Performance of an Auto-Reduced Nickel Catalyst for Auto-Thermal Reforming of Dodecane
Abstract
:1. Introduction
- Steam reforming (SR): CnHm + nH2O ↔ nCO + (n + m/2)H2
- Partial oxidation (POx): CnHm + (n/2)O2 ↔ nCO + (m/2)H2
- Auto thermal reforming (ATR): CnHm + (n/4)O2 + (n/2)H2O ↔ nCO + (n + m)/2H2
2. Results and Discussion
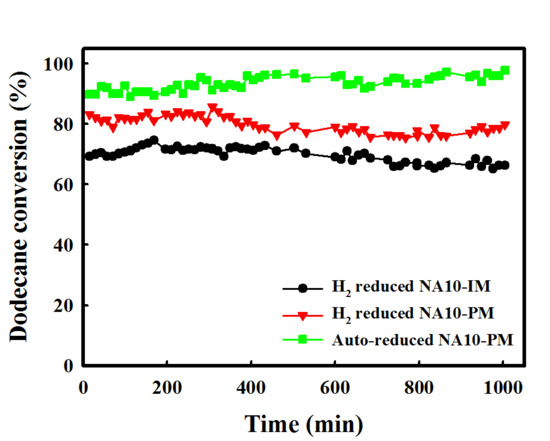
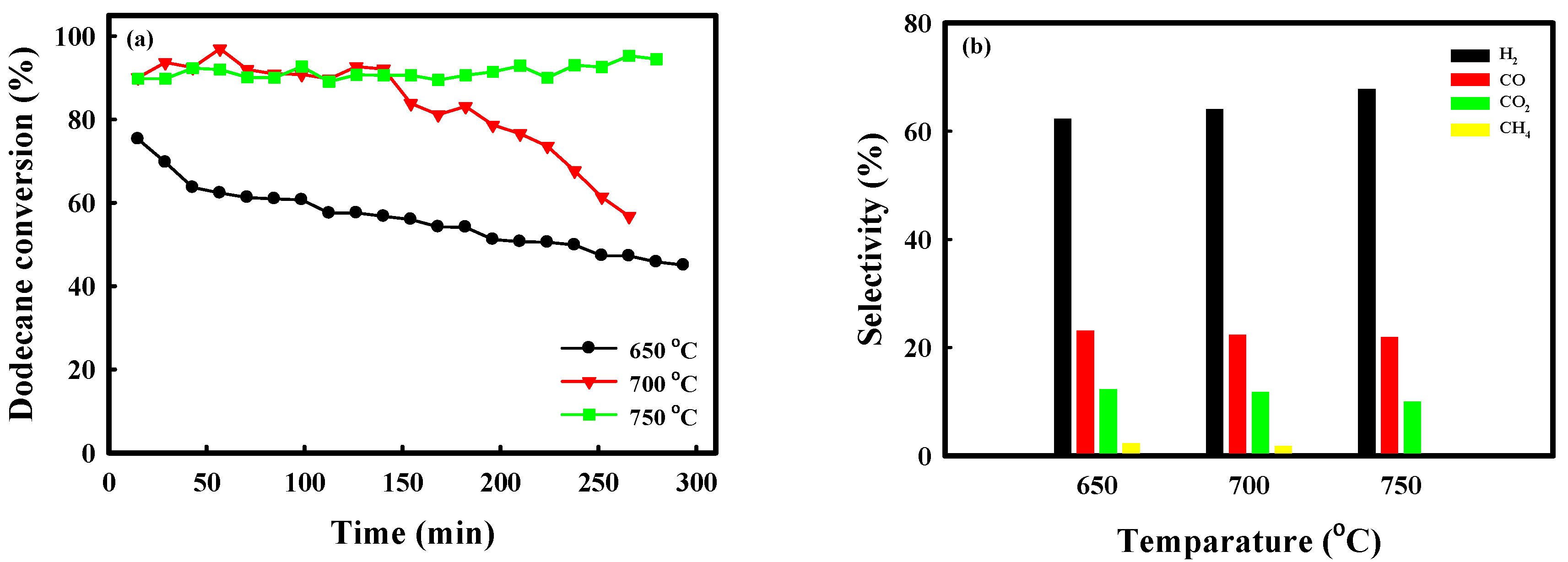
2.1. Catalytic Performance Comparison of NA10-PM with Various Catalysts
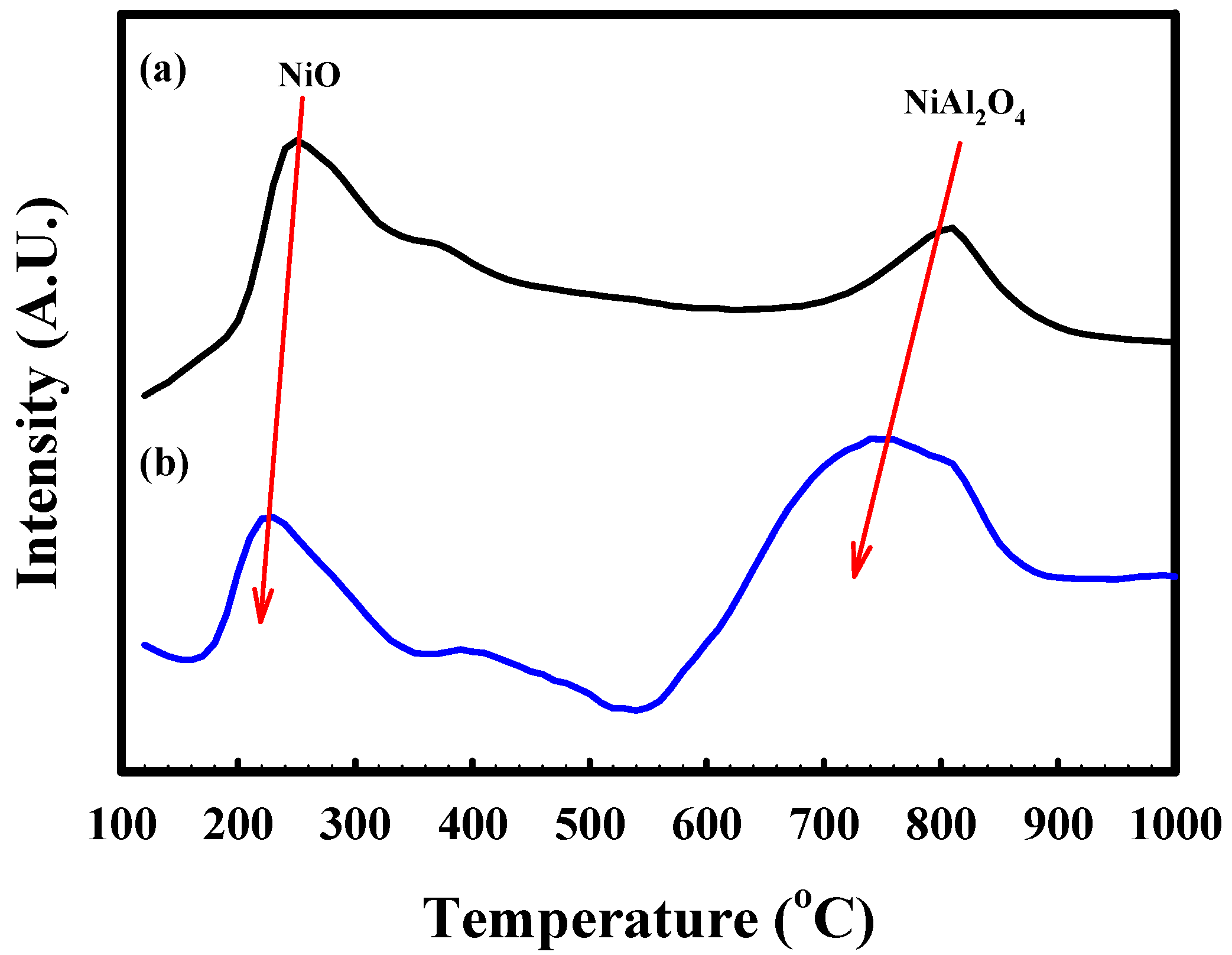
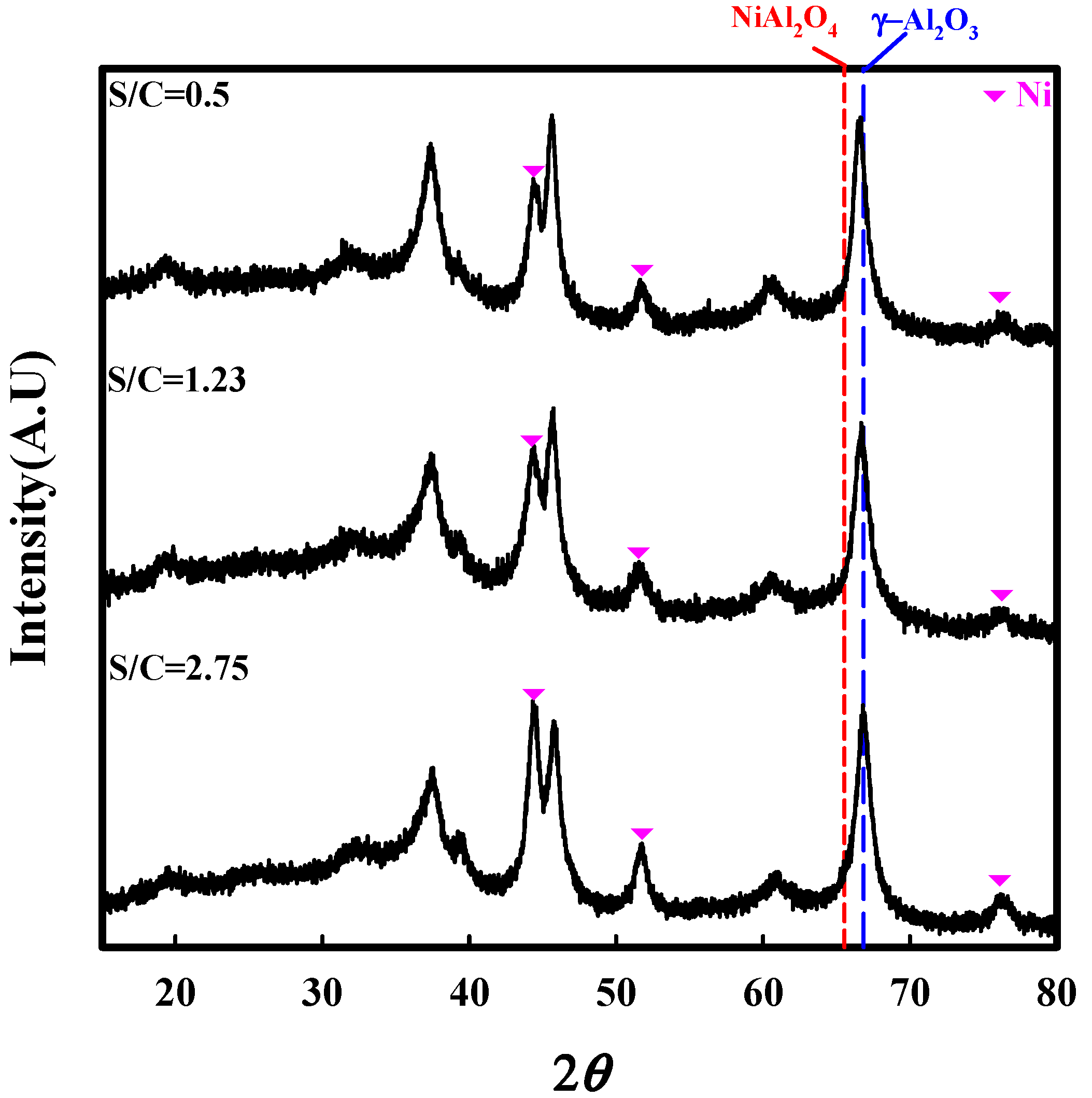
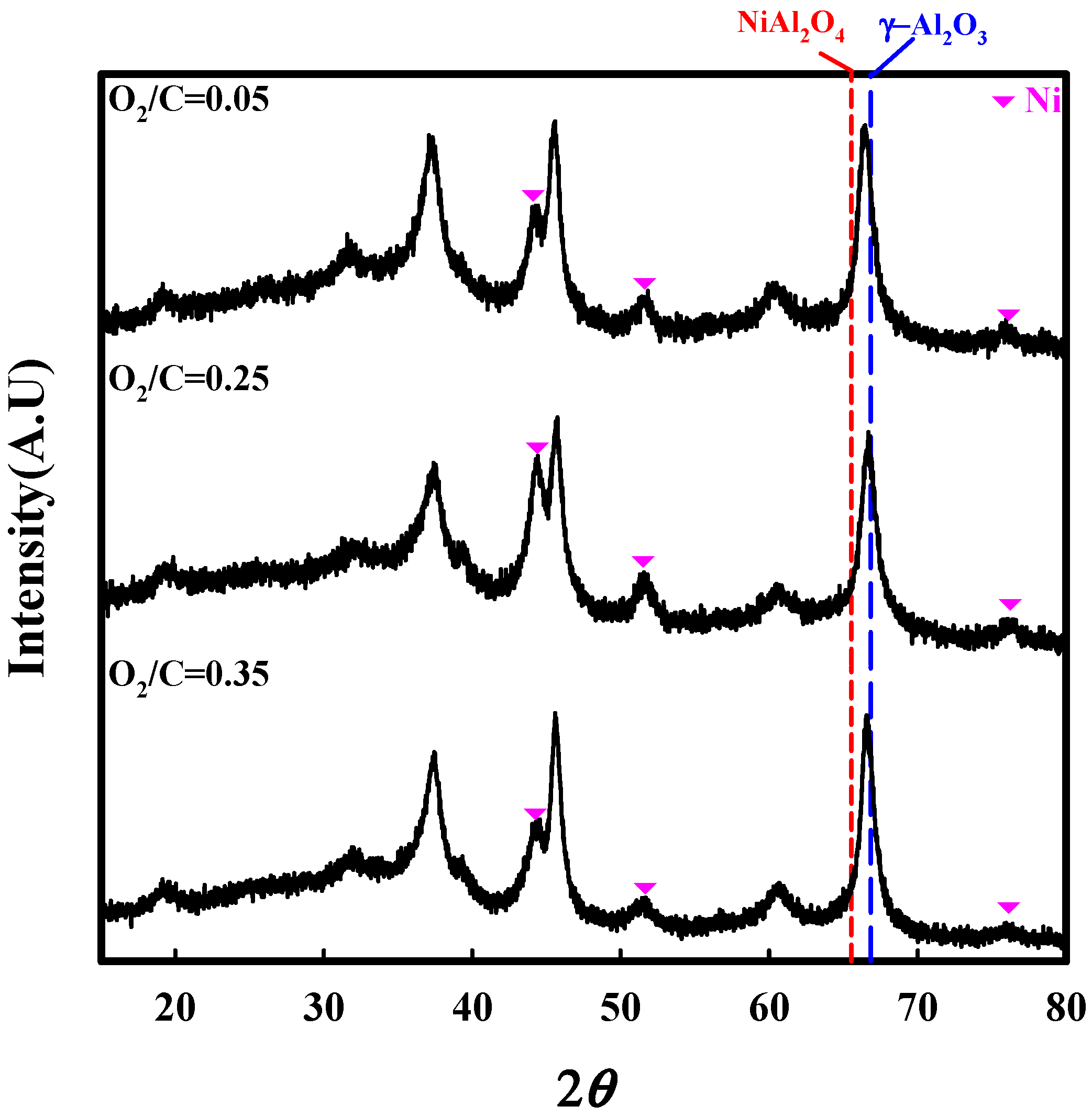
2.2. Characterization
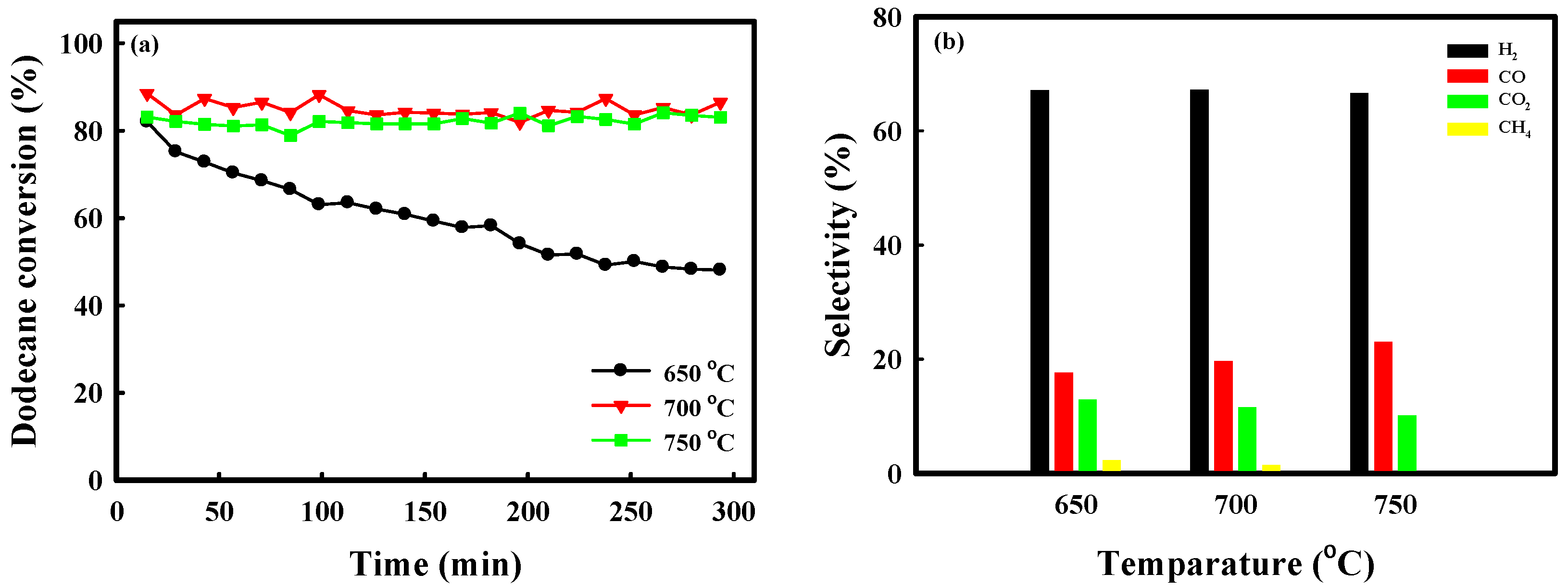
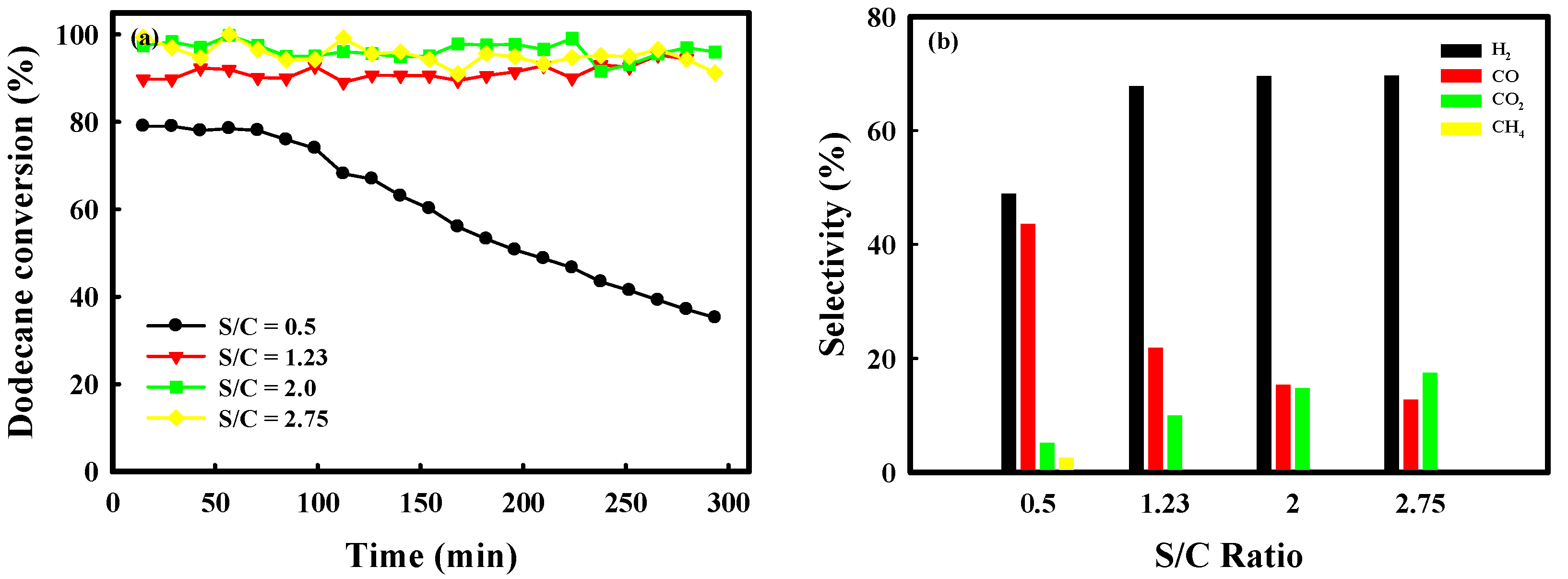
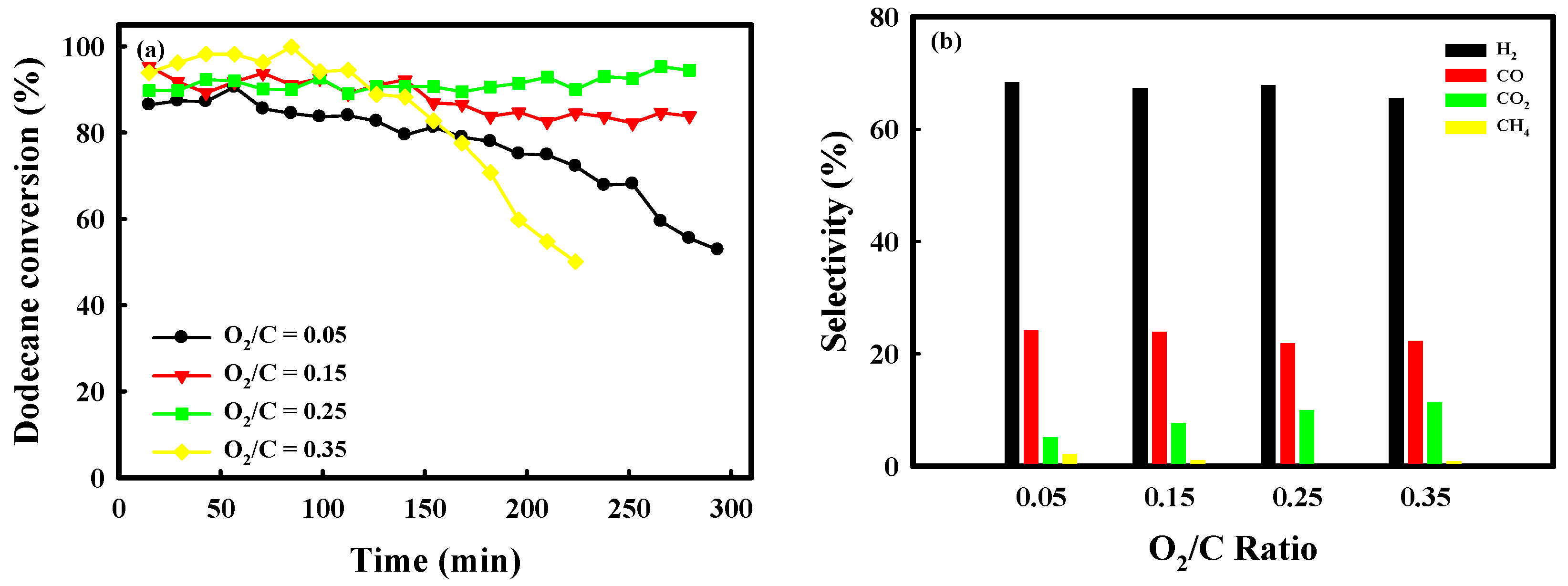
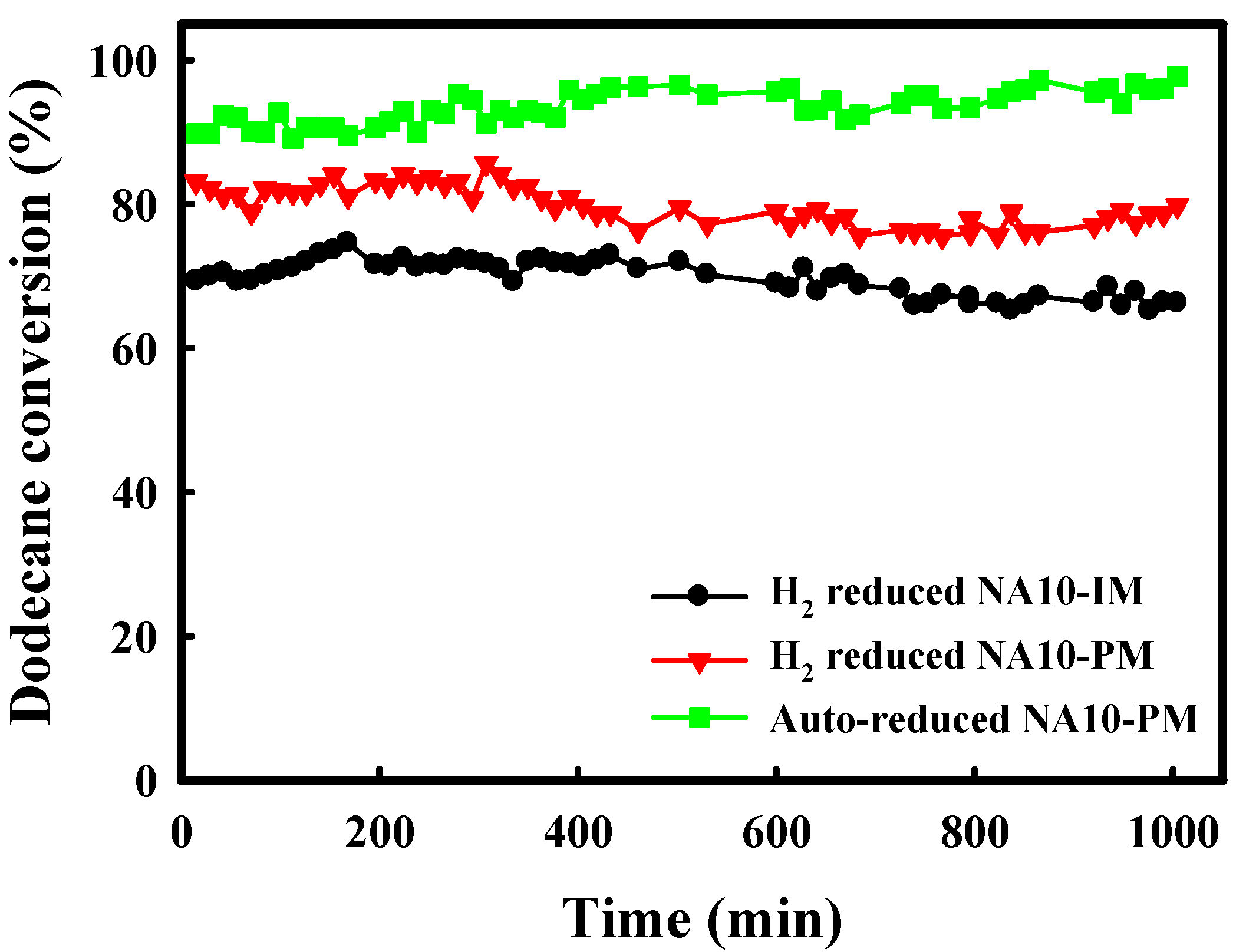
2.3. Catalytic Performance of Auto-Reduced NA10-PM Catalyst
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization
3.3. Catalytic Reforming Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aicher, T.; Lenz, B.; Gschnell, F.; Groos, U.; Federici, F.; Caprile, L.; Parodi, L. Fuel processors for fuel cell APU applications. J. Power Sources 2006, 154, 503–508. [Google Scholar] [CrossRef]
- Lindström, B.; Karlsson, J.; Ekdunge, P.; De Verdier, L.; Häggendal, B.; Dawody, J.; Nilsson, M.; Pettersson, L.J. Diesel fuel reformer for automotive fuel cell applications. Int. J. Hydrogen Energy 2009, 34, 3367–3381. [Google Scholar] [CrossRef]
- Zur Megede, D. Fuel processors for fuel cell vehicles. J. Power Sources 2002, 106, 35–41. [Google Scholar] [CrossRef]
- Clarke, S.H.; Dicks, A.L.; Pointon, K.; Smith, T.A.; Swann, A. Catalytic aspects of the steam reforming of hydrocarbons in internal reforming fuel cells. Catal. Today 1997, 38, 411–423. [Google Scholar] [CrossRef]
- Kaila, R.; Krause, A. Autothermal reforming of simulated gasoline and diesel fuels. Int. J. Hydrogen Energy 2006, 31, 1934–1941. [Google Scholar] [CrossRef] [Green Version]
- Palm, C.; Cremer, P.; Peters, R.; Stolten, D. Small-scale testing of a precious metal catalyst in the autothermal reforming of various hydrocarbon feeds. J. Power Sources 2002, 106, 231–237. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Liu, H.; Zhou, X. Self-sustained electrochemical promotion catalysts for partial oxidation reforming of heavy hydrocarbons. Int. J. Hydrogen Energy 2012, 37, 17928–17935. [Google Scholar] [CrossRef]
- Lakhapatri, S.L.; Abraham, M.A. Deactivation due to sulfur poisoning and carbon deposition on Rh-Ni/Al2O3 catalyst during steam reforming of sulfur-doped n-hexadecane. Appl. Catal. A Gen. 2009, 364, 113–121. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Y.; Li, Y.; Wang, X.; Song, C. Influence of sulfur on the carbon deposition in steam reforming of liquid hydrocarbons over CeO2-Al2O3 supported Ni and Rh catalysts. Appl. Catal. A Gen. 2011, 394, 32–40. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zheng, Z.; Zhao, C. Hydrogen production from chemical looping reforming of ethanol using Ni/CeO2 nanorod oxygen carrier. Catalysts 2018, 8, 257. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Renewable hydrogen from ethanol reforming over CeO2-SiO2 based catalysts. Catalysts 2017, 7, 226. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; Krause, T.R. Rare earth-first-row transition metal perovskites as catalysts for the autothermal reforming of hydrocarbon fuels to generate hydrogen. Appl. Catal. A Gen. 2008, 334, 311–320. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Seo, J.G.; Youn, M.H.; Song, J.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over ordered mesoporous nickel–alumina catalyst. Int. J. Hydrogen Energy 2012, 37, 17967–17977. [Google Scholar] [CrossRef]
- Chen, X.; Gould, B.D.; Schwank, J.W. n-Dodecane reforming over monolith-based Ni catalysts: Sem study of axial carbon distribution profile. Appl. Catal. A Gen. 2009, 356, 137–147. [Google Scholar] [CrossRef]
- Gould, B.D.; Chen, X.; Schwank, J.W. Dodecane reforming over nickel-based monolith catalysts. J. Catal. 2007, 250, 209–221. [Google Scholar] [CrossRef]
- Gould, B.D.; Chen, X.; Schwank, J.W. n-Dodecane reforming over nickel-based monolith catalysts: Deactivation and carbon deposition. Appl. Catal. A Gen. 2008, 334, 277–290. [Google Scholar] [CrossRef]
- Cheng, F.; Dupont, V. Steam reforming of bio-compounds with auto-reduced nickel catalyst. Catalysts 2017, 7, 114. [Google Scholar] [CrossRef]
- Gould, B.D.; Tadd, A.R.; Schwank, J.W. Nickel-catalyzed autothermal reforming of jet fuel surrogates: N-Dodecane, tetralin, and their mixture. J. Power Sources 2007, 164, 344–350. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Jia, L.; Kameyama, H. Trace Ru-doped anodic alumina-supported Ni catalysts for steam reforming of kerosene: Activity performance and electrical-heating possibility. Fuel Process. Technol. 2011, 92, 2341–2347. [Google Scholar] [CrossRef]
- Modafferi, V.; Panzera, G.; Baglio, V.; Frusteri, F.; Antonucci, P. Propane reforming on Ni–Ru/GDC catalyst: H2 production for IT-SOFCs under SR and ATR conditions. Appl. Catal. A Gen. 2008, 334, 1–9. [Google Scholar] [CrossRef]
- Zhong, X.; Xie, W.; Wang, N.; Duan, Y.; Shang, R.; Huang, L. Dolomite-derived Ni-based catalysts with Fe modification for hydrogen production via auto-thermal reforming of acetic acid. Catalysts 2016, 6, 85. [Google Scholar] [CrossRef]
- Jung, S.Y.; Ju, D.G.; Lim, E.J.; Lee, S.C.; Hwang, B.W.; Kim, J.C. Study of sulfur-resistant Ni–Al-based catalysts for autothermal reforming of dodecane. Int. J. Hydrogen Energy 2015, 40, 13412–13422. [Google Scholar] [CrossRef]
- Lee, W.S.; Ju, D.G.; Jung, S.Y.; Lee, S.C.; Ha, D.S.; Hwang, B.W.; Kim, J.C. n-Dodecane autothermal reforming properties of Ni-Al based catalysts prepared by various methods. Top. Catal. 2017, 60, 727–734. [Google Scholar] [CrossRef]
- Amano, F.; Tanaka, T.; Funabiki, T. Auto-reduction of Cu(II) species supported on Al2O3 to Cu (i) by thermovacuum treatment. J. Mol. Catal. A Chem. 2004, 221, 89–95. [Google Scholar] [CrossRef]
- Cheng, F.; Dupont, V. Nickel catalyst auto-reduction during steam reforming of bio-oil model compound acetic acid. Int. J. Hydrogen Energy 2013, 38, 15160–15172. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, Y.; Kim, Y.; Mingle, K.; Lauterbach, J.; Blom, D.A.; Vogt, T.; Lee, Y. Ethylene epoxidation catalyzed by Ag nanoparticles on Ag-LSX zeolites formed by pressure-and temperature-induced auto-reduction. Chem. Eur. J. 2018, 24, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Hildebrandt, P.; Ludwig, B. Induction of photochemical auto-reduction of Cytochrome-c oxidase by an organic peroxide. Biochim. Biophys. Acta (BBA) Bioenergetics 2000, 1459, 125–130. [Google Scholar] [CrossRef]
- Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction kinetics of Cu-, Ni-, and Fe-based oxygen carriers using syngas (CO + H2) for chemical-looping combustion. Energy Fuels 2007, 21, 1843–1853. [Google Scholar] [CrossRef]
- Bang, Y.; Seo, J.G.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni–La–Al2O3 aerogel catalysts: Effect of La content. Int. J. Hydrogen Energy 2011, 36, 8307–8315. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Bang, Y.; Song, I.K. Effect of Ni/Al atomic ratio of mesoporous Ni–Al2O3 aerogel catalysts on their catalytic activity for hydrogen production by steam reforming of liquefied natural gas (LNG). Int. J. Hydrogen Energy 2010, 35, 12174–12181. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158, 190–201. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, Q.; Jiang, Z.; Hou, B.; Li, H. Characterization of aerogel Ni/Al2O3 catalysts and investigation on their stability for CH4-CO2 reforming in a fluidized bed. Fuel Process. Technol. 2009, 90, 113–121. [Google Scholar] [CrossRef]
- Cheekatamarla, P.K.; Lane, A.M. Catalytic autothermal reforming of diesel fuel for hydrogen generation in fuel cells: I. Activity tests and sulfur poisoning. J. Power Sources 2005, 152, 256–263. [Google Scholar] [CrossRef]

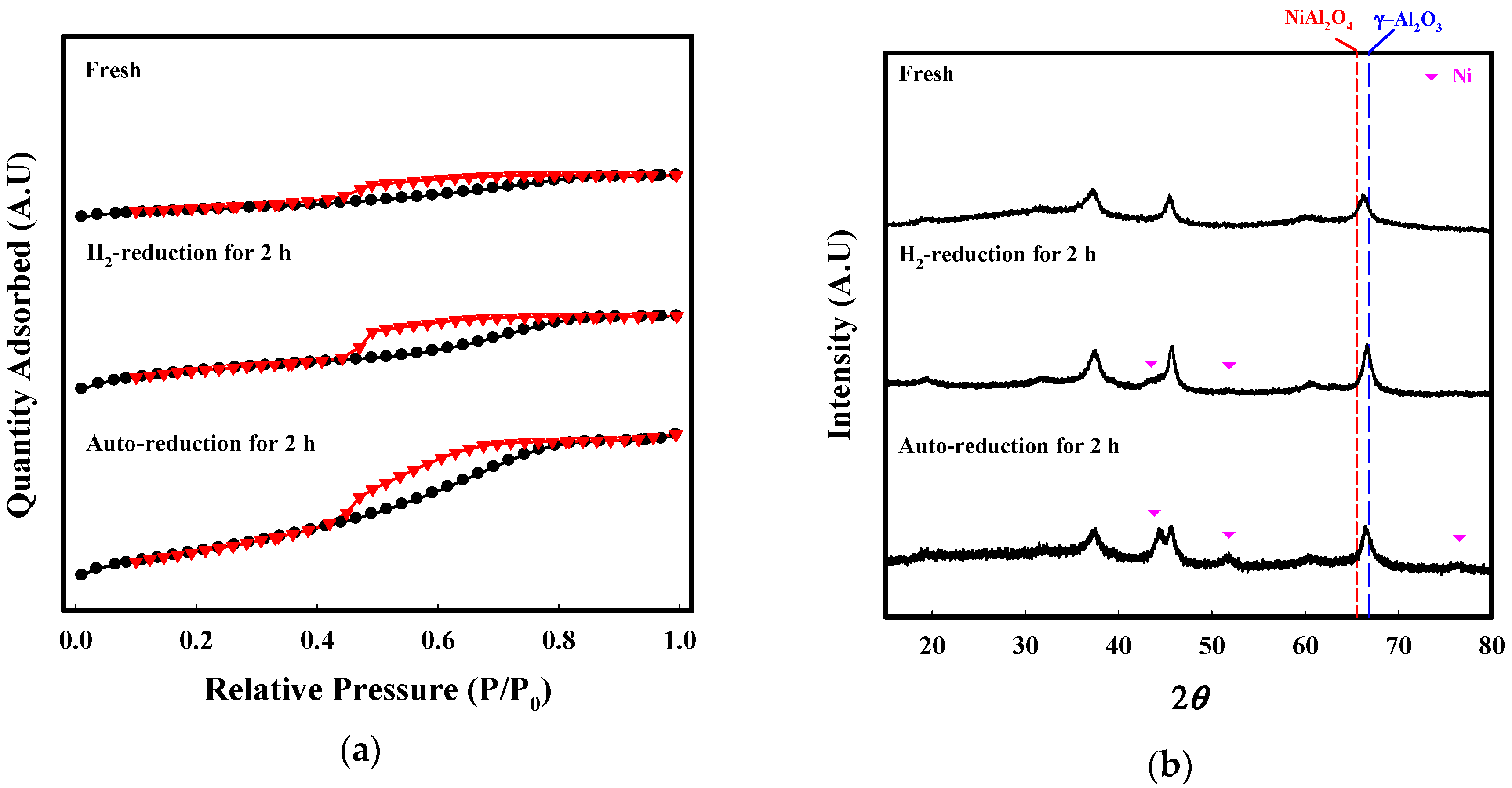
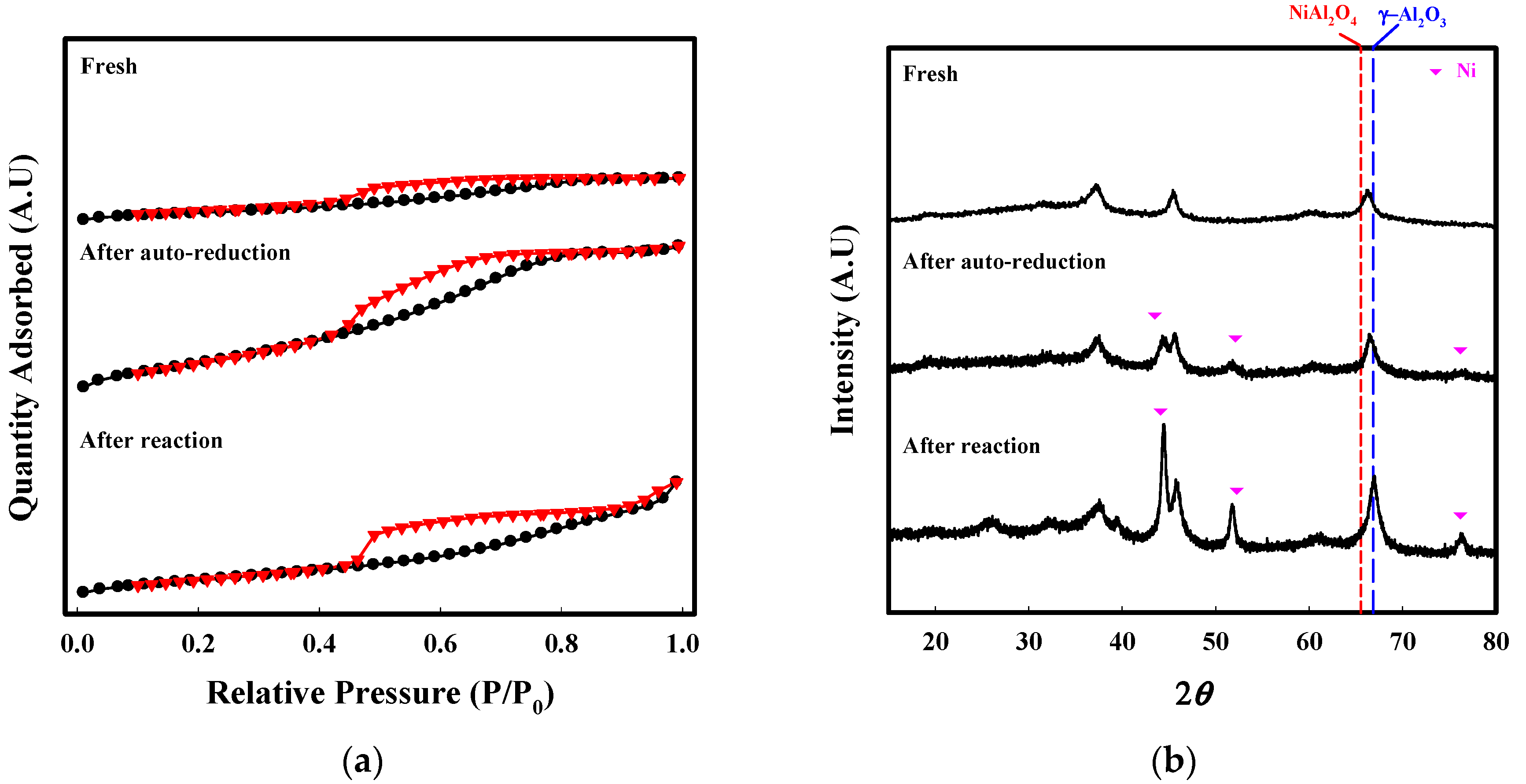
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | |
|---|---|---|---|
| Fresh | 28.1 | 0.04 | 3.8 |
| H2-reduction | 78.1 | 0.06 | 3.8 |
| Auto-reduction | 96.6 | 0.12 | 4.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.B.; Ju, D.G.; Jung, S.Y.; Ha, D.S.; Chae, H.J.; Lee, S.C.; Kim, J.C. Performance of an Auto-Reduced Nickel Catalyst for Auto-Thermal Reforming of Dodecane. Catalysts 2018, 8, 371. https://doi.org/10.3390/catal8090371
Jo SB, Ju DG, Jung SY, Ha DS, Chae HJ, Lee SC, Kim JC. Performance of an Auto-Reduced Nickel Catalyst for Auto-Thermal Reforming of Dodecane. Catalysts. 2018; 8(9):371. https://doi.org/10.3390/catal8090371
Chicago/Turabian StyleJo, Seong Bin, Dong Geon Ju, Suk Yong Jung, Dong Su Ha, Ho Jin Chae, Soo Chool Lee, and Jae Chang Kim. 2018. "Performance of an Auto-Reduced Nickel Catalyst for Auto-Thermal Reforming of Dodecane" Catalysts 8, no. 9: 371. https://doi.org/10.3390/catal8090371