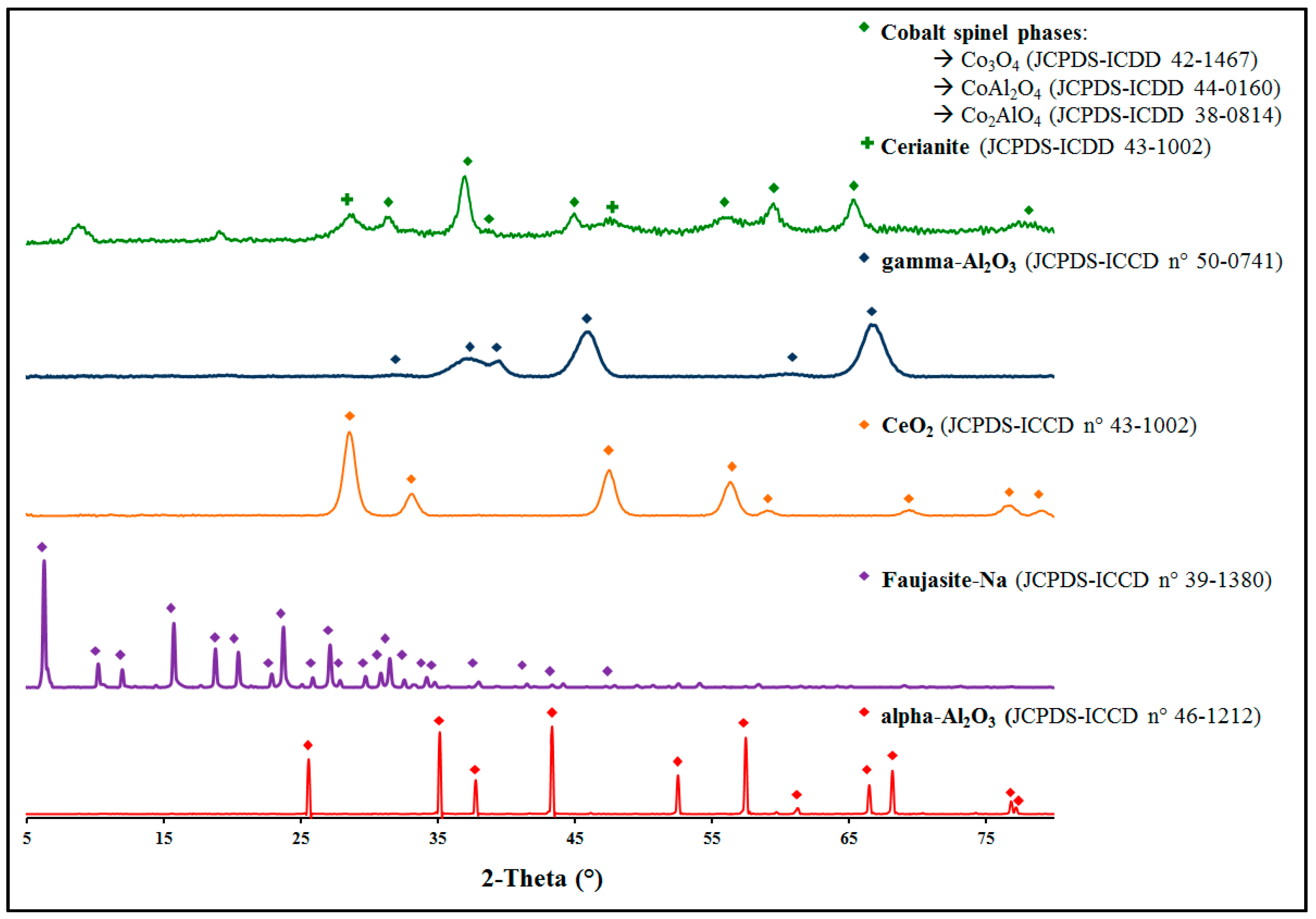
2.2. Toluene Oxidation
As a first set of experiments, the materials of interest were used to investigate the total oxidation of toluene, and the light-off curves obtained are shown in
Figure 2 for all materials.
The light-off curves of Pd-based catalysts have been presented in a previous study (Pd/α-Al
2O
3, Pd/HY, Pd/CeO
2 and Pd/γ-Al
2O
3) [
3].
T50 and
T100 are defined as the temperature when 50% and 100% conversion, respectively, was observed. These values of
T50 and
T100 for toluene oxidation are reported in
Table 2. The test conducted with pure SiC shows only a slight toluene conversion from 360 °C, with a maximum of 5% at 400 °C. This can be explained by a thermal decomposition. Indeed, the test performed under the same conditions with the empty reactor leads to the same result. The results show that the mixed oxide has a lower performance than palladium-based catalysts. However, with a
T50 value of 249 °C, the CoAlCeO material shows excellent activity for the toluene total oxidation. Moreover, in view of the activity values (
A) in
Table 2, it is possible to observe that the mixed oxide has a similar activity to that of Pd/CeO
2 and Pd/γ-Al
2O
3 catalysts, highlighting the possibility of such catalysts replacing Pd-based materials in the VOCs removal processes.
As for palladium-based materials, the CoAlCeO mixed oxide was investigated regarding its properties to form byproducts, in particular benzene. In addition to the toluene conversion,
Figure 2 shows the benzene emission profiles (as dashed lines) as a function of toluene conversion (solid lines) and temperature. The results show that the four palladium-based catalysts present similar emission profiles, with a maximum value at around 10 ppm. By contrast, the emission profile of the CoAlCeO mixed oxide revealed a maximum value of less than 1 ppm, namely an order of magnitude lower as compared to the other catalysts. In order to compare the benzene emissions of each material, the methodology presented in a previous study has been used [
3], where the emission profiles are characterized by seven parameters. However, only the most relevant parameters are presented in this paper:
Qmax: maximum quantity emitted observed of the considered byproduct (ppm);
Tf: temperature at which the byproduct is totally oxidized (°C);
P: persistence of byproduct, corresponding to the difference between Tf and T100 (°C).
Table 2 reports the values of
Qmax,
Tf and
P. Regarding Pd/α-Al
2O
3, Pd/HY, Pd/CeO
2, and Pd/γ-Al
2O
3 catalysts, the
Qmax values present a maximum amount of emitted benzene between 7 and 14 ppm. These values are relatively low as compared to the concentration of oxidized toluene. However, by taking into account the toxicity of this compound and its strict regulations, these values remain important. On the contrary, the CoAlCeO catalyst presents the lowest emissions with a
Qmax value of 0.9 ppm. Concerning the
P values, they are positive (on average 40 °C), which clearly indicates that benzene is not fully oxidized when the toluene total oxidation (
T100) is reached. Therefore, this shows that benzene is a crucial limitation to the catalytic process of toluene oxidation: it is then necessary to consider this byproduct in order to set the working temperature of the process so as to oxidize all organic compounds. Both materials Pd/HY and CoAlCeO stand with the lowest
P values of 7 and 20 °C respectively, sign of their improved performance for benzene oxidation.
To achieve real VOCs total oxidation, the working temperature must be set assuming the total oxidation of the reactant and its byproducts. This fact is supported by the toxic and regulatory aspects related to benzene. Therefore, the classification of the catalysts by their performance must be reassessed. Subsequently, according to the T50 and T100, the ranking is as follows, from the most efficient to the least efficient: Pd/γ-Al2O3 > Pd/α-Al2O3 > Pd/CeO2 > Pd/HY > CoAlCeO. Considering the emissions of benzene, as Tf, this classification may be reviewed as follows: Pd/γ-Al2O3 > Pd/HY > CoAlCeO > Pd/CeO2 > Pd/α-Al2O3. The difference between the T100 value of Pd/γ-Al2O3 and Pd/HY catalysts is then reduced from 42 °C to 5 °C by considering the Tf value corresponding to the temperature at which toluene is totally converted. Thus, the performances of these two materials are very close. Moreover, if the CoAlCeO catalyst exhibits lower performance considering only T50 and T100 values, it is by far the lowest benzene emitter and consequently, accounting for this point, CoAlCeO is among the best catalysts for toluene oxidation.
2.3. Butanone Oxidation
After analyzing the efficiency of the different catalytic materials for toluene oxidation, these materials have been studied in the framework of butanone (MEK) oxidation with a similar methodology. The light-off curves obtained for all materials are shown in
Figure 3.
The values of
T50 and
T100 for MEK oxidation are reported in
Table 3 in comparison with values corresponding to the toluene oxidation. The test conducted with SiC shows a slight conversion from 260 °C, with a MEK conversion of 20% at 400 °C. As in the case of toluene, this observation can be explained by thermal decomposition. However, this phenomenon is more important because ketone function promotes the oxidation of the molecule. For the remaining materials, firstly, the curves show greater reactivity of MEK as compared to toluene. Indeed, besides the Pd/α-Al
2O
3 catalyst, the light-off curves are shifted to lower temperatures. This effect is particularly important for the CoAlCeO catalyst with a difference of 71 °C in the
T50 values. Moreover, the MEK conversion at low temperature is particularly marked for the Pd/γ-Al
2O
3 and CoAlCeO catalysts. Therefore, from an activity point of view, the classification observed for the toluene oxidation on these materials is different, taking into account only the values of
T100. In the case of toluene, the performance sequence is: Pd/γ-Al
2O
3 > Pd/α-Al
2O
3 > Pd/CeO
2 > Pd/HY > CoAlCeO. For the MEK oxidation however, the performance sequence turns as follows: CoAlCeO > Pd/γ-Al
2O
3 > Pd/HY > Pd/CeO
2 > Pd/αAl
2O
3, where the CoAlCeO catalyst is now the most efficient catalyst.
Figure 3 compares the light-off curves of toluene and MEK and clearly shows a shift of the light-off curves towards lower temperatures for MEK (dashed lines) relative to toluene (full lines). However, it is also possible to notice that these light-off curves are extended over a wider range of temperature in case of MEK oxidation. This is particularly highlighted by considering the differences between
T90 and
T10 values for each light-off curve (given in
Table 3). In fact, this difference is on average 38 °C for toluene oxidation while it is on average 92 °C for MEK oxidation. For Pd/γ-Al
2O
3, Pd/CeO
2 and Pd/HY, the difference between the two light-off curves are substantially reduced and even reversed at high conversion: toluene indeed becomes easier to oxidize than MEK beyond a certain conversion. In addition, the light-off curves of MEK of these three catalysts exhibit an inflection point between 30% and 50% of conversion. From this point, light-off curves are stretched towards higher temperatures. In the case of Pd/α-Al
2O
3, no strong inflection point was evidenced, but the light-off curve stretches progressively with increasing temperatures. On the contrary, the light-off curve of the CoAlCeO material shows a similar behavior for both VOCs. The most important observation from this figure is the fact that the CoAlCeO catalyst achieves 100% conversion for the lowest temperature, with an important difference of approximately 65 °C with Pd/γ-Al
2O
3 and Pd/HY materials.
The profiles of the light-off curves for the MEK oxidation can be explained by the formation of byproducts. Indeed, the generation of byproducts in the case of oxygenated compounds’ oxidation is relatively well known, and byproducts of MEK oxidation were identified as a result of various studies made on its partial oxidation and total oxidation [
8,
9,
12,
27,
28]. Firstly, studies of McCullagh et al. [
27,
28], Álvarez-Galván et al. [
12] and Arzamendi et al. [
8] have identified three oxidation pathways that lead to several oxygenated compounds in C
2 and C
4: acetic acid, acetaldehyde, diacetyl (butane-2,3-dione) and methyl vinyl ketone. Secondly, a study realized by Machold et al. [
9] has highlighted a fourth method of oxidation that leads to the formation of propanal, propanoic acid, and methanoic acid. The study of the byproducts has been applied for MEK oxidation. A microGC analysis permitted the detection of four peaks corresponding to oxidation by-products. Injection of organic standards allowed the identification of five compounds, in agreement with the literature: acetic acid (AcOOH), acetaldehyde (AcO), propionic acid (PrOOH), diacetyl (DAC), and methyl vinyl ketone (MVK). In fact, with acetic acid and acetaldehyde being co-eluted, it is not possible to differentiate and quantify them separately by micro-GC. Nevertheless, in contrast to acetic acid, acetaldehyde is carcinogenic and thus an unwanted product. Consequently, the corresponding chromatographic peak was assigned to acetaldehyde in order to consider the worst results in terms of byproduct emissions. The different byproducts have been quantified and their emission profiles have been determined as a function of MEK conversion and temperature, and are represented in
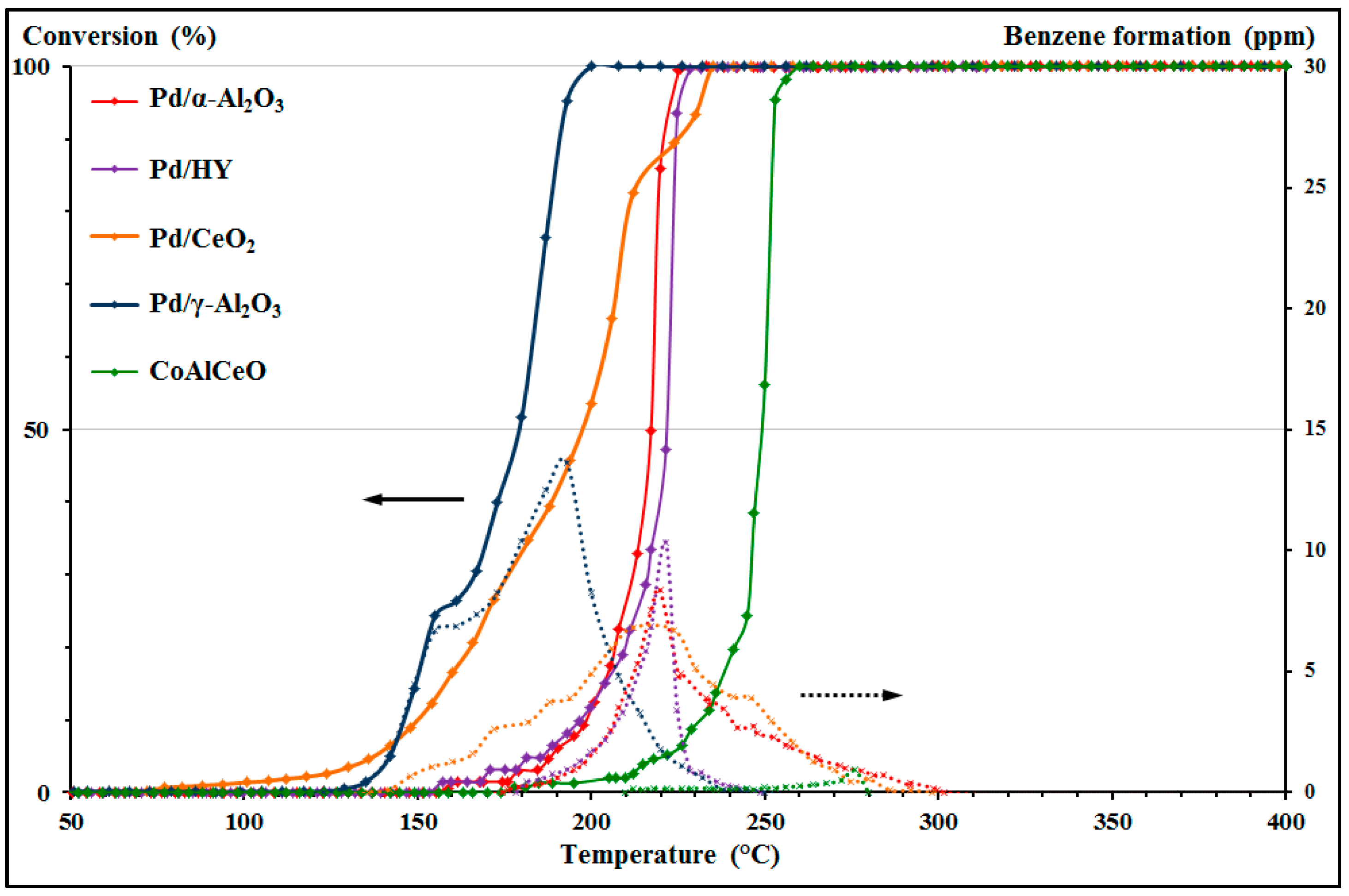
Figure 4 for Pd/γ-Al
2O
3 (
Figure 4a) and CoAlCeO (
Figure 4b).
For the two materials Pd/γ-Al
2O
3 and CoAlCeO illustrate the two behaviors observed for the MEK oxidation. For Pd/γ-Al
2O
3,
Figure 4a shows that the light-off curve is influenced by the generation of byproducts. In fact, the inflection point at 50% conversion is consistent with an emission peak of a byproduct: propionic acid. For the Pd/CeO
2 and Pd/HY materials, this inflection point is consistent with the emission peak of acetaldehyde. A hypothesis for this behavior is that these byproducts induce a partial inhibition of the MEK oxidation by blocking the catalytic sites. For CoAlCeO, the emission profiles are more important, especially for acetaldehyde (
Figure 4b). However, despite this larger amount of byproducts, the material does not show any inhibitory effect on the MEK oxidation. The parameters used to characterize the benzene emission profiles were also applied on the byproducts’ oxidation of MEK and are presented in
Table 4.
Table 4 reports the values of
Qmax,
Tf and
P for MEK byproducts. The profiles show that acetaldehyde is the major byproduct of the MEK oxidation, except for Pd/γ-Al
2O
3. Moreover, it is possible to distinguish two emission profiles. The first can be observed for Pd/α-Al
2O
3, Pd/HY, and Pd/γ-Al
2O
3, where the maximum quantities issued for each byproduct are between 1 and 15 ppm. The second concerns Pd/CeO
2 and CoAlCeO, where these values remain on the same order of magnitude for propionic acid, methyl vinyl ketone and diacetyl, but acetaldehyde is emitted in a much larger quantity, up to 56 ppm. Finally, except for the two materials with alumina support, the byproducts seem to be fully oxidized before reaching the total conversion of MEK (
T100). For Pd/α-Al
2O
3 and Pd/γ-Al
2O
3,
Table 4 shows a persistence of acetaldehyde. Nevertheless, this persistence is very low (6–12 °C) and has a small impact on the performance of these materials. Therefore, the sequence of performance established in relation to activities (
T100) remains the same after considering the byproducts: CoAlCeO > Pd/γ-Al
2O
3 > Pd/HY > Pd/CeO
2 > Pd/α-Al
2O
3.
2.4. Toluene/MEK Mixture Oxidation
The next step towards application of industrial exhaust is to study the behavior of the different catalytic materials for the total oxidation of a binary mixture (toluene/MEK 1000/1000 ppm). The results presented in this section are discussed according to two aspects: (i) the light-off curves of VOCs alone and in mixture are compared to assess mixing effects between toluene and butanone for each catalyst; (ii) the light-off curves of the binary mixture are compared in order to differentiate the performance of each catalyst. Two methods are used to calculate the VOC conversion. The first is shown in Equation (1) and denoted “
XTotal Carbon”. This allows for tracing the light-off curve in total carbon without taking into account the pressure drop and adsorption/desorption effects. Subsequently, the light-off curve is only influenced by chemical phenomena. However, this method gives a general idea and does not allow following the conversion of each VOC in the mixture. The second method is a conversion calculation taking into account the initial and outgoing quantity of VOCs. However, the light-off curve is influenced by physical phenomena (pressure drop, adsorption/desorption phenomena). The formula is given by Equation (2) and is denoted “
XVOC”. Thus, the first method has been used to compare oxidation performances between catalysts, and the second to compare the oxidation performances between VOCs alone and in mixture.
where:
Ri,0 is the initial mole percentage of VOCs;
Ri,T is the mole percentage of VOCs at the temperature T;
Pi,T is the mole percentage of byproducts at the temperature T;
CO2,T is the mole percentage of carbon dioxide at the temperature T;
Xi is the number of carbon atoms in the compound i.
Concerning the mixing effects, two behaviors have been identified: inhibitory effects as well as beneficial effects. These are more or less marked as a function of the materials used and the temperature. As for the influence of MEK byproducts, these effects are more important on Pd/γ-Al
2O
3 and CoAlCeO catalysts. The light-off curves of the binary mixture for these catalysts are shown in
Figure 5, and the corresponding
T50 values are reported in
Table 5.
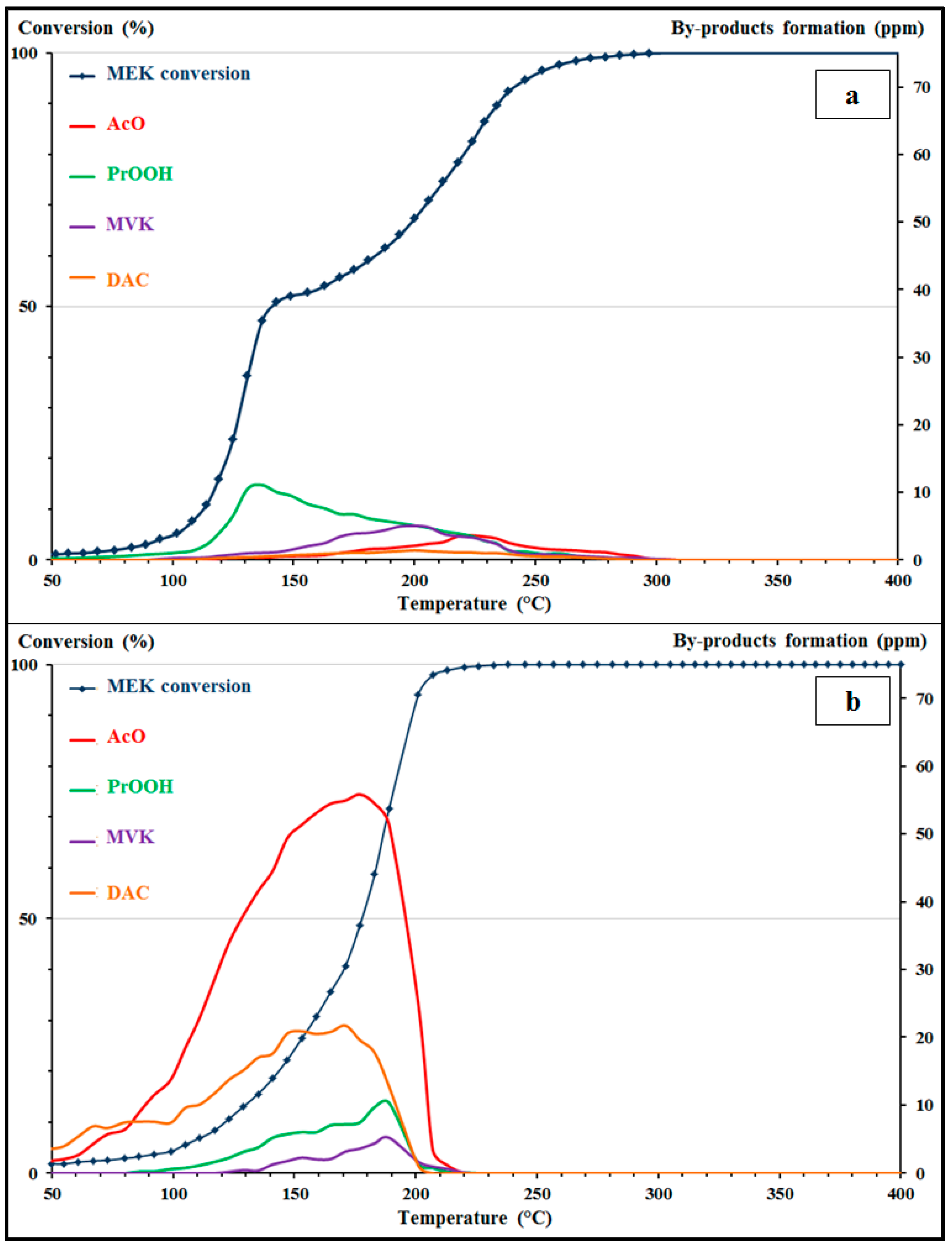
In the case of Pd/γ-Al
2O
3 catalyst (
Figure 5a), a modification of the reactivity is revealed for both VOCs. For MEK oxidation in mixture, a shift of the light-off curve to higher temperatures is observed as compared to the light-off curve of MEK alone. Indeed, the shift between the curves begins at 100 °C on a conversion range of 0 to 70%, with a value of 63 °C at
T50. Beyond 70% of conversion, both light-off curves are reversed, MEK being then slightly more reactive in the presence of toluene. For toluene oxidation in mixture, a shift in the conversion curve is also observed with the light-off curve of toluene alone. Consequently, the light-off curves of VOCs in a binary mixture show inhibitory effects for both compounds. This can be explained by competitive reactions between the two molecules in the adsorption/desorption steps, causing the blocking of the active sites of the catalyst. This phenomenon has already been observed in the literature in mixtures of hydrocarbons and oxygenated compounds [
7,
13,
29,
30]. These inhibitory phenomena are also observed for Pd/HY and Pd/CeO
2.
For the CoAlCeO material (
Figure 5b), the results also show a change in the reactivity of both VOCs. For MEK oxidation in mixture, a shift of the light-off curve to higher temperatures is observed. However, this effect between the two curves is more regular than with Pd/γ-Al
2O
3. A shift of 28 °C at
T50 with the light-off curve of MEK alone is observed. In contrast, a beneficial effect is observed on the toluene oxidation in mixture. Indeed, the light-off curve of toluene in mixture is clearly shifted to lower temperatures (a difference of 30 °C at
T50 with the toluene-alone light-off curve). This behavior can be explained by an additive effect, as has been highlighted in the studies of Beauchet et al. [
31,
32] for the oxidation of xylene/isopropanol mixtures. It has been shown that an intermediate compound was formed during the oxidation of this mixture, isopropyldimethylbenzene, which is obtained from the alkylation of xylene with propene, an oxidation byproduct of isopropanol. This aromatic intermediate compound possesses more alkyl chains than xylene and a ternary carbon atom, therefore its reactivity is probably higher than that of xylene. Hence, the formation of this intermediate compound lowers the temperature of xylene oxidation due to the indirect increase of its reactivity. Is has to be noted that in the case of xylene and isopropanol, the number of their respective byproducts is restricted [
31,
33], leading to a facilitated identification of these intermediates during the oxidation of xylene/isopropanol mixtures. In this work, both VOCs have a greater number of byproducts with almost similar structures and chemical properties [
3,
8,
9,
33]: the identification of a potential byproduct from the addition of toluene and MEK is then more difficult. Additional analyses were performed with a Omnistar Quadrupole Mass Spectrometer (QMS-200) (Pfeiffer Vacuum, Asslar, Germany), configured in “Bargraph” mode to scan all
m/
z fragments between 1 and 199. These analyses have failed to detect compounds other than those already known and mentioned. As a consequence, if an additional compound is formed between the toluene and MEK, its lifetime is probably too short to be detected by ex situ analysis. Enhanced catalytic activity has also been observed on the Pd/α-Al
2O
3 catalyst, but the difference was not significant.
Concerning the oxidation of the binary mixture, the light-off curves obtained are shown in
Figure 6 for all materials. The values of
T50 and
T100 are reported in
Table 6, as well as the values for the oxidation of toluene for comparison.
The test conducted with SiC shows a slight conversion of the binary mixture from 225 °C, with a maximum of 9.9% at 400 °C. This partial conversion is derived from the thermal decomposition of VOCs, primarily of MEK, as has been observed for blank experiments performed with pure VOCs. In addition, partial conversion of the mixture begins at 225 °C instead of 260 °C and 360 °C, respectively, for MEK and toluene used on their own. However, partial conversion of the mixture reached a maximum of 9.9% (198 ppm) at 400 °C instead of 20.6% (206 ppm) and 5.7% (57 ppm), respectively, for MEK and toluene. Thus, the binary mixture seems to be reactive at lower temperatures, but with more limited conversion rates at high temperatures. As a first proof, the tests show light-off curves much less stretched than in the case of MEK oxidation. In addition, mixing effects observed for palladium-based catalysts do not significantly modify their performance. Indeed, given the T50 values, they remain close to those observed during toluene oxidation, with only a small temperature difference. Nevertheless, the mixing effects observed for the CoAlCeO mixed oxide have a strong impact on its activity: this is the least active catalyst for oxidation of toluene alone, without considering the byproduct oxidation. For the binary mixture, the presence of the MEK significantly lowers the value of T50 and this material exhibits performance fully comparable to palladium-based catalysts. As a second proof, beyond 95% of conversion, the light-off curves significantly stretch, which is correlated with a strong persistence of the residual traces of VOCs in the flow, especially for MEK. Therefore, the T100 values for the binary mixture oxidation are more important for all materials with respect to the oxidation of toluene alone, with an average shift of 88 °C. This phenomenon was expected since the total VOC concentration is higher in the binary mixture (2000 ppm instead of 1000 ppm). An important point is that only CoAlCeO mixed oxide presents a reduced impact of this phenomenon with only 30 °C difference on its T100 value. As a conclusion, this oxide corresponds to one of the best catalyst for the total abatement of the binary mixture. According to the T50 and T100, the performance sequence observed for the binary mixture oxidation is the following: CoAlCeO = Pd/γ-Al2O3 > Pd/CeO2 > Pd/α-Al2O3 > Pd/HY.
Regarding the effect of byproducts on the behavior of the mixture, their profiles were followed according to the conversion and temperature. The results show the emission of all byproducts already detected during the study of toluene and MEK oxidation: benzene, acetaldehyde (AcO), propionic acid (PrOOH), methyl vinyl ketone (MVK), and diacetyl (DAC). Additionally, the emission profiles present significant changes due to the mixing effects. Indeed, the persistence,
P, and the maximum quantity emitted observed
Qmax, are significantly changed.
Figure 7 shows the emission profiles of the byproducts from the binary mixture oxidation on Pd/CeO
2.
This catalyst exhibits the most remarkable and representative impacts of mixing effects on byproducts’ emission profiles.
Table 7 reports values of
Qmax,
Tf, and
P for the binary mixture, as well as the values for oxidation of toluene and MEK alone for comparison.
The mixing effects mainly impact on the values of
P and
Qmax. Regarding the persistence,
P, the values decrease significantly for several compounds. This observation indicates that byproducts are eliminated at a lower temperature. This is particularly true for benzene whose
P value is +63 °C for toluene oxidation instead of −77 °C for binary mixture oxidation. As mentioned before, this effect is especially beneficial due to the toxicity of this compound. Propionic acid is the only byproduct here whose persistence increases significantly, although the value is still negative. Concerning
Qmax values, the emission peaks of benzene, acetaldehyde, and propionic acid are observed. This proves that even though these compounds are more easily oxidized, they are also emitted in larger amounts. In a different way to these three compounds, the values of
Qmax observed for the methyl vinyl ketone and diacetyl show very slight variations, and these two compounds are present as minority byproducts. This observation is also carried out for all materials studied on the binary mixture. To simplify the comparison between materials, only benzene, acetaldehyde, and propionic acid will be considered thereafter.
Table 8 reports the
Qmax,
Tf, and
P values of these three compounds for the five materials. This table highlights the significant reduction in the persistence of benzene.
Indeed, except for the two materials with an alumina support, the persistence of benzene is negative, but has nonetheless been greatly reduced since these values have increased from +6 °C to +35 °C and +44 °C instead of +67 °C, respectively, for the oxidation of toluene alone. Regarding acetaldehyde and propionic acid, the tests show a relative increase in Qmax values as compared to those for MEK alone. Similarly, the persistence of acetaldehyde emissions is greatly reduced for all materials except Pd/HY. For the persistence of propionic acid, the behavior is more variable, though: a slight increase is observed for Pd/α-Al2O3 and Pd/CeO2, while a decrease is observed for Pd/HY, Pd/γ-Al2O3 and CoAlCeO. Considering these facts, the study of the total oxidation of the binary mixture highlights the two most efficient catalytic materials: the commercial formulation Pd/γ-Al2O3 and the mixed oxide CoAlCeO. Indeed, these materials show the same performance in terms of activity with a T100 to 290 °C for both materials. Considering the byproducts, the mixed oxide has a slight advantage over Pd/γ-Al2O3 since the byproducts are completely eliminated at 290 °C. Considering this fact, the performance sequence is as follows: CoAlCeO > Pd/γ-Al2O3 > Pd/CeO2 > Pd/HY > Pd/αAl2O3.
2.5. Oxidation of a Simulated Industrial Exhaust
The literature on VOCs’ oxidation systematically studied models exhausts where VOCs’ concentration and flow rate are perfectly controlled, while only a few articles focus on the study of VOCs mixtures [
7,
13,
14,
15,
16,
17,
18,
31,
32]. As in this study, these articles highlight different inhibitory or beneficial effects derived from interactions between compounds. However, a binary or ternary mixture studied at the laboratory level remains far from the reality of an industrial effluent, which can be made up of many more compounds (10 to 50 compounds) with variable compositions and concentrations over time. To get closer to industrial applications, we further studied a more complex mixture of VOCs that was established based on the composition of an industrial effluent and will therefore be referred to as a simulated industrial exhaust. In the industry, part of the manufacturing process is to apply lacquers and paints to metal surfaces, the consequence being that industry emits significant amounts of gaseous effluents with a high concentration of VOCs and a high flow rate. These effluents contain mostly aromatic compounds, mainly toluene, and oxygenated compounds, mainly MEK, as well as traces of paraffins (C
9–C
11). The liquid mixture used here to simulate the industrial effluent of VOCs was constituted by selecting six aromatic compounds (toluene, o-xylene, ethylbenzene, propylbenzene, 4-ethylbenzene, and 1,4diethylbenzene), three oxygenates (butanone, butanol, butoxyethanol), and a paraffin (decane). The concentration of each substance is fixed to a concentration of 10 vol% in the liquid phase. This mixture was studied on the same experimental setup as in previous studies, the mixture being placed in a saturator. The temperature of the saturator was set so as to fix a value of 7000 ppm carbon equivalent (ppm eq. C) in a 100 mL·min
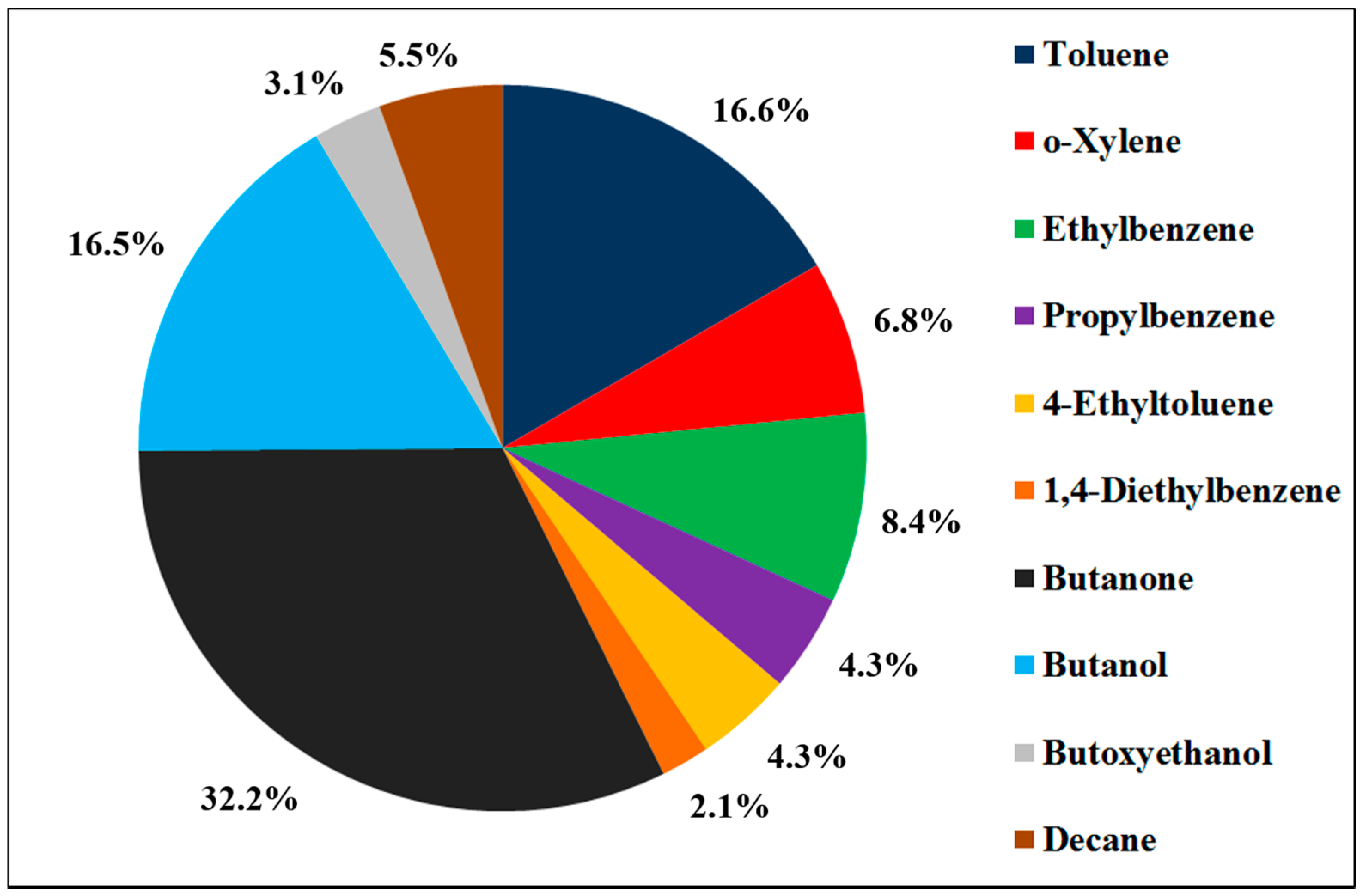
−1 air flow, with a total hydrocarbons analyzer COSMA Graphite 655 (Environnement SA, Poissy, France). The gas phase composition determined by microGC is given in
Figure 8.
In order to place themselves under real measurement conditions, the abatement rate of VOCs mixture is measured with a total hydrocarbons analyzer (COSMA Graphite 655), equivalent to a flame ionization detector (FID). Indeed, VOC measurements on industrial effluents are performed with this type of device, and this measuring method conforms to a French regulatory norm (NF X 43-301). Furthermore, although the microGC is a suitable tool for measuring VOCs’ oxidation, this technique reaches its limits with regards to the detection and separation of the various molecules that can be emitted or generated during the process. The abatement curves obtained by the total hydrocarbons analyser (measurements given in equivalent carbon (eq. C)) are comparable to light-off curves determined by microGC. Nevertheless, microGC was used as a complementary analytic device, as well as trapping on cartridge Tenax TA (Supelco, Bellefonte, PA, USA) and Mass Spectrometer QMS-200 (Pfeiffer Vacuum, Asslar, Germany), for speciation analysis. Finally, this study has been conducted with the two catalysts presenting the best performances: Pd/γ-Al
2O
3 and CoAlCeO. These two materials appear to be most effective for the abatement of the VOCs studied and their resulting byproducts. This choice is relevant for two main reasons: Firstly, Pd/γ-Al
2O
3 is an efficient commercial catalyst that is sold for such industrial applications and is thus suitable as a benchmark. Secondly, CoAlCeO is an alternative formulation exhibiting an efficiency similar to or even better than the commercial material. In addition, this material is not made of noble metals and its cost is considerably lower as compared to the commercial formulation. The abatement curves obtained, expressed in ppm carbon equivalent (ppm eq. C) versus temperature, are given in
Figure 9. The
T50 values are reported in
Table 9, where the values for the oxidation of toluene and binary mixture are also reported for comparison.
The test performed on the SiC shows a partial conversion of the complex mixture, with a maximum of 28.1% at 400 °C. This relatively high conversion is due to the thermal decomposition of oxygenates (MEK, butan-1-ol and butoxyethanol). This was confirmed by microGC analyses that highlight some byproducts: acetaldehyde, propionic acid, methyl vinyl ketone and diacetyl, which are derived from the MEK oxidation and butanal and 1-butene, which are derived from the butan-1-ol conversion (partial oxidation and dehydration, respectively). In addition, analyses do not show the presence of CO2, which confirms partial oxidation only. The results show an abatement of 99.2% and 100% of the complex mixture for Pd/γ-Al2O3 and CoAlCeO, respectively. In addition, the abatement curves do not exhibit a sigmoidal profile characteristic of the light-off curves. The conversion observed between 0% and 20% is due to the decomposition of oxygenates, mainly butan-1-ol and butoxyethanol, leading to the corresponding aldehydes and acids (butanal, acetaldehyde, and acetic acid). The degradation of aromatic compounds is only starting at 155 and 175 °C for CoAlCeO and Pd/γ-Al2O3, respectively. Then, the mixture conversion abruptly increases from 30 to 95% for CoAlCeO and from 55 to 95% for Pd/γ-Al2O3 in just seconds. This is accompanied by an overpressure/depression phenomenon, confirmed by mass spectrometry, and an immediate increase of 30 °C of the reactor temperature. This is characteristic of a deflagration effect, which explains the appearance of abatement curves. This phenomenon is slower on Pd/γ-Al2O3, and also more visible. Moreover, a peak of methane emission is measured by the total hydrocarbons analyzer. Finally, the system returns to a steady state, with a slight loss of conversion, before the degradation of the residual organic fraction is performed until the maximum conversion is reached.
Concerning the performance, Pd/γ-Al2O3 presents a T50 values around 40 °C greater than in the binary mixture case, highlighting a more significant inhibition effect. In addition, conversion of the complex mixture stabilizes at 99.2% at 325 °C. Additional analyses at 325 (T99), 350, and 400 °C show that the remaining organic fraction is predominantly composed of decane and benzene, as well as a small proportion of MEK. For CoAlCeO, the T50 value is lowered once more in comparison with the binary mixture and toluene oxidation, but to a lower extent with only 5 °C on T50 between the complex and binary mixture. Moreover, conversion of the complex mixture reaches 100% from 357 °C. Additional analysis at 325 (T99) and 350 °C show that the residual organic fraction is composed of MEK and toluene, with MEK being predominant. The same analysis conducted at 400 °C confirms a total abatement of the mixture. Therefore, the CoAlCeO mixed oxide is highlighted as a promising alternative material to the commercial Pd/γ-Al2O3 catalyst, with the latter currently being used for industrial units’ VOCs treatment. Indeed, CoAlCeO shows the best performance for the complex mixture oxidation considering the rate and temperature of VOCs abatement. In addition, no toxic compounds seem to be emitted at high conversion by this catalyst.