, E.; Lotfy, S. Enhancement of fibrinolytic enzyme production from Bacillus subtilis via immobilization process onto radiation synthesized starch/dimethylaminoethyl methacrylate hydrogel. Carbohydr. Polym. 2012, 87, 1369–1374. [Google Scholar] [CrossRef]
- Chang, Y.K.; Chu, L. A simple method for cell disruption by immobilization of lysozyme on the extrudate-shaped NaY zeolite. Biochem. Eng. J. 2007, 35, 37–47. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Z.M. Immobilization of lipase on chemically modified bimodal ceramic foams for olive oil hydrolysis. Chem. Eng. J. 2008, 144, 103–109. [Google Scholar] [CrossRef]
- Kahraman, M.V.; Bayramoǧlu, G.; Kayaman-Apohan, N.; Güngör, A. UV-curable methacrylated/fumaric acid modified epoxy as a potential support for enzyme immobilization. React. Funct. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Daoud, F.B.O.; Kaddour, S.; Sadoun, T. Adsorption of cellulase Aspergillus niger on a commercial activated carbon: Kinetics and equilibrium studies. Colloids Surfaces B Biointerfaces 2010, 75, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Schreier, P. Enzymes and flavour biotechnology. In Biotechnology of Aroma Compounds; Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.-O., Eriksson, K.-E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 51–72. [Google Scholar]
- Garlapati, V.K.; Banerjee, R. Solvent-Free Synthesis of Flavour Esters through Immobilized Lipase Mediated Transesterification. Enzyme Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Lipase-catalyzed polyester synthesis—A green polymer chemistry. Proc. Japan Acad. Ser. B 2010, 86, 338–365. [Google Scholar] [CrossRef]
- Pellis, A.; Vastano, M.; Quartinello, F.; Herrero Acero, E.; Guebitz, G.M. His-Tag Immobilization of Cutinase 1 From Thermobifida cellulosilytica for Solvent-Free Synthesis of Polyesters. Biotechnol. J. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Tyrikos-Ergas, T.; Shirke, A.N.; Zou, Y.; Dooley, A.L.; Pavlidis, I.V.; Gross, R.A. Revealing Cutinases’ Capabilities as Enantioselective Catalysts. ACS Catal. 2018, 8, 7944–7951. [Google Scholar] [CrossRef]
- Langrand, G.; Rondot, N.; Triantaphylides, C.; Baratti, J. Short chain flavour esters synthesis by microbial lipases. Biotechnol. Lett. 1990, 12, 581–586. [Google Scholar] [CrossRef]
- Azudin, N.Y.; Mashitah, M.D.; Abd Shukor, S.R. Optimization of Isoamyl Acetate Production in a Solvent-Free System. J. Food Qual. 2013, 36, 441–446. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Maneffa, A.J.; Sipponen, M.H.; Clark, J.H.; Farmer, T.J. Elucidating enzymatic polymerisations: Chain-length selectivity of Candida antarctica lipase B towards various aliphatic diols and dicarboxylic acid diesters. Eur. Polym. J. 2018, 106, 79–84. [Google Scholar] [CrossRef]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Pellis, A.; Daxbacher, A.; Nyanhongo, G.S.; Guebitz, G.M. Polymerization of various lignins via immobilized Myceliophthora thermophila Laccase (MtL). Polymers 2016, 8, 280. [Google Scholar] [CrossRef]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Ferrario, V.; Cespugli, M.; Corici, L.; Guarneri, A.; Zartl, B.; Herrero Acero, E.; Ebert, C.; Guebitz, G.M.; Gardossi, L. Fully renewable polyesters: Via polycondensation catalyzed by Thermobifida cellulosilytica cutinase 1: An integrated approach. Green Chem. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- Manoel, E.A.; Ribeiro, M.F.P.; Dos Santos, J.C.S.; Coelho, M.A.Z.; Simas, A.B.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Accurel MP 1000 as a support for the immobilization of lipase from Burkholderia cepacia: Application to the kinetic resolution of myo-inositol derivatives. Process. Biochem. 2015, 50, 1557–1564. [Google Scholar] [CrossRef]
- Almeida, R.V.; Branco, R.V.; Peixoto, B.; da Silva Lima, C.; Alqueres, S.M.C.; Martins, O.B.; Antunes, O.A.C.; Freire, D.M.G. Immobilization of a recombinant thermostable esterase (Pf2001) from Pyrococcus furiosus on microporous polypropylene: Isotherms, hyperactivation and purification. Biochem. Eng. J. 2008, 39, 531–537. [Google Scholar] [CrossRef]
- Su, A.; Shirke, A.; Baik, J.; Zou, Y.; Gross, R. Immobilized cutinases: Preparation, solvent tolerance and thermal stability. Enzyme Microb. Technol. 2018, 116, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Lopez, J.; Beckman, E.J.; Russell, A.J. Biocatalytic solvent-free polymerization to produce high molecular weight polyesters. Biotechnol. Prog. 1997, 13, 318–325. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]

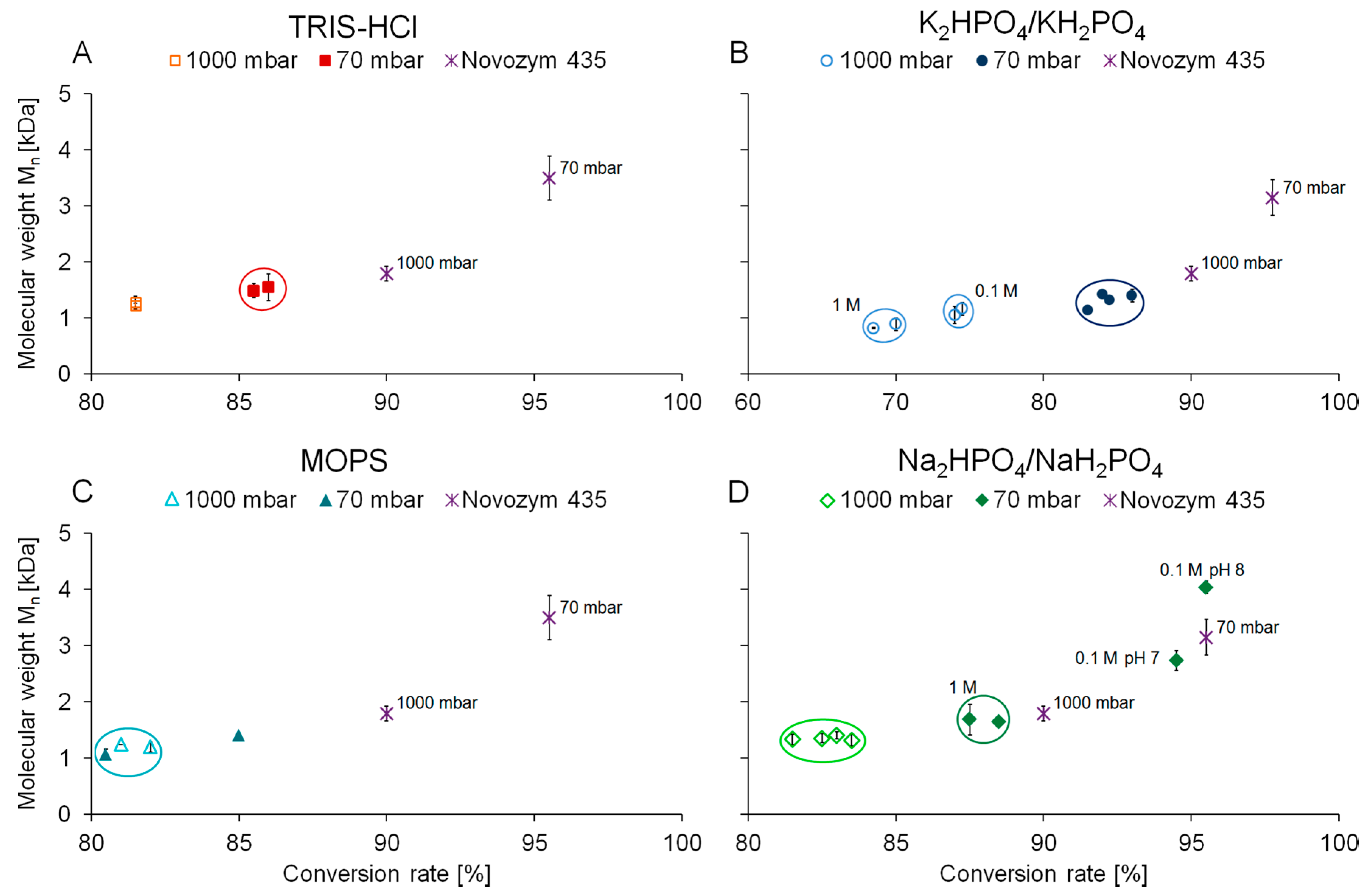

| Pressure [mbar] | Buffer | Conversion rate [%] | Stdv. | Molecular weight Mn [Da] | Stdv. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | salts | pH | ||||||||||
| 70 | 1 | K2HPO4KH2PO4 | 7 | 86.0 | 1.0 | 1399.0 | 110.0 | |||||
| 0.1 | 84.5 | 0.5 | 1309.0 | 41.0 | ||||||||
| 1 | 8 | 83.0 | 0.0 | 1135.0 | 27.0 | |||||||
| 0.1 | 84.0 | 1.0 | 1417.5 | 0.5 | ||||||||
| 1000 | 1 | 7 | 68.5 | 4.5 | 816.5 | 13.5 | ||||||
| 0.1 | 74.0 | 4.0 | 1052.5 | 152.5 | ||||||||
| 1 | 8 | 70.0 | 7.0 | 889.5 | 112.5 | |||||||
| 0.1 | 74.5 | 5.5 | 1162.5 | 113.5 | ||||||||
| 70 | 1 | TRIS-HCl | 7 | 85.5 | 0.5 | 1487.5 | 126.5 | |||||
| 0.1 | 86.0 | 3.0 | 1548.0 | 242.0 | ||||||||
| 1000 | 1 | 81.5 | 0.5 | 1228.5 | 32.5 | |||||||
| 0.1 | 81.5 | 1.5 | 1276.5 | 114.5 | ||||||||
| 70 | 1 | Na2HPO4NaH2PO4 | 87.5 | 2.5 | 1686.0 | 273.0 | ||||||
| 0.1 | 94.5 | 0.5 | 2734.5 | 174.5 | ||||||||
| 1 | 8 | 88.5 | 0.5 | 1648.5 | 77.5 | |||||||
| 0.1 | 95.5 | 0.5 | 4033.0 | 116.0 | ||||||||
| 1000 | 1 | 7 | 83.5 | 2.5 | 1317.5 | 104.5 | ||||||
| 0.1 | 83.0 | 1.0 | 1405.0 | 67.0 | ||||||||
| 1 | 8 | 82.5 | 1.5 | 1353.5 | 79.5 | |||||||
| 0.1 | 81.5 | 0.5 | 1334.5 | 90.5 | ||||||||
| 1 | MOPS | 7 | 82.0 | 2.0 | 1195.5 | 95.5 | ||||||
| 0.1 | 81.0 | 0.0 | 1241.5 | 0.5 | ||||||||
| 70 | 1 | 80.5 | 0.5 | 1063.0 | 16.0 | |||||||
| 0.1 | 85.0 | 0.0 | 1401.0 | 53.0 | ||||||||
| 1000 | Novozym 435 | 90.0 | 0.0 | 1791.0 | 127.0 | |||||||
| 70 | 95.5 | 0.5 | 3148.5 | 315.5 | ||||||||
| Buffer | Molarities [M] | pH |
|---|---|---|
| K2HPO4\KH2PO4 | 1/0.1 | 7 |
| 8 | ||
| Na2HPO4\NaH2PO4 | 1/0.1 | 7 |
| 8 | ||
| Tris- HCl | 1/0.1 | 7 |
| MOPS | 1/0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinberger, S.; Pellis, A.; Comerford, J.W.; Farmer, T.J.; Guebitz, G.M. Efficient Physisorption of Candida Antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis. Catalysts 2018, 8, 369. https://doi.org/10.3390/catal8090369
Weinberger S, Pellis A, Comerford JW, Farmer TJ, Guebitz GM. Efficient Physisorption of Candida Antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis. Catalysts. 2018; 8(9):369. https://doi.org/10.3390/catal8090369
Chicago/Turabian StyleWeinberger, Simone, Alessandro Pellis, James W. Comerford, Thomas J. Farmer, and Georg M. Guebitz. 2018. "Efficient Physisorption of Candida Antarctica Lipase B on Polypropylene Beads and Application for Polyester Synthesis" Catalysts 8, no. 9: 369. https://doi.org/10.3390/catal8090369






