Catalytic Activity Studies of Vanadia/Silica–Titania Catalysts in SVOC Partial Oxidation to Formaldehyde: Focus on the Catalyst Composition
Abstract
:1. Introduction
2. Results and Discussion
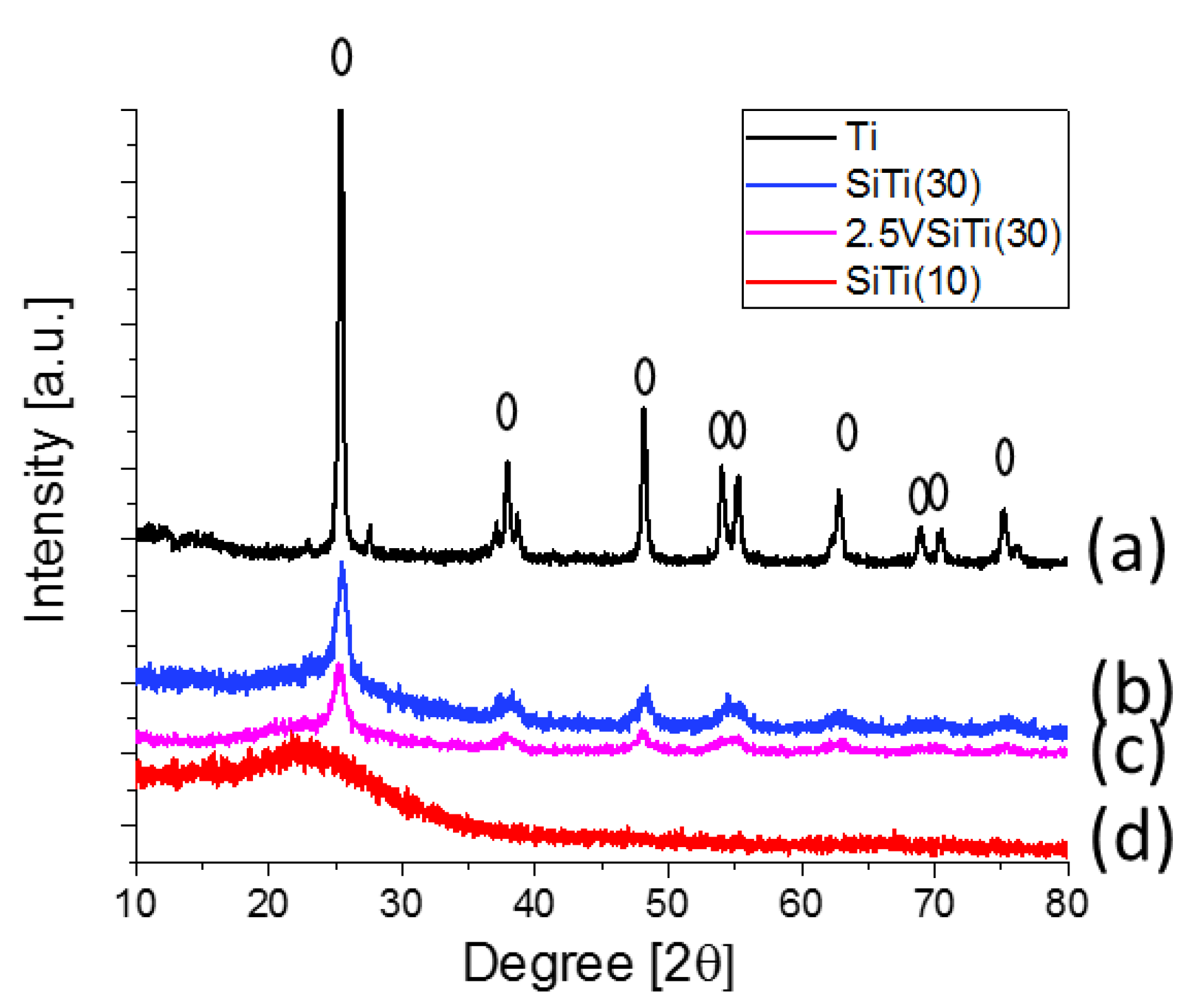
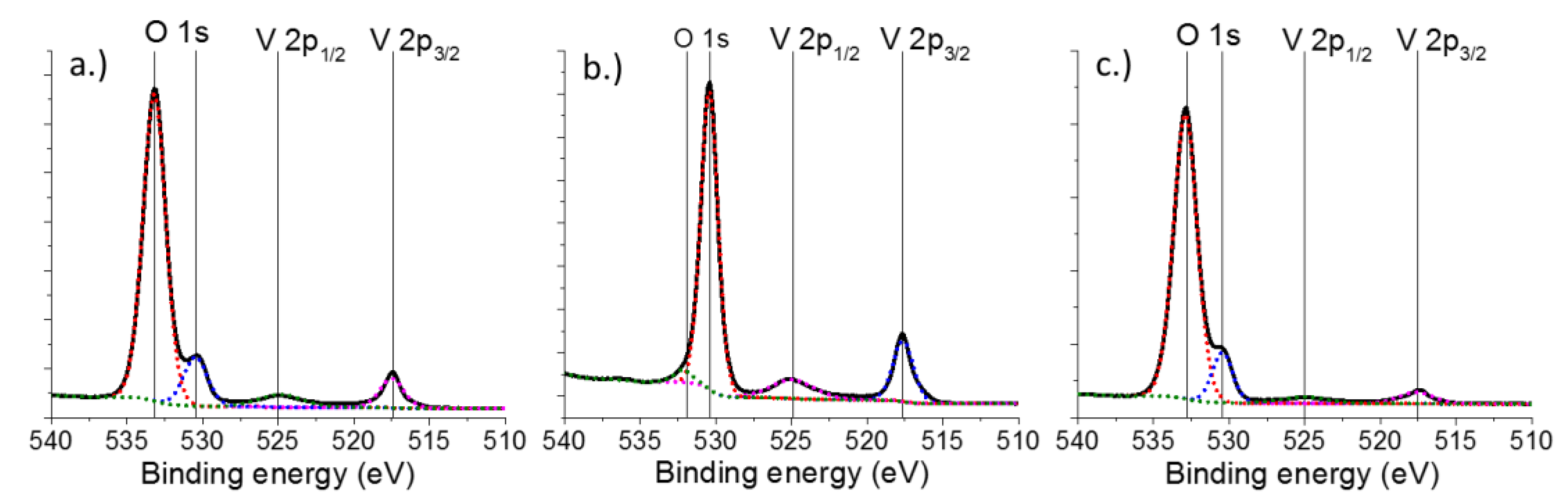
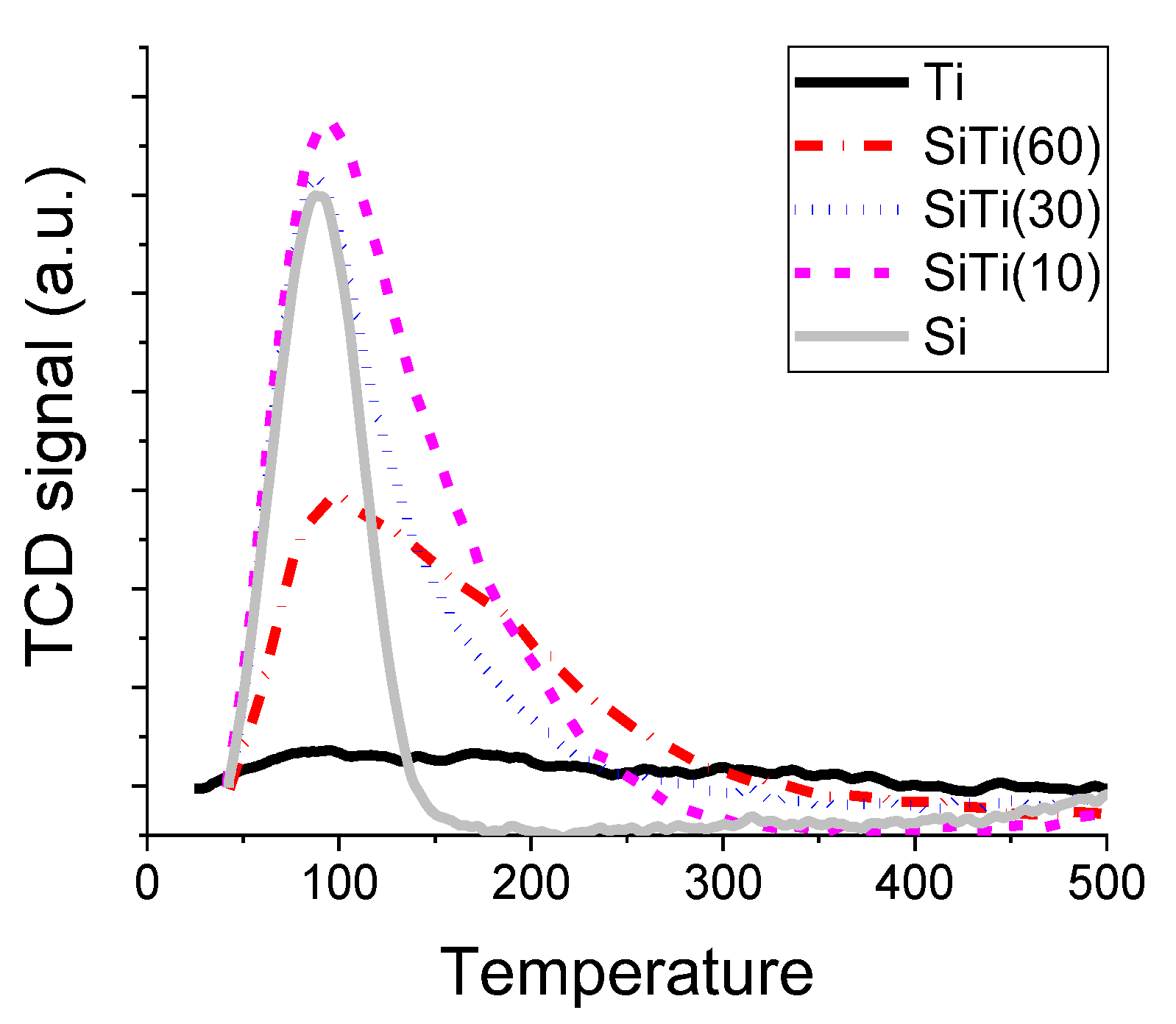
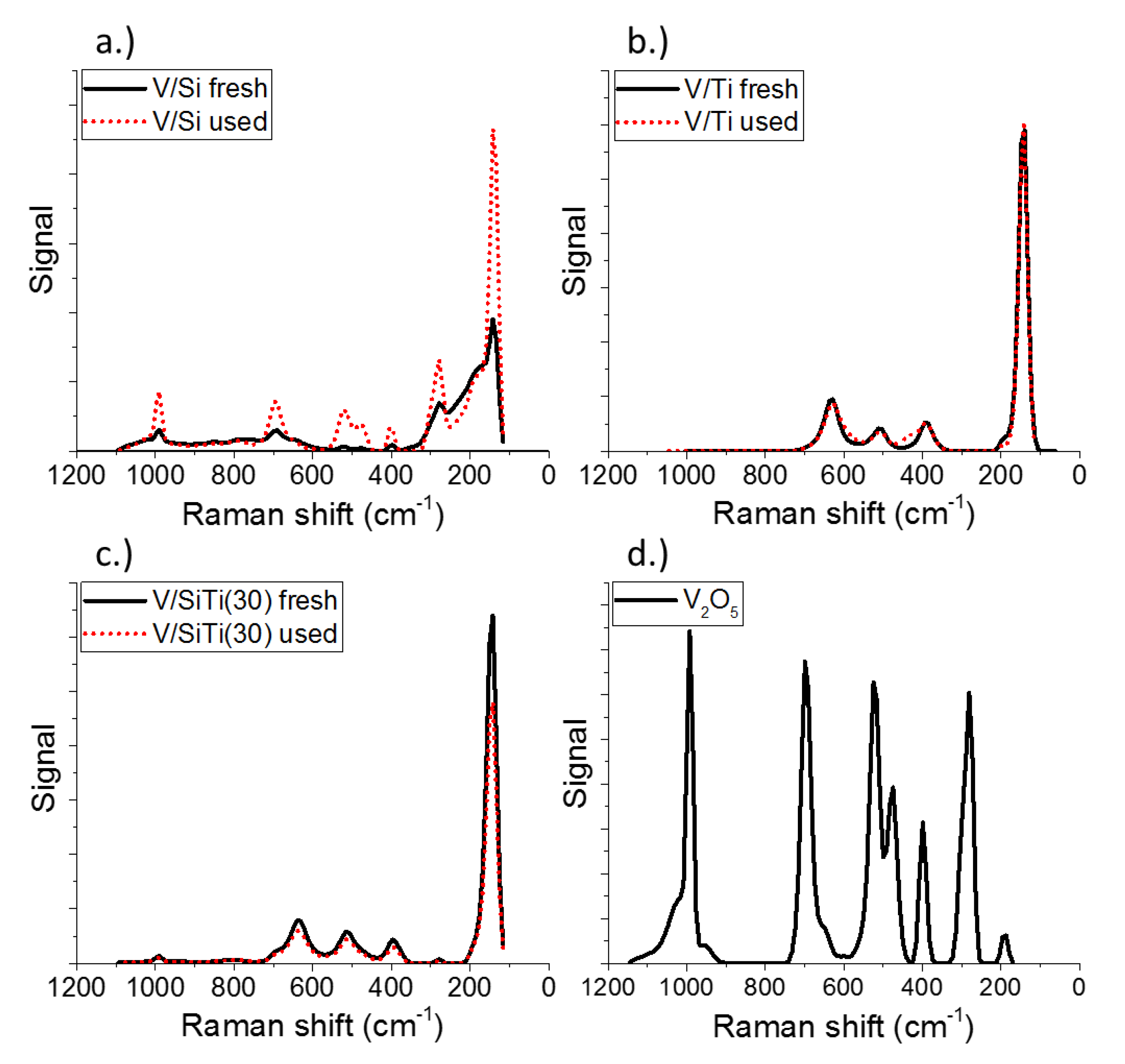
2.1. Characterization of Catalysts
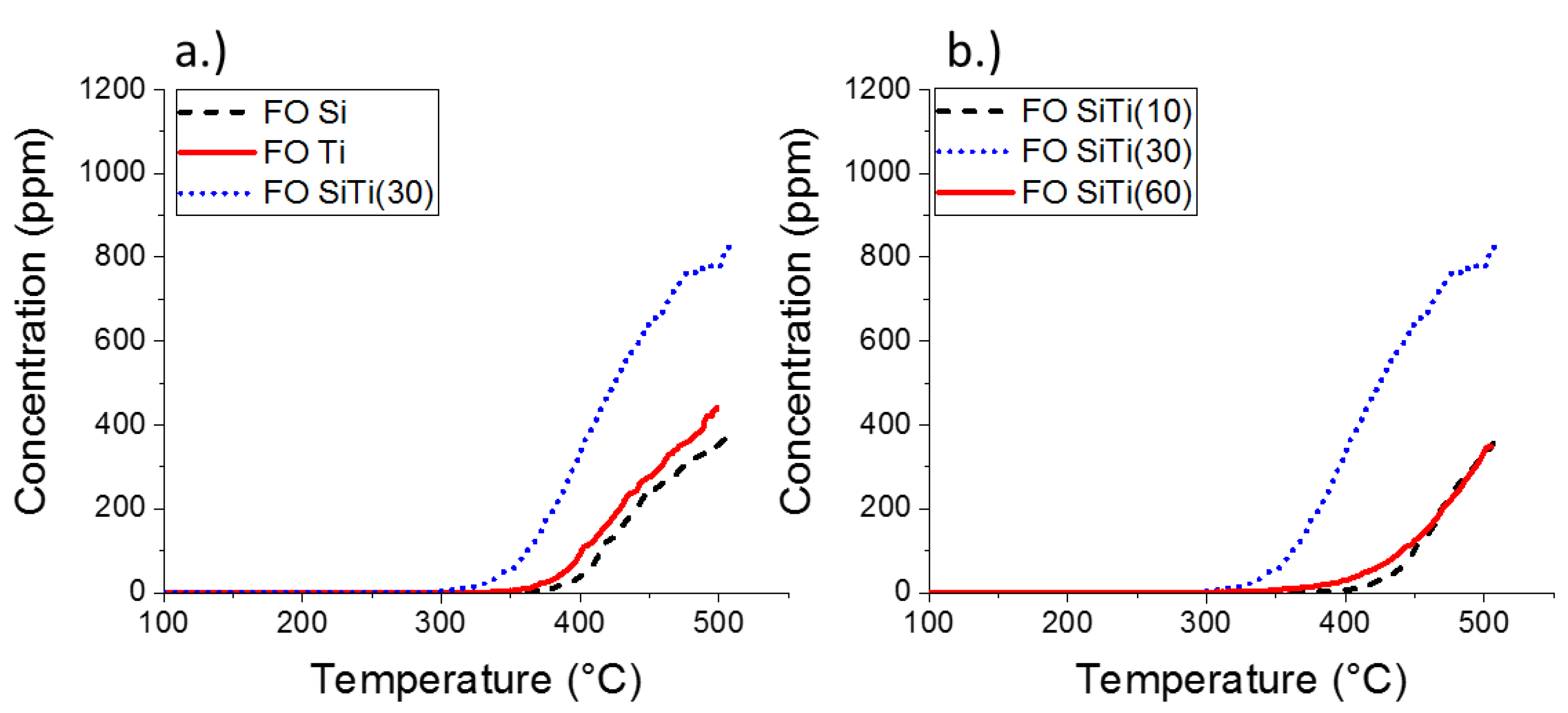
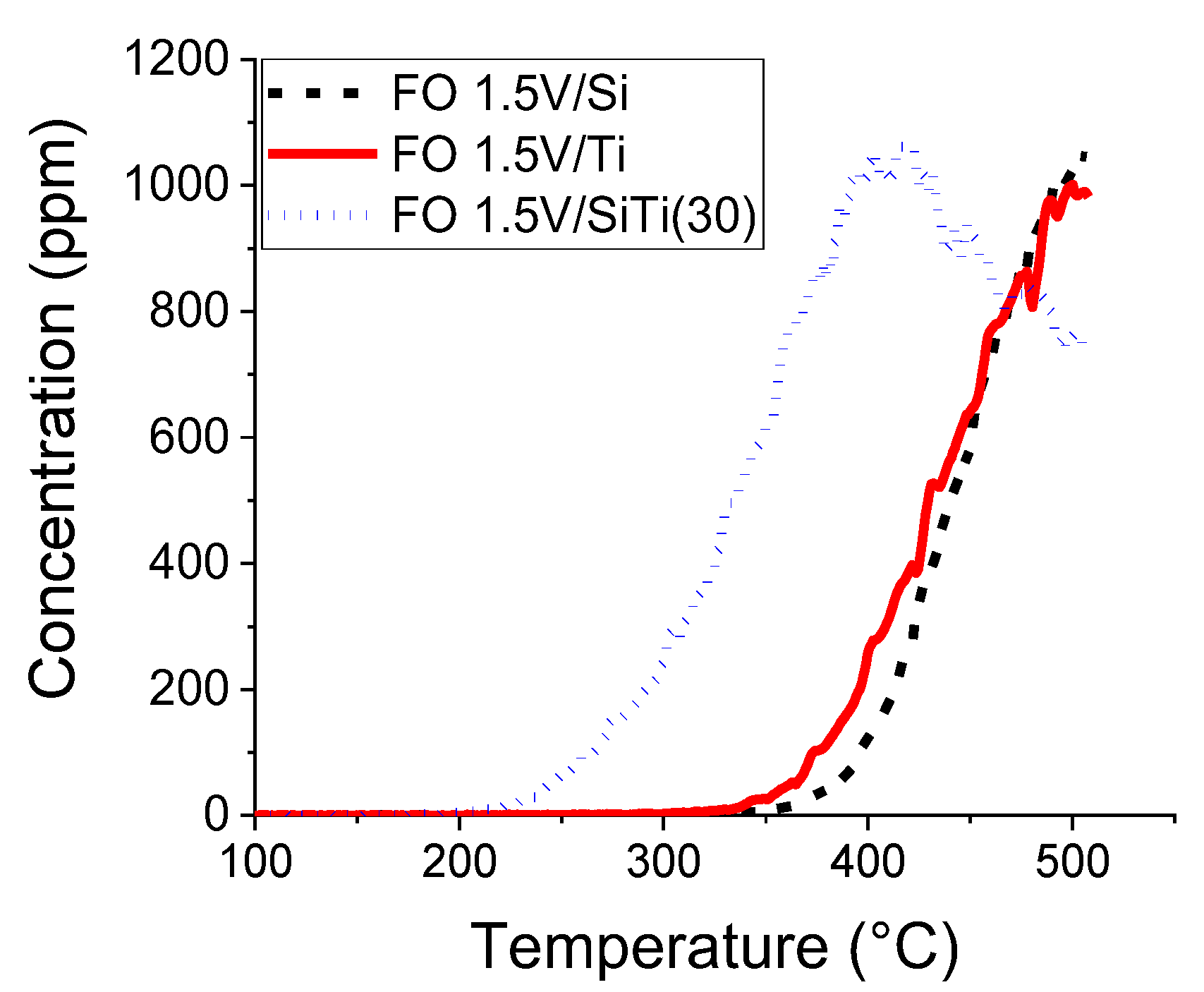
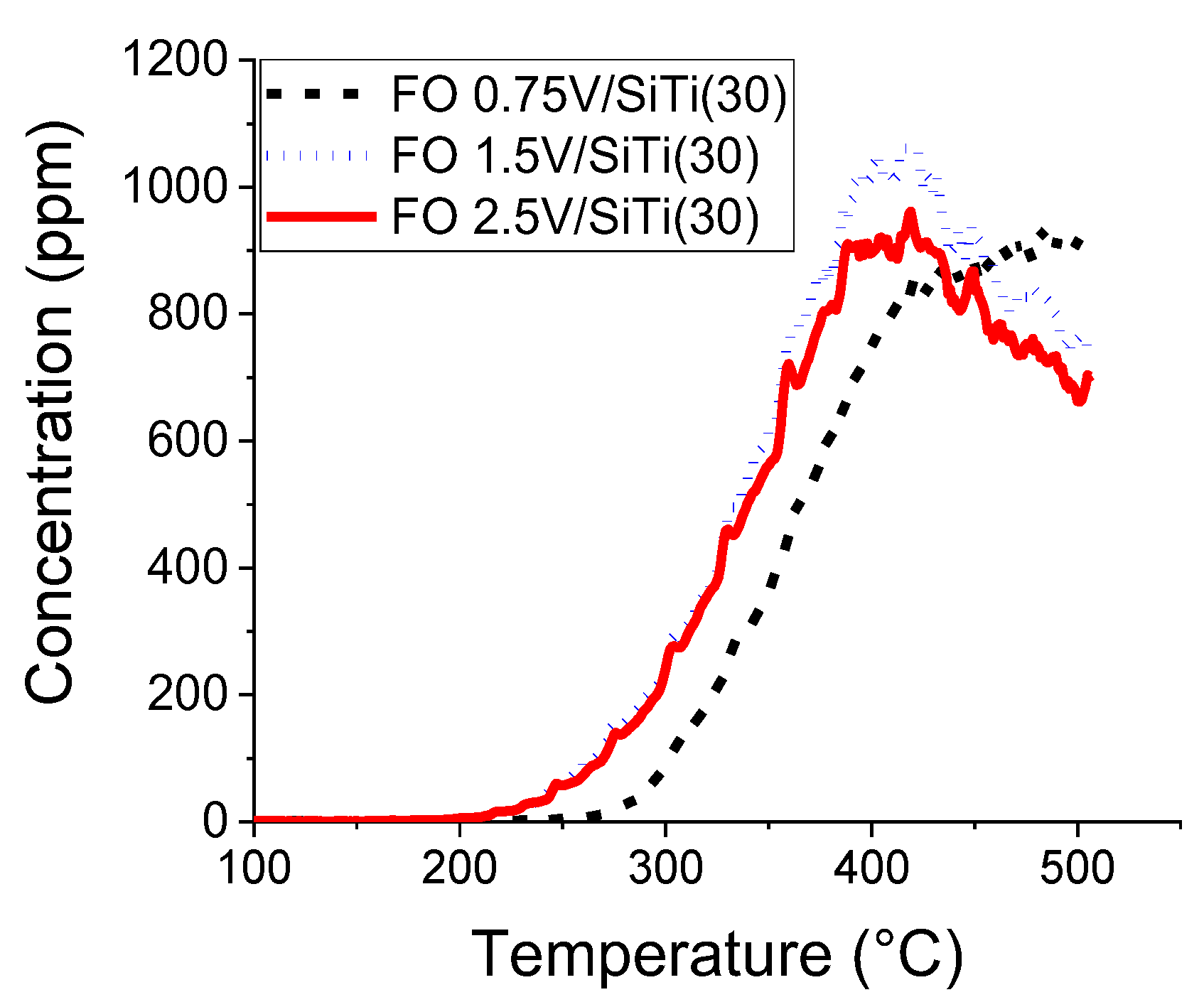
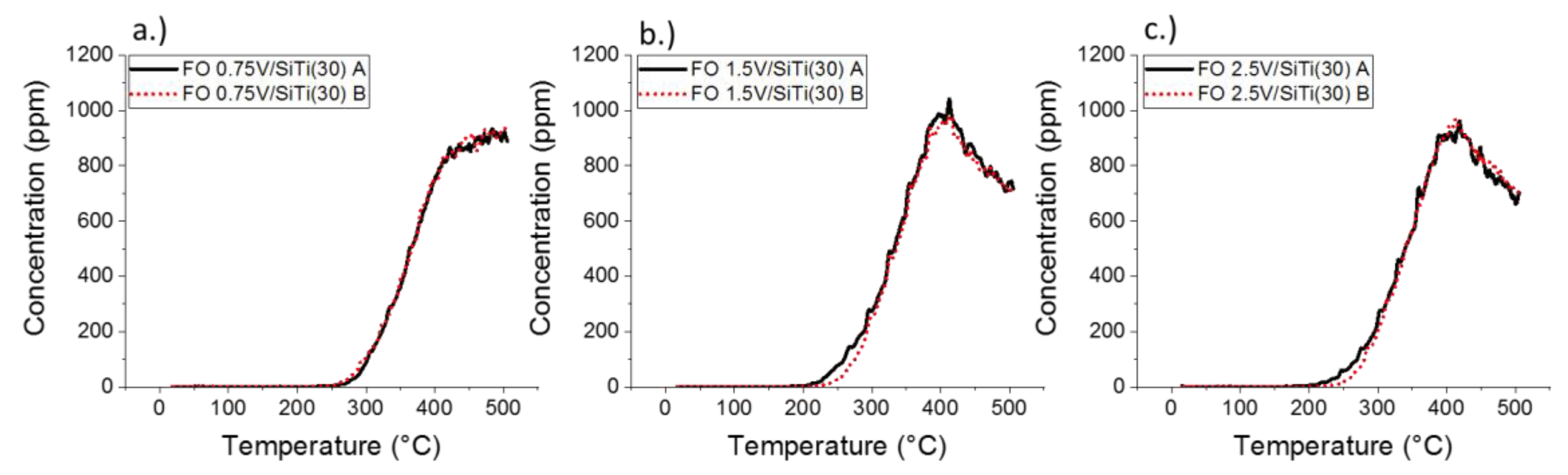
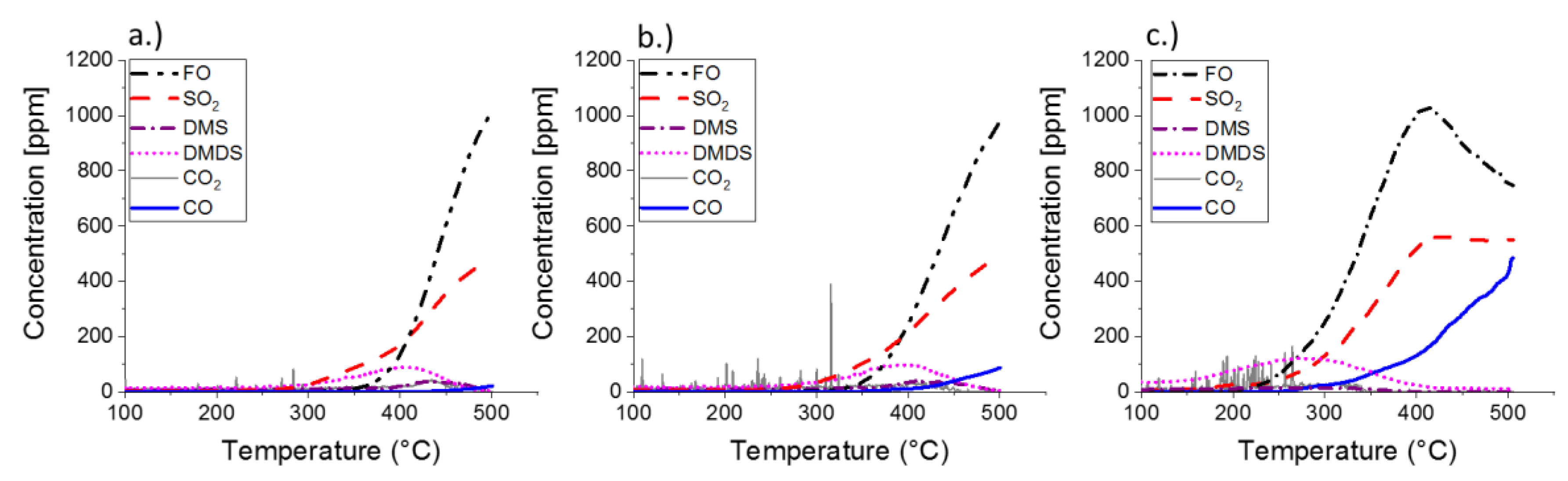
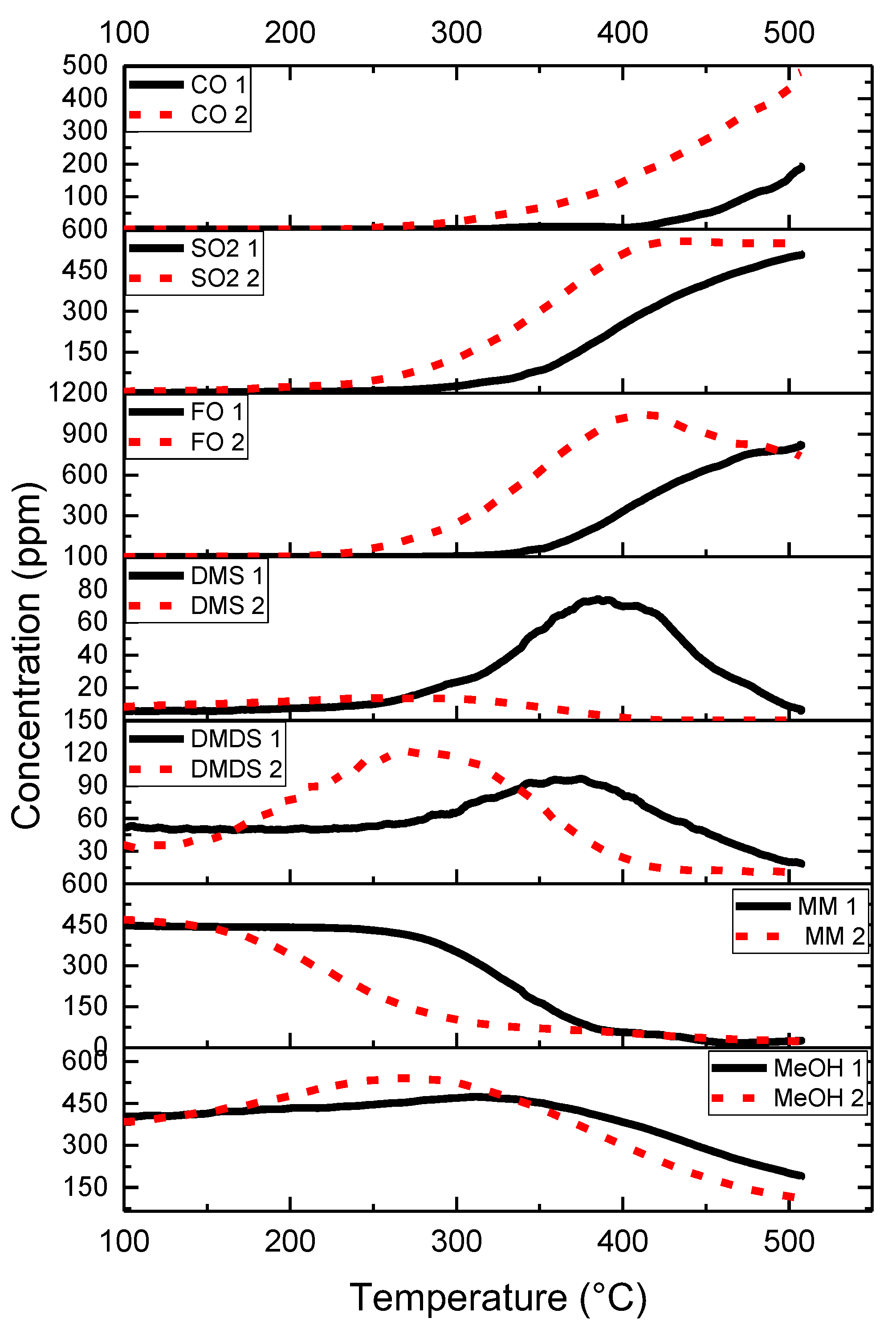
2.2. Results of Catalytic Oxidation Tests
3. Materials and Methods
3.1. Characterization of Materials
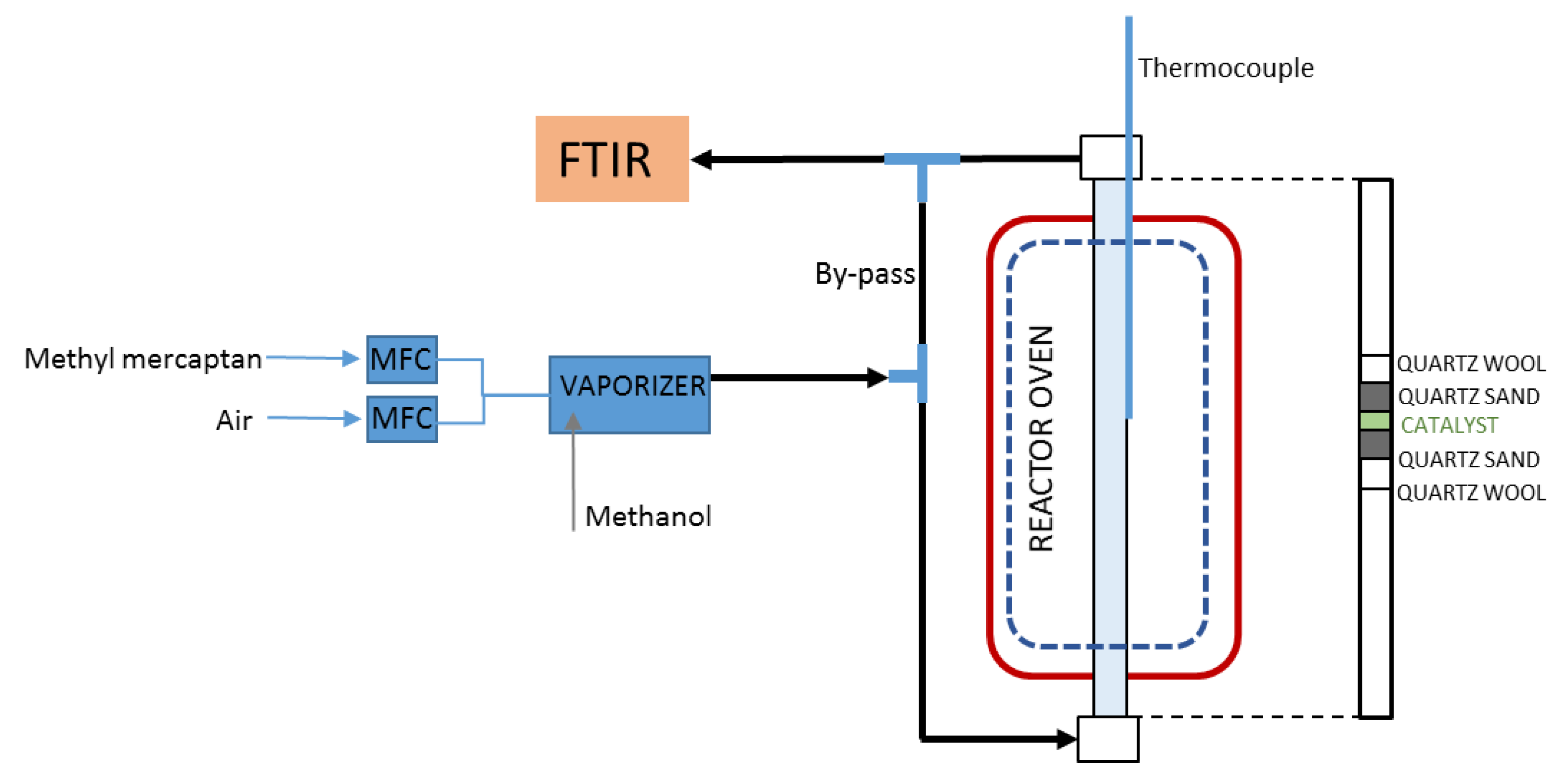
3.2. Catalytic Oxidation Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Itoh, M.; Hattori, H.; Tanabe, K. The acidic properties of TiO2-SiO2 and its catalytic activities for the amination of phenol, the hydration of ethylene and the isomerization of butene. J. Catal. 1974, 35, 225–231. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Shpiro, E.S.; Apijok, J. XPS and XAES study of TiO2-SiO2 mixed oxide system. J. Phys. Chem. 1993, 97, 5668–5672. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Topsoe, H.; Dumesic, J.A. Vanadia/Titania Catalysts for Selective Catalytic Reduction (SCR) of Nitric-Oxide by Ammonia. J. Catal. 1995, 151, 226–240. [Google Scholar] [CrossRef]
- Klein, S.; Thorimbert, S.; Maier, W.F. Amorphous Microporous Titania–Silica Mixed Oxides: Preparation, Characterization, and Catalytic Redox Properties. J. Catal. 1996, 163, 476–488. [Google Scholar] [CrossRef]
- Walters, J.K.; Rigden, J.S.; Dirken, P.J.; Smith, M.E.; Howells, W.S.; Newport, R.J. An atomic-scale study of the role of titanium in TiO2:SiO2 sol–gel materials. Chem. Phys. Lett. 1997, 264, 539–544. [Google Scholar] [CrossRef]
- Watson, R.B.; Ozkan, U.S. K/Mo Catalysts Supported over Sol–Gel Silica–Titania Mixed Oxides in the Oxidative Dehydrogenation of Propane. J. Catal. 2000, 191, 12–29. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kuma, R.; Masaki, S.; Sugishima, N. TiO2-SiO2 and V2O5/TiO2-SiO2 catalyst: Physico-chemical characteristics and catalytic behavior in selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2005, 60, 173–179. [Google Scholar] [CrossRef]
- Morosanova, E.I. Silica and silica–titania sol–gel materials: Synthesis and analytical application. Talanta 2012, 102, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Nizar, U.K.; Efendi, J.; Yuliati, L.; Gustiono, D.; Nur, H. A new way to control the coordination of titanium (IV) in the sol–gel synthesis of broom fibers-like mesoporous alkyl silica–titania catalyst through addition of water. Chem. Eng. J. 2013, 222, 23–31. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. The molecular structures and reactivity of V2O5/TiO2/SiO2 catalysts. Catal. Lett. 1992, 13, 9–19. [Google Scholar] [CrossRef]
- Galán-Fereres, M.; Mariscal, R.; Alemany, L.J.; Fierro, J.L.G.; Anderson, J.A. Ternary V–Ti–Si catalysts and their behaviour in the CO + NO reaction. J. Chem. Soc. Faraday Trans. 1994, 90, 3711–3718. [Google Scholar] [CrossRef]
- Quaranta, N.E.; Soria, J.; Cortés Corberán, V.; Fierro, J.L.G. Selective Oxidation of Ethanol to Acetaldehyde on V2O5/TiO2/SiO2 Catalysts. J. Catal. 1997, 171, 1–13. [Google Scholar] [CrossRef]
- Reiche, M.A.; Ortelli, E.; Baiker, A. Vanadia grafted on TiO2–SiO2, TiO2 and SiO2 aerogels Structural properties and catalytic behaviour in selective reduction of NO by NH3. Appl. Catal. B Environ. 1999, 23, 187–203. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Fierro, J.L.G.; Wachs, I.E. Structural Characteristics and Reactivity/Reducibility Properties of Dispersed and Bilayered V2O5/TiO2/SiO2 Catalysts. J. Phys. Chem. B 1999, 103, 618–629. [Google Scholar] [CrossRef]
- Burcham, L.J.; Deo, G.; Gao, X.; Wachs, I.E. In situ IR, Raman, and UV-Vis DRS spectroscopy of supported vanadium oxide catalysts during methanol oxidation. Top. Catal. 2000, 11–12, 85–100. [Google Scholar] [CrossRef]
- Monaci, R.; Rombi, E.; Solinas, V.; Sorrentino, A.; Santacesaria, E.; Colon, G. Oxidative dehydrogenation of propane over V2O5/TiO2/SiO2 catalysts obtained by grafting titanium and vanadium alkoxides on silica. Appl. Catal. A Gen. 2001, 214, 203–212. [Google Scholar] [CrossRef]
- Dias, C.R.; Portela, M.F.; Bañares, M.A.; Galán-Fereres, M.; López-Granados, M.; Peña, M.A.; Fierro, J.L.G. Selective oxidation of o-xylene over ternary V-Ti-Si catalysts. Appl. Catal. A Gen. 2002, 224, 141–151. [Google Scholar] [CrossRef]
- Keränen, J.; Guimon, C.; Iiskola, E.; Auroux, A.; Niinistö, L. Atomic layer deposition and surface characterization of highly dispersed titania/silica-supported vanadia catalysts. Catal. Today 2003, 78, 149–157. [Google Scholar] [CrossRef]
- Burgess, T.L.; Gibson, A.G.; Furstein, S.J.; Wachs, I.E. Converting waste gases from pulp mills into value-added chemicals. Environ. Prog. 2002, 21, 137–141. [Google Scholar] [CrossRef]
- Wachs, I.E. Treating Methanol-Containing Waste Gas Streams. U.S. Patent 5907066, 6 March 2001. [Google Scholar]
- Koivikko, N.; Laitinen, T.; Ojala, S.; Pitkäaho, S.; Kucherov, A.; Keiski, R.L. Formaldehyde production from methanol and methyl mercaptan over titania and vanadia based catalysts. Appl. Catal. B Environ. 2011, 103, 72–78. [Google Scholar] [CrossRef]
- Wang, Q.; Madix, R.J. Partial oxidation of methanol to formaldehyde on a model supported monolayer vanadia catalyst: Vanadia on TiO2(1 1 0). Surf. Sci. 2002, 496, 51–63. [Google Scholar] [CrossRef]
- Goodrow, A.; Bell, A.T. A theoretical investigation of the selective oxidation of methanol to formaldehyde on isolated vanadate species supported on titania. J. Phys. Chem. C 2008, 112, 13204–13214. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Bell, A.T. Mechanistic Studies of Methanol Oxidation to Formaldehyde on Isolated Vanadate Sites Supported on MCM-48 Mechanistic Studies of Methanol Oxidation to Formaldehyde on Isolated Vanadate Sites Supported on MCM-48. J. Phys. Chem. C 2007, 111, 420–430. [Google Scholar] [CrossRef]
- Bronkema, J.L.; Bell, A.T. Mechanistic studies of methanol oxidation to formaldehyde on isolated vanadate sites supported on high surface area zirconia. J. Phys. Chem. C 2008, 112, 6404–6412. [Google Scholar] [CrossRef]
- Vining, W.C.; Strunk, J.; Bell, A.T. Investigation of the structure and activity of VOx/ZrO2/SiO2 catalysts for methanol oxidation to formaldehyde. J. Catal. 2011, 281, 222–230. [Google Scholar] [CrossRef]
- Burcham, L.J.; Wachs, I.E. The origin of the support effect in supported metal oxide catalysts: In situ infrared and kinetic studies during methanol oxidation. Catal. Today 1999, 49, 467–484. [Google Scholar] [CrossRef]
- Kropp, T.; Paier, J.; Sauer, J. Oxidative dehydrogenation of methanol at ceria-supported vanadia oligomers. J. Catal. 2017, 352, 382–387. [Google Scholar] [CrossRef]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Determination of Metal Dispersion and Surface Composition in Supported Cu–Pt Catalysts. J. Catal. 2002, 208, 381–392. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis science of supported vanadium oxide catalysts. Dalt. Trans. 2013, 42, 11762–11769. [Google Scholar] [CrossRef] [PubMed]
- Chary, K.V.R.; Kishan, G.; Lakshmi, K.S.; Ramesh, K. Studies on dispersion and reactivity of vanadium oxide catalysts supported on titania. Langmuir 2000, 16, 7192–7199. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454, 41–44. [Google Scholar] [CrossRef]
- Odriozola, J.A.; Soria, J.; Somorjai, G.A.; Heinemann, H.; de la Garcia Banda, J.F.; Lopez Granados, M.; Conesa, J.C. Adsorption of nitric oxide and ammonia on vanadia-titania catalysts: ESR and XPS studies of adsorption. J. Phys. Chem. 1991, 95, 240–246. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Fierro, J.L.G.; Banares, M.A.; Wachs, I.E. Preparation and in-situ Spectroscopic Characterization of Molecularly Dispersed Titanium Oxide on Silica. J. Phys. Chem. B 1998, 102, 5653–5666. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Weckhuysen, B.M.; Wachs, I.E. In Situ Spectroscopic Investigation of Molecular Structures of Highly Dispersed Vanadium Oxide on Silica under Various Conditions. J. Phys. Chem. B 1998, 102, 10842–10852. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Predicting molecular structures of surface metal oxide species on oxide supports under ambient conditions. J. Phys. Chem. 1991, 95, 5889–5895. [Google Scholar] [CrossRef]
- Blasco, T.; Nieto, J.M.L. Oxidative dyhydrogenation of short chain alkanes on supported vanadium oxide catalysts. Appl. Catal. A Gen. 1997, 157, 117–142. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 98th ed.; Internet Version 2018; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Laitinen, T.; Ojala, S.; Koivikko, N.; Mouammine, A.; Keiski, R.L. Stability of a vanadium-based catalyst in the partial oxidation of a mixture of methanol and methyl mercaptan. In Proceedings of the 17th Nordic Symposium on Catalysis, Lund, Sweden, 14–16 June 2016. [Google Scholar]
- Tanabe, K.; Sumiyoshi, T.; Shibata, K.; Kiyoura, T.; Kitagawa, J. A new hypothesis regarding the surface acidity of binary metal oxides. Bull. Chem. Soc. Jpn. 1974, 47, 1064–1066. [Google Scholar] [CrossRef]
- Keränen, J.; Carniti, P.; Gervasini, A.; Iiskola, E.; Auroux, A.; Niinistö, L. Preparation by atomic layer deposition and characterization of active sites in nanodispersed vanadia/titania/silica catalysts. Catal. Today 2004, 91–92, 67–71. [Google Scholar] [CrossRef]
- Mouammine, A.; Ojala, S.; Pirault-Roy, L.; Bensitel, M.; Keiski, R.; Brahmi, R. Catalytic partial oxidation of methanol and methyl mercaptan: Studies on the selectivity of TiO2 and CeO2 supported V2O5 catalysts. Top. Catal. 2013, 56, 650–657. [Google Scholar] [CrossRef]
- Went, G.T.; Oyama, S.T.; Bell, A.T. Laser Raman spectroscopy of supported vanadium oxide catalysts. J. Phys. Chem. 1990, 94, 4240–4246. [Google Scholar] [CrossRef]
- Vuurman, M.A.; Wachs, I.E.; Hirt, A.M. Structural determination of supported vanadium pentoxide-tungsten trioxide-titania catalysts by in situ Raman spectroscopy and X-ray photoelectron spectroscopy. J. Phys. Chem. 1991, 95, 9928–9937. [Google Scholar] [CrossRef]
- Wachs, I.E.; Deo, G.; Weckhuysen, B.M.; Andreini, A.; Vuurman, M.A.; de Boer, M.; Amiridis, M.D. Selective Catalytic Reduction of NO with NH3 over Supported Vanadia Catalysts. J. Catal. 1996, 161, 211–221. [Google Scholar] [CrossRef]
- Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. In Ulmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; Volume 15, pp. 735–768. [Google Scholar]

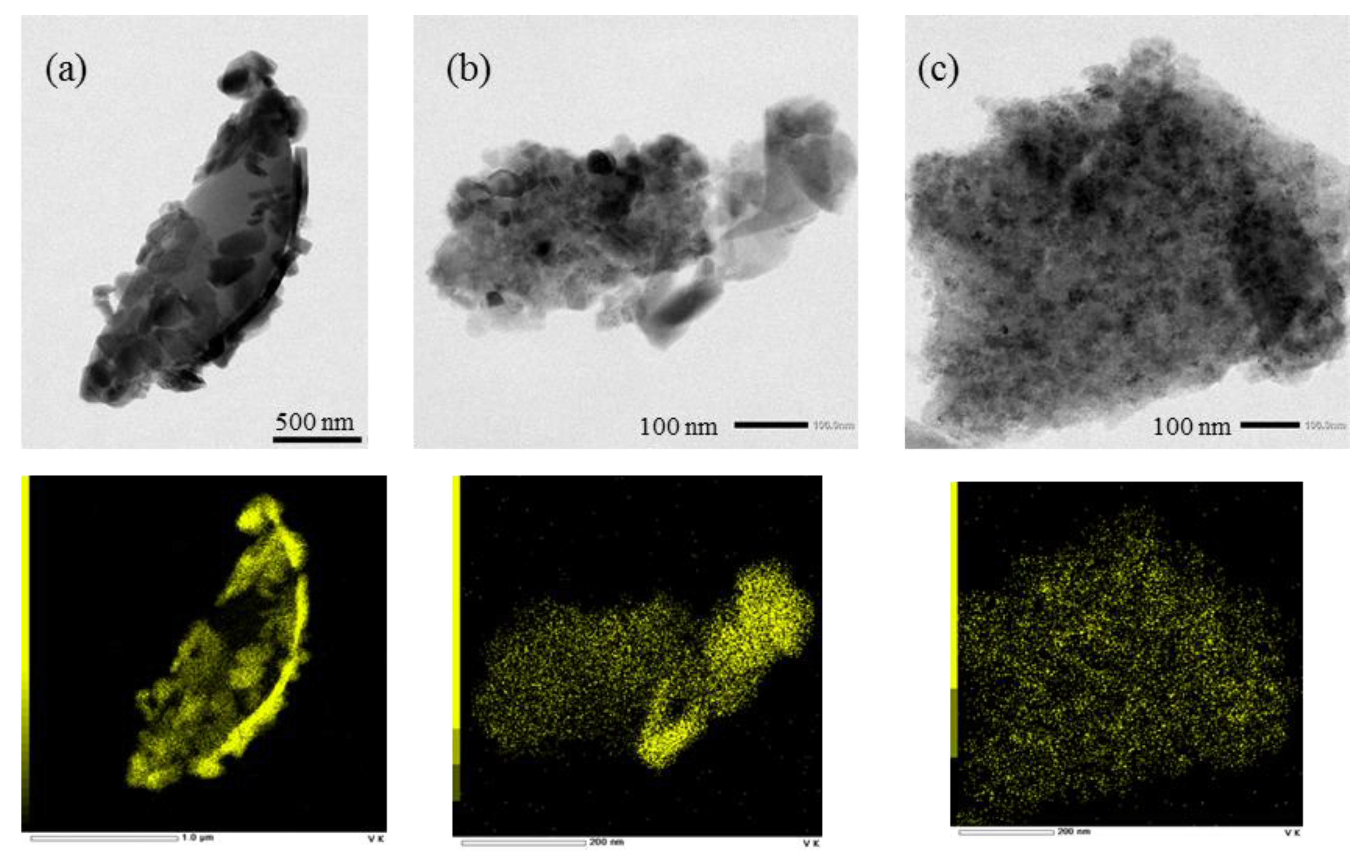
| Support | Surface Area | Total Pore Volume | Pore Size | XRF [%] | Surface Loading | ||
|---|---|---|---|---|---|---|---|
| [m2 g−1] | [cm3 g−1] | [nm] | V2O5 | SiO2 | TiO2 | Vatom nm−2 | |
| Si | 225 | 0.1 | 1.9 | - | ~100 | - | - |
| Ti | 10 | 0.02 | 6.8 | - | - | ~100 | - |
| SiTi(10) | 560 | 0.28 | 2.0 | - | 70 | 9 | - |
| SiTi(30) | 590 | 0.35 | 2.3 | - | 57 | 27 | - |
| SiTi(60) | 220 | 0.11 | 2.1 | - | 35 | 56 | - |
| Catalyst | |||||||
| 1.5V/Si | 140 | 0.06 | 1.9 | 1.56 | 98 | - | 0.74 |
| 1.5V/Ti | 10 | 0.01 | 7.3 | 1.44 | - | 98 | 9.54 |
| 0.75V/SiTi(30) | 560 | 0.33 | 2.3 | 0.65 | 58 | 27 | 0.08 |
| 1.5V/SiTi(30) | 500 | 0.3 | 2.3 | 1.7 | 66 | 31 | 0.23 |
| 2.5V/SiTi(30) | 470 | 0.28 | 2.3 | 2.4 | 66 | 31 | 0.34 |
| Catalyst | V 2p [eV] | O 1s [eV] | Si 2p [eV] | Ti 2p [eV] | |||
|---|---|---|---|---|---|---|---|
| V 2p3/2 | V 2p1/2 | Ti 2p1/2 | Ti 2p3/2 | ||||
| 1.5V/Si | 517.49 | 524.85 | 533.17 | 530.46 | 103.86 | - | - |
| (1.86) | (3.37) | (1.78) | (1.58) | (1.66) | - | - | |
| 1.5V/Ti | 517.62 | 524.94 | 531.92 | 530.41 | - | 464.84 | 459.19 |
| (1.58) | (3.37) | (1.64) | (1.29) | - | (2.13) | (1.27) | |
| 1.5V/SiTi(30) | 517.64 | 525.06 | 532.88 | 530.43 | 103.60 | 465.00 | 459.20 |
| (2.67) | (3.37) | (1.86) | (1.40) | (1.65) | (2.27) | (2.02) | |
| Catalyst | Temperature A * | Temperature B # | Formaldehyde Concentration at Temp. B | Formaldehyde Concentration at 500 °C |
|---|---|---|---|---|
| [°C] | [°C] | [ppm] | [ppm] | |
| SiO2 | 380 | 500 | 360 | 360 |
| TiO2 | 360 | 500 | 440 | 440 |
| SiTi(10) | 410 | 500 | 330 | 330 |
| SiTi(30) | 315 | 500 | 780 | 780 |
| SiTi(60) | 365 | 500 | 340 | 340 |
| 0.75%V/SiTi(30) | 215 | 480 | 930 | 918 |
| 1.5%V/SiTi(30) | 215 | 415 | 1060 | 760 |
| 1.5%VSi | 350 | 500 | 1030 | 1030 |
| 1.5%VTi | 335 | 500 | 1000 | 1000 |
| 2.5%V/SiTi(30) | 215 | 420 | 960 | 660 |
| Support/Catalyst | Abbreviation |
|---|---|
| SiO2 | Si |
| TiO2 | Ti |
| SiO2 + 10%TiO2 | SiTi(10) |
| SiO2 + 30%TiO2 | SiTi(30) |
| SiO2 + 60%TiO2 | SiTi(60) |
| 0.75%V2O5/ SiO2+30%TiO2 | 0.75V/SiTi(30) |
| 1.5%V2O5/SiO2 | 1.5V/Si |
| 1.5%V2O5/TiO2 | 1.5V/Ti |
| 1.5%V2O5/SiO2 + 30%TiO2 | 1.5V/SiTi(30) |
| 2.5%V2O5/SiO2 + 30%TiO2 | 2.5V/SiTi(30) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koivikko, N.; Laitinen, T.; Mouammine, A.; Ojala, S.; Keiski, R.L. Catalytic Activity Studies of Vanadia/Silica–Titania Catalysts in SVOC Partial Oxidation to Formaldehyde: Focus on the Catalyst Composition. Catalysts 2018, 8, 56. https://doi.org/10.3390/catal8020056
Koivikko N, Laitinen T, Mouammine A, Ojala S, Keiski RL. Catalytic Activity Studies of Vanadia/Silica–Titania Catalysts in SVOC Partial Oxidation to Formaldehyde: Focus on the Catalyst Composition. Catalysts. 2018; 8(2):56. https://doi.org/10.3390/catal8020056
Chicago/Turabian StyleKoivikko, Niina, Tiina Laitinen, Anass Mouammine, Satu Ojala, and Riitta L. Keiski. 2018. "Catalytic Activity Studies of Vanadia/Silica–Titania Catalysts in SVOC Partial Oxidation to Formaldehyde: Focus on the Catalyst Composition" Catalysts 8, no. 2: 56. https://doi.org/10.3390/catal8020056