Olefins from Biomass Intermediates: A Review
Abstract
:1. Introduction
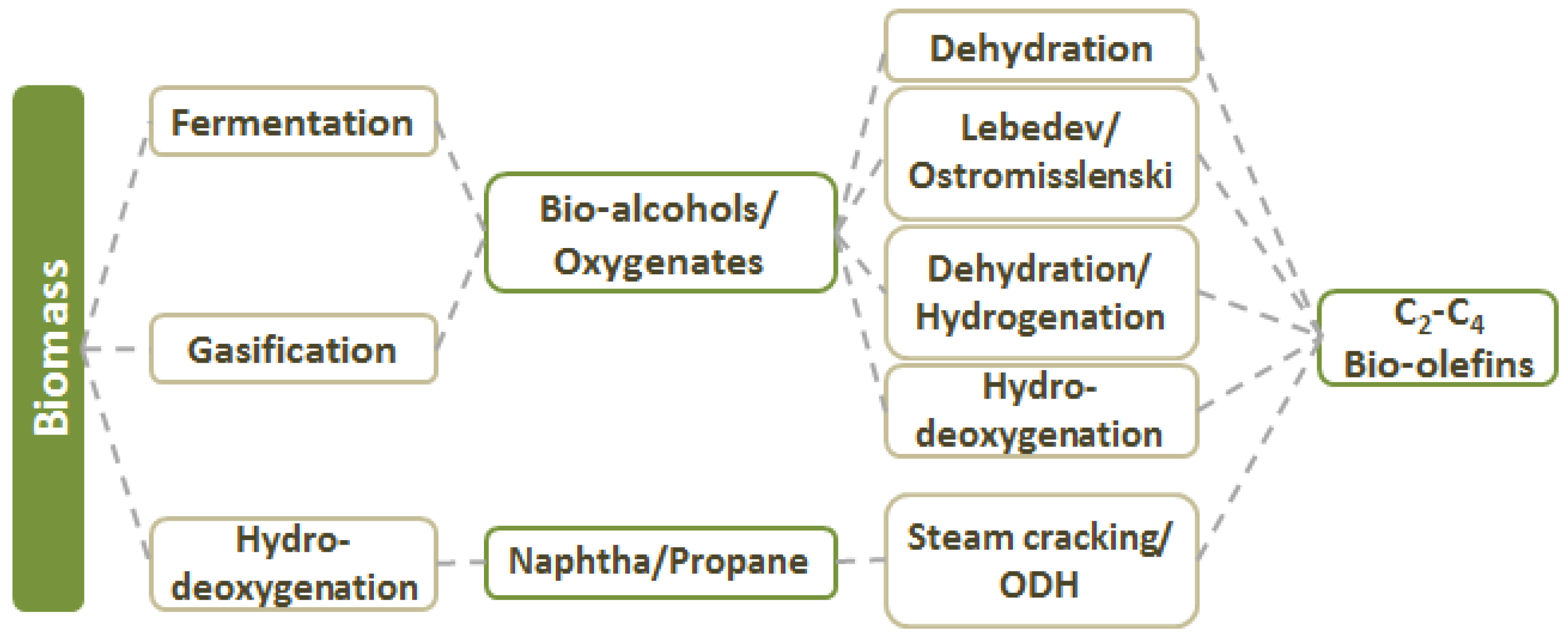
2. Olefins from Biomass
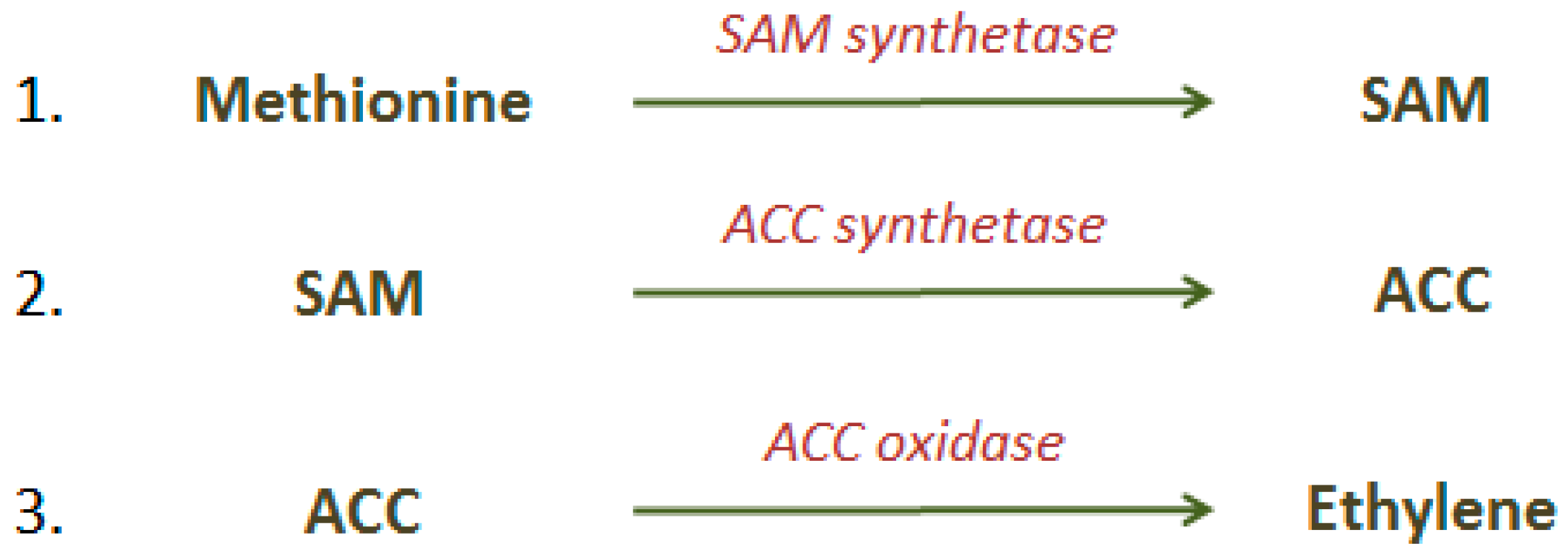
2.1. Ethylene (C2H4)
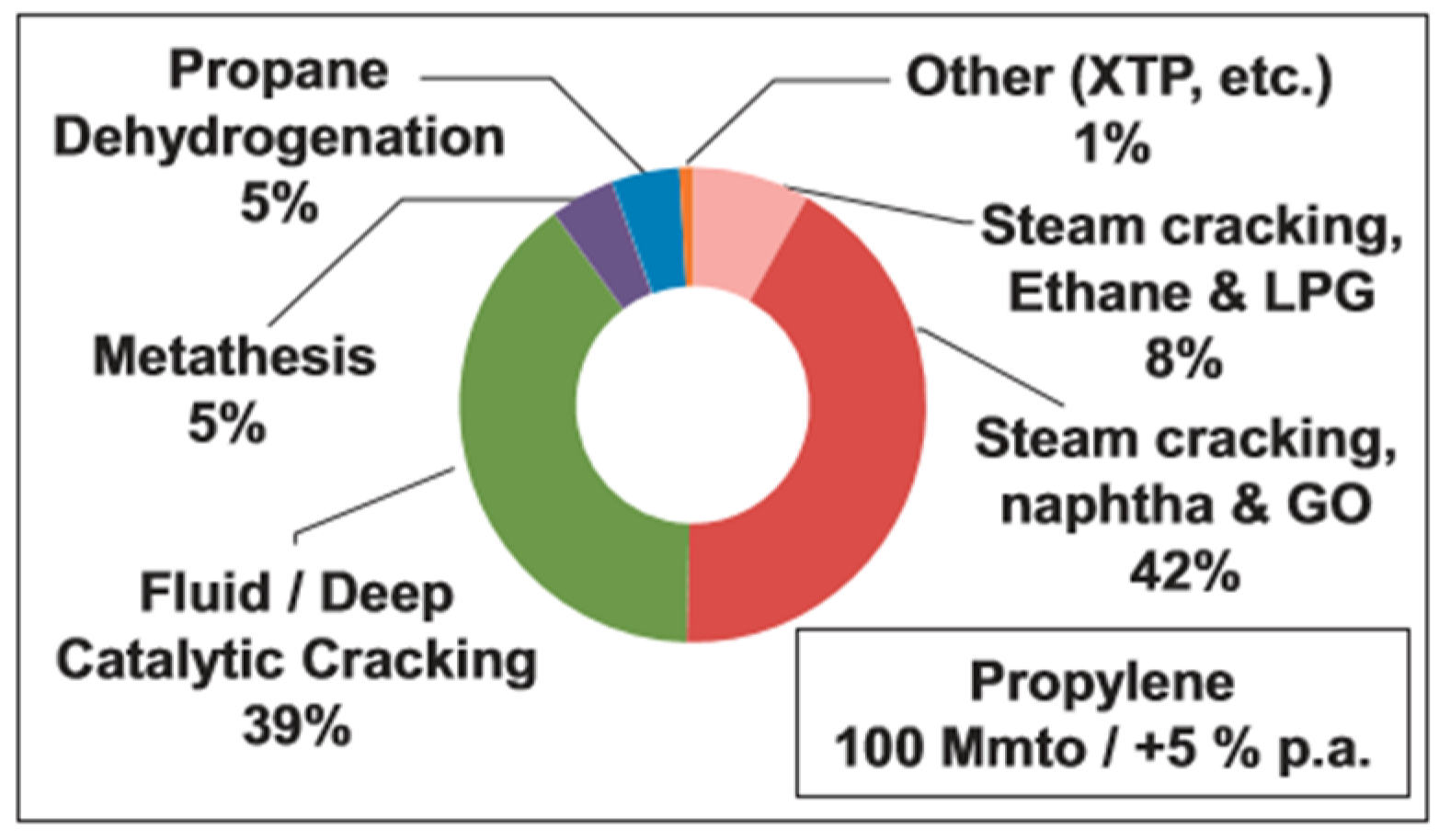
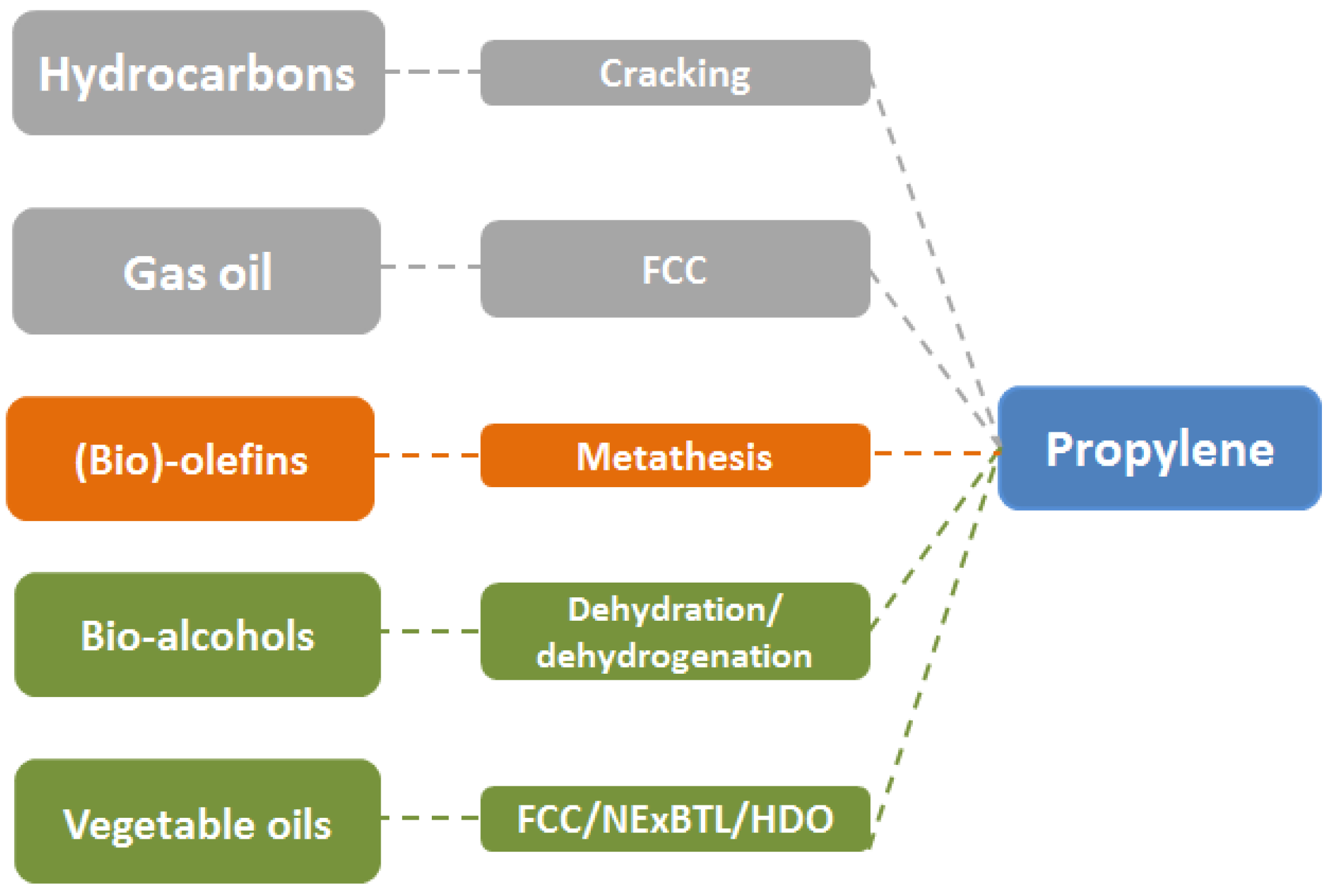
2.2. Propylene (C3H6)
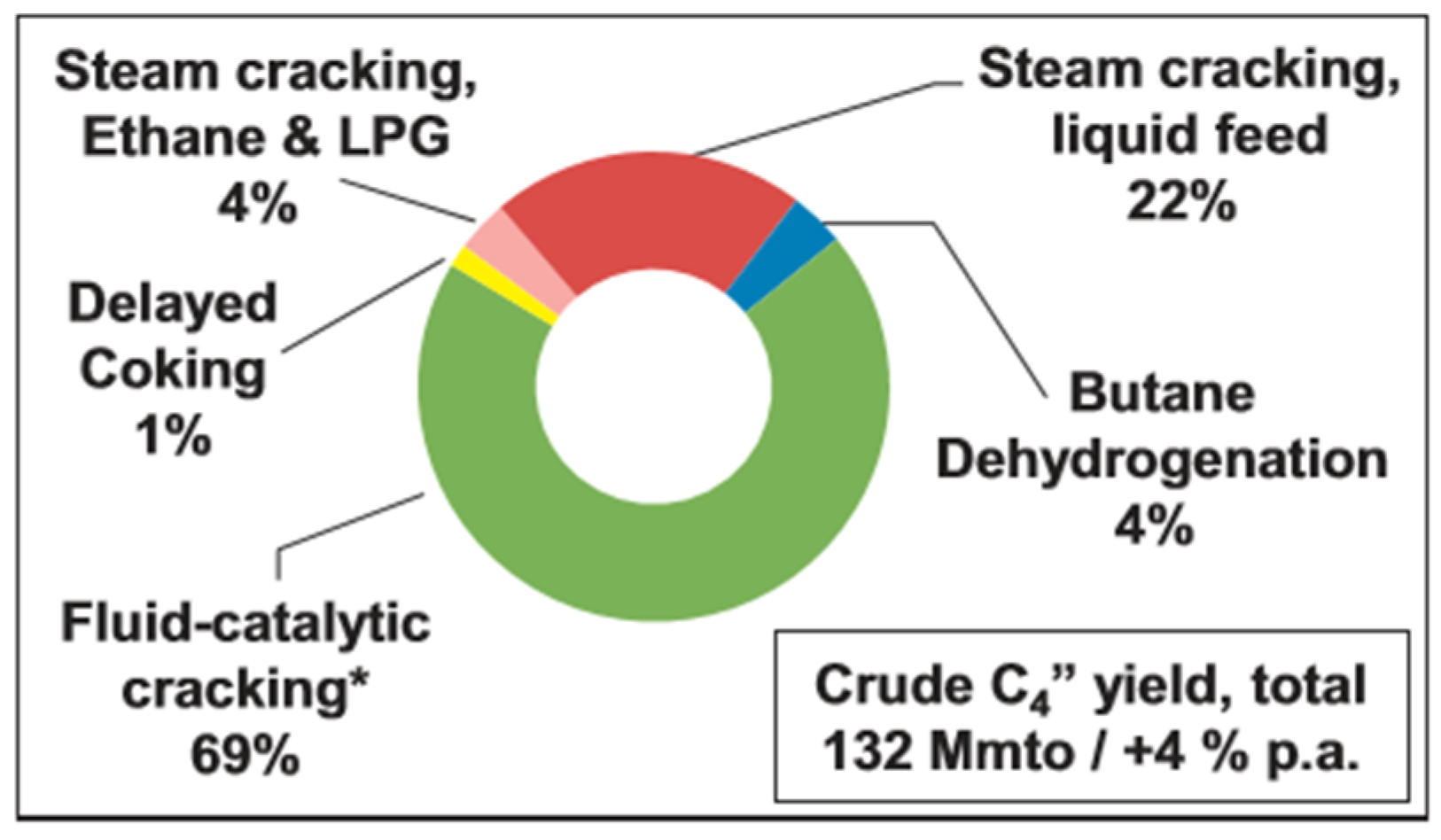
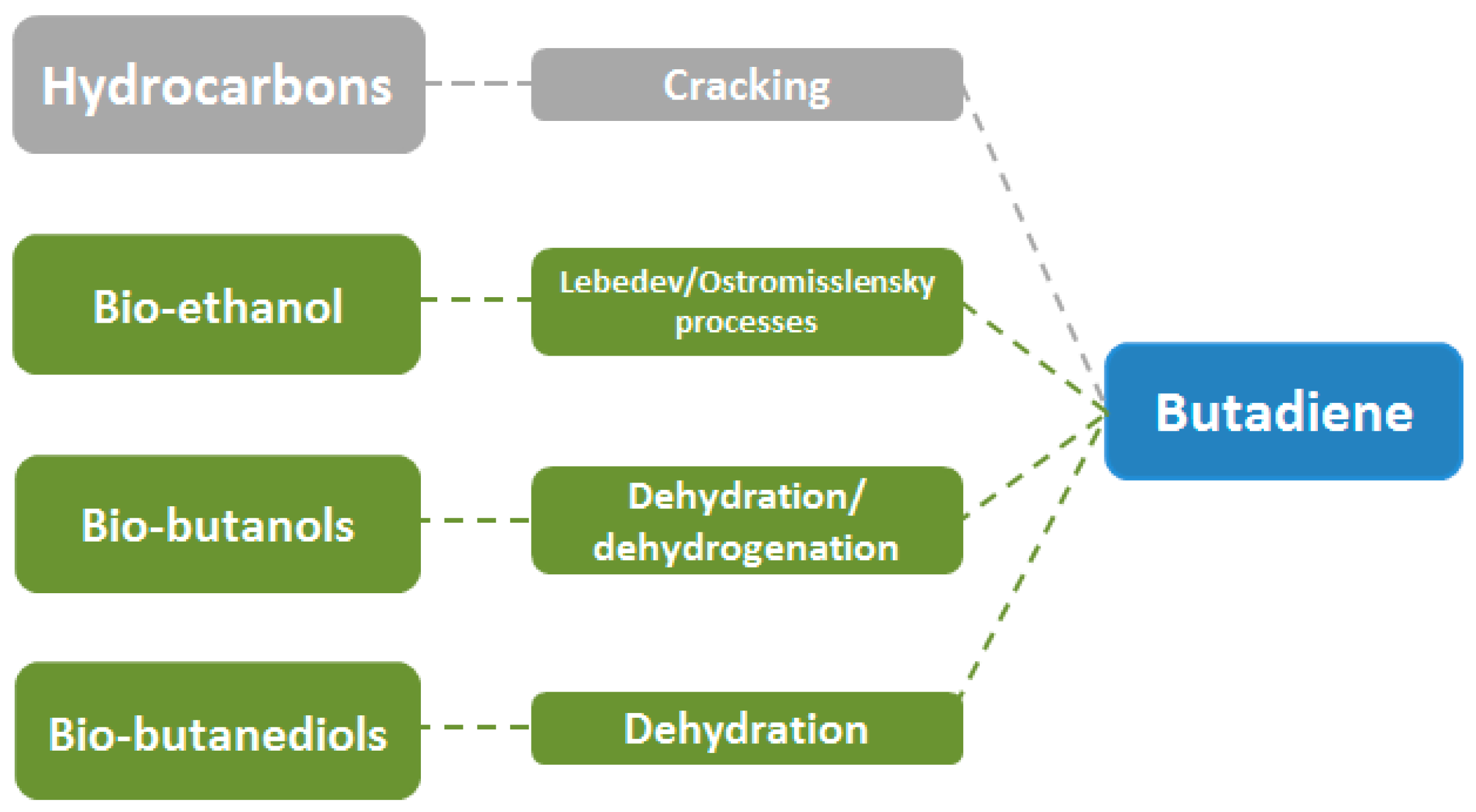
2.3. Butadiene (C4H6)
3. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- BP. Statistical Review of World Energy; BP: London, UK, 2017. [Google Scholar]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Bender, M. An Overview of Industrial Processes for the Production of Olefins—C4 Hydrocarbons. ChemBioEng Rev. 2014, 1, 136–147. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Processes; Gulf Professional Publishing: Houston, TX, USA, 2009. [Google Scholar]
- Weissermel, K.; Arpe, H. Oxidation Products Ethylene. In Industrial Organic Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1978; pp. 145–192. ISBN 9783527619191. [Google Scholar]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527306732. [Google Scholar]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Chang, C.D. Methanol Conversion to Light Olefins. Catal. Rev. 1984, 26, 323–345. [Google Scholar] [CrossRef]
- Haro, P.; Trippe, F.; Stahl, R.; Henrich, E. Bio-syngas to gasoline and olefins via DME—A comprehensive techno-economic assessment. Appl. Energy 2013, 108, 54–65. [Google Scholar] [CrossRef]
- McManus, M.T. The Plant Hormone Ethylene; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 1444330039. [Google Scholar]
- Ding, J.; Hua, W. Game Changers of the C3 Value Chain: Gas, Coal, and Biotechnologies. Chem. Eng. Technol. 2013, 36, 83–90. [Google Scholar] [CrossRef]
- Eisele, P.; Killpack, R. Propene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527306732. [Google Scholar]
- Sahebdelfar, S.; Zangeneh, F.T. Dehydrogenation of Propane to Propylene Over Pt-Sn/Al2O3 Catalysts: The influence of operating conditions on product selectivity. Iran. J. Chem. Eng. 2010, 7, 51–57. [Google Scholar]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Chen, D.; Moljord, K.; Holmen, A. A methanol to olefins review: Diffusion, coke formation and deactivation on SAPO type catalysts. Microporous Mesoporous Mater. 2012, 164, 239–250. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J. Top Value Added Chemicals from Biomass. Volume 1-Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Washington, DC, USA, 2004.
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chem. Int. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef] [PubMed]
- Siirola, J.J. The impact of shale gas in the chemical industry. AIChE J. 2014, 60, 810–819. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Chieregato, A.; Ochoa, J.V.; Cavani, F. Olefins from Biomass. In Chemicals and Fuels from Bio-Based Building Blocks; Cavani, F., Albonetti, S., Basile, F., Gandini, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Lynch, S.; Eckert, C.; Yu, J.; Gill, R.; Maness, P.-C. Overcoming substrate limitations for improved production of ethylene in E. coli. Biotechnol. Biofuels 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, W.J. Ethylene as a metabolic product of the pathogenic fungus, Blastomyces dermatitidis. Arch Biochem. 1948, 17, 225–233. [Google Scholar] [PubMed]
- Eckert, C.; Xu, W.; Xiong, W.; Lynch, S.; Ungerer, J.; Tao, L.; Gill, R.; Maness, P.-C.; Yu, J. Ethylene-forming enzyme and bioethylene production. Biotechnol. Biofuels 2014, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Wu, L.X.; Xu, F.; Lv, J.; Jin, H.J.; Chen, S.F. Metabolic engineering for ethylene production by inserting the ethylene-forming enzyme gene (efe) at the 16S rDNA sites of Pseudomonas putida KT2440. Bioresour. Technol. 2010, 101, 6404–6409. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.N.; Tao, L.; Davis, R.; Voulis, N.; Angenent, L.T.; Ungerer, J.; Yu, J. Techno-economic analysis of a conceptual biofuel production process from bioethylene produced by photosynthetic recombinant cyanobacteria. Green Chem. 2016, 18, 6266–6281. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Fan, D.; Dai, D.J.; Wu, H.S. Ethylene formation by catalytic dehydration of ethanol with industrial considerations. Materials 2013, 6, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kosaric, N.; Duvnjak, Z.; Farkas, A.; Sahm, H.; Bringer-Meyer, S.; Goebel, O.; Mayer, D.; Kosaric, N.; Duvnjak, Z.; Farkas, A.; et al. Ethanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–72. ISBN 9783527306732. [Google Scholar]
- Althoff, J.; Biesheuvel, K.; De Kok, A.; Pelt, H.; Ruitenbeek, M.; Spork, G.; Tange, J.; Wevers, R. Economic Feasibility of the Sugar Beet-to-Ethylene Value Chain. ChemSusChem 2013, 6, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Fukumoto, M.; Kimura, A. Process for the Manufacture of Ethylene by Dehydration of Ethanol. EP 2594546 A1, 17 November 2011. [Google Scholar]
- Chen, Y.; Wu, Y.; Tao, L.; Dai, B.; Yang, M.; Chen, Z.; Zhu, X. Dehydration reaction of bio-ethanol to ethylene over modified SAPO catalysts. J. Ind. Eng. Chem. 2010, 16, 717–722. [Google Scholar] [CrossRef]
- Wu, L.; Shi, X.; Cui, Q.; Wang, H.; Huang, H. Effects of the SAPO-11 synthetic process on dehydration Of ethanol to ethylene. Front. Chem. Sci. Eng. 2011, 5, 60–66. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, R.; Yang, X. Effect of P content on the catalytic performance of P-modified HZSM-5 catalysts in dehydration of ethanol to ethylene. Catal. Lett. 2008, 124, 384–391. [Google Scholar] [CrossRef]
- Patrick, G.B.; Partington, S.R. Process for Preparing Ethene. U.S. Patent 8822748 B2, 8 October 2008. [Google Scholar]
- Lanzafame, P.; Centi, G.; Perathoner, S. Evolving scenarios for biorefineries and the impact on catalysis. Catal. Today 2014, 234, 2–12. [Google Scholar] [CrossRef]
- Kang, M.; DeWilde, J.F.; Bhan, A. Kinetics and Mechanism of Alcohol Dehydration on γ-Al2O3: Effects of Carbon Chain Length and Substitution. ACS Catal. 2015, 5, 602–612. [Google Scholar] [CrossRef]
- Christiansen, M.A.; Mpourmpakis, G.; Vlachos, D.G. DFT-driven multi-site microkinetic modeling of ethanol conversion to ethylene and diethyl ether on γ-Al2O3 (1 1 1). J. Catal. 2015, 323, 121–131. [Google Scholar] [CrossRef]
- Potter, M.E.; Cholerton, M.E.; Kezina, J.; Bounds, R.; Carravetta, M.; Manzoli, M.; Gianotti, E.; Lefenfeld, M.; Raja, R. Role of Isolated Acid Sites and Influence of Pore Diameter in the Low-Temperature Dehydration of Ethanol. ACS Catal. 2014, 4, 4161–4169. [Google Scholar] [CrossRef]
- DeWilde, J.F.; Czopinski, C.J.; Bhan, A. Ethanol Dehydration and Dehydrogenation on γ-Al2O3: Mechanism of Acetaldehyde Formation. ACS Catal. 2014, 4, 4425–4433. [Google Scholar] [CrossRef]
- Kochar, N.K.; Merims, R.; Padia, A.S. Ethylene from Ethanol. Chem. Eng. Prog. 1981, 6, 66–70. [Google Scholar]
- Ondrey, G. The Launch of a New Bioethylene-Production Process; Chemical Engineering: New York, NY, USA, 2014. [Google Scholar]
- Chen, G.; Li, S.; Jiao, F.; Yuan, Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal. Today 2007, 125, 111–119. [Google Scholar] [CrossRef]
- Zhan, N.; Hu, Y.; Li, H.; Yu, D.; Han, Y.; Huang, H. Lanthanum-phosphorous modified HZSM-5 catalysts in dehydration of ethanol to ethylene: A comparative analysis. Catal. Commun. 2010, 11, 633–637. [Google Scholar] [CrossRef]
- Ciftci, A.; Varisli, D.; Tokay, K.C.; Sezgi, N.A.; Dogu, T. Dimethyl ether, diethyl ether & ethylene from alcohols over tungstophosphoric acid based mesoporous catalysts. Chem. Eng. J. 2012, 207–208, 85–93. [Google Scholar]
- Varisli, D.; Dogu, T.; Dogu, G. Silicotungstic acid impregnated MCM-41-like mesoporous solid acid catalysts for dehydration of ethanol. Ind. Eng. Chem. Res. 2008, 47, 4071–4076. [Google Scholar] [CrossRef]
- Chang, C.D.; Silvestri, A.J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 1977, 47, 249–259. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjorgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Güllü, D.; Demirbas, A. Biomass to methanol via pyrolysis process. Energy Convers. Manag. 2001, 42, 1349–1356. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, J. Methanol to olefins conversion catalysts Curr. Opin. Solid State Mater. Sci. 1999, 4, 80–84. [Google Scholar] [CrossRef]
- Galindo, C.P.; Badr, O. Renewable hydrogen utilisation for the production of methanol. Energy Convers. Manag. 2007, 48, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.E.; Noceti, R.P.; Joseph, R.D. New developments in the photocatalytic conversion of methane to methanol. Catal. Today 2000, 55, 259–267. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Zhao, S.; Guo, W.; Wang, R.; Ying, M. Characteristics and performance of SAPO-34 catalyst for methanol-to-olefin conversion. Appl. Catal. 1990, 64, 31–40. [Google Scholar] [CrossRef]
- Raffelt, K.; Henrich, E.; Koegel, A.; Stahl, R.; Steinhardt, J.; Weirich, F. The BTL2 Process of Biomass Utilization Entrained-Flow Gasification of Pyrolyzed Biomass Slurries. Appl. Biochem. Biotechnol. 2006, 129, 153–164. [Google Scholar] [CrossRef]
- Henrich, E.; Dahmen, N.; Dinjus, E. Cost estimate for biosynfuel production via biosyncrude gasification. Biofuels Bioprod. Biorefin. 2009, 3, 28–41. [Google Scholar] [CrossRef]
- Wright, M.M.; Brown, R.C.; Boateng, A.A. Distributed processing of biomass to bio-oil for subsequent production of Fischer-Tropsch liquids. Biofuels Bioprod. Biorefin. 2008, 2, 229–238. [Google Scholar] [CrossRef]
- Kvisle, S.; Fuglerud, T.; Kolboe, S.; Olsbye, U.; Lillerud, K.P.; Vora, B.V.; Kvisle, S.; Fuglerud, T.; Kolboe, S.; Olsbye, U.; et al. Methanol-to-Hydrocarbons. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527610044. [Google Scholar]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Akah, A.; Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl. Petrochem. Res. 2015, 5, 377–392. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gong, Y.; Zhang, L.; Liu, Y.; Dou, T.; Xu, J.; Deng, F. Hydrothermal treatment on ZSM-5 extrudates catalyst for methanol to propylene reaction: Finely tuning the acidic property. Fuel Process. Technol. 2015, 129, 130–138. [Google Scholar] [CrossRef]
- Banks, R.L.; Kukes, S.G. New developments and concepts in enhancing activities of heterogeneous metathesis catalysts. J. Mol. Catal. 1985, 28, 117–131. [Google Scholar] [CrossRef]
- Knifton, J.F.; Sanderson, J.R.; Stockton, M.E. Tert-butanol dehydration to isobutylene via reactive distillation. Catal. Lett. 2001, 73, 55–57. [Google Scholar] [CrossRef]
- Kozlowski, J.T.; Davis, R.J. Heterogeneous catalysts for the guerbet coupling of alcohols. ACS Catal. 2013, 3, 1588–1600. [Google Scholar] [CrossRef]
- Dowson, G.R.M.; Haddow, M.F.; Lee, J.; Wingad, R.L.; Wass, D.F. Catalytic conversion of ethanol into an advanced biofuel: Unprecedented selectivity for n-butanol. Angew. Chem. Int. Ed. 2013, 52, 9005–9008. [Google Scholar] [CrossRef] [PubMed]
- HOOD, A.D., Jr.; Bridges, R.S. Staged Propylene Production Process. WO 2014110125 A1, 17 July 2014. [Google Scholar]
- Mol, J.C. Industrial applications of olefin metathesis. J. Mol. Catal. A Chem. 2004, 213, 39–45. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Xin, W.; Bai, J.; Xie, S.; Wang, Q.; Xu, L. Metathesis of ethene and 2-butene to propene on W/Al2O3-HY catalysts with different HY contents. J. Mol. Catal. A Chem. 2005, 226, 61–68. [Google Scholar] [CrossRef]
- Bouchmella, K.; Hubert Mutin, P.; Stoyanova, M.; Poleunis, C.; Eloy, P.; Rodemerck, U.; Gaigneaux, E.M.; Debecker, D.P. Olefin metathesis with mesoporous rhenium-silicium-aluminum mixed oxides obtained via a one-step non-hydrolytic sol-gel route. J. Catal. 2013, 301, 233–241. [Google Scholar] [CrossRef]
- Busca, G. Heterogeneous Catalytic Materials: Solid State Chemistry, Surface Chemistry and Catalytic Behaviour; Elsevier: Warsaw, Poland, 2014. [Google Scholar]
- Popoff, N.; Mazoyer, E.; Pelletier, J.; Gauvin, R.M.; Taoufik, M. Expanding the scope of metathesis: A survey of polyfunctional, single-site supported tungsten systems for hydrocarbon valorization. Chem. Soc. Rev. 2013, 42, 9035–9054. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Inaba, M.; Takahara, I.; Murata, K. Conversion of ethanol to propylene by H-ZSM-5 with Si/Al2 ratio of 280. Catal. Lett. 2010, 136, 14–19. [Google Scholar] [CrossRef]
- Kazuhisa, M.; Takahara, I.; Inaba, M. Propane Formation by Aqueous-Phase Reforming of Glycerol over Pt/H-ZSM5 Catalysts. React. Kinet. Catal. Lett. 2008, 93, 59–66. [Google Scholar]
- Lin, B.; Zhang, Q.; Wang, Y. Catalytic conversion of ethylene to propylene and butenes over H-ZSM-5. Ind. Eng. Chem. Res. 2009, 48, 10788–10795. [Google Scholar] [CrossRef]
- Huangfu, J.; Mao, D.; Zhai, X.; Guo, Q. Remarkably enhanced stability of HZSM-5 zeolite co-modified with alkaline and phosphorous for the selective conversion of bio-ethanol to propylene. Appl. Catal. A Gen. 2016, 520, 99–104. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, D.; Zhai, X. Selective conversion of bio-ethanol to propene over nano-HZSM-5 zeolite: Remarkably enhanced catalytic performance by fluorine modification. Fuel Process. Technol. 2017, 167, 50–60. [Google Scholar] [CrossRef]
- Hayashi, F.; Iwamoto, M. Yttrium-modified ceria as a highly durable catalyst for the selective conversion of ethanol to propene and ethene. ACS Catal. 2013, 3, 14–17. [Google Scholar] [CrossRef]
- Hayashi, F.; Tanaka, M.; Lin, D.; Iwamoto, M. Surface structure of yttrium-modified ceria catalysts and reaction pathways from ethanol to propene. J. Catal. 2014, 316, 112–120. [Google Scholar] [CrossRef]
- Iwamoto, M.; Mizuno, S.; Tanaka, M. Direct and selective production of propene from bio-ethanol on Sc-loaded In2O3 catalysts. Chem. A Eur. J. 2013, 19, 7214–7220. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wang, F.; Mu, X.; Chen, K.; Takahashi, A.; Nakamura, I.; Fujitani, T. Catalytic performance of H-ZSM-5 zeolites for conversion of ethanol or ethylene to propylene: Effect of reaction pressure and SiO2/Al2O3 ratio. Catal. Commun. 2017, 91, 62–66. [Google Scholar] [CrossRef]
- Xue, F.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Direct conversion of bio-ethanol to propylene in high yield over the composite of In2O3 and zeolite beta. Green Chem. 2017. [Google Scholar] [CrossRef]
- Hulteberg, C.; Brandin, J. Process for Preparing Lower Hydrocarbons from Glycerol. U.S. Patent 20110224470 A1, 15 September 2011. [Google Scholar]
- Souza, F.J.C.; Gambetta, R.; de Araujo Mota, C.J.; da Conceicao Goncalves, V.L. Preparation of Heterogeneous Catalysts Used in Selective Hydrogenation of Glycerin to Propene, and a Process for the Selective Hydrogenation of Glycerin to Propene. U.S. Patent 8841497 B2, 24 June 2009. [Google Scholar]
- Blass, S.D.; Hermann, R.J.; Persson, N.E.; Bhan, A.; Schmidt, L.D. Conversion of glycerol to light olefins and gasoline precursors. Appl. Catal. A Gen. 2014, 475, 10–15. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, J.; Zhang, Q.; Liu, Y.M.; He, H.Y.; Fan, K.N.; Cao, Y. Propylene from renewable resources: Catalytic conversion of glycerol into propylene. ChemSusChem 2014, 7, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Zacharopoulou, V.; Vasiliadou, E.S.; Lemonidou, A.A. One-step propylene formation from bio-glycerol over molybdena-based catalysts. Green Chem. 2015, 17, 903–912. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S. Efficient production of propylene in the catalytic conversion of glycerol. Appl. Catal. B Environ. 2015, 174, 13–20. [Google Scholar] [CrossRef]
- Deshpande, R.; Davis, P.; Pandey, V.; Kore, N. Dehydroxylation of Crude Alcohol Streams Using a Halogen-Based Catalyst. WO 2013090076 A1, 20 June 2013. [Google Scholar]
- Zacharopoulou, V.; Vasiliadou, E.; Lemonidou, A.A. Exploring the reaction pathways of bio-glycerol hydro-deoxygenation to propene over Molybdena-based catalysts. ChemSusChem 2017. [Google Scholar] [CrossRef] [PubMed]
- Pyl, S.P.; Schietekat, C.M.; Reyniers, M.F.; Abhari, R.; Marin, G.B.; Van Geem, K.M. Biomass to olefins: Cracking of renewable naphtha. Chem. Eng. J. 2011, 176–177, 178–187. [Google Scholar] [CrossRef]
- Ramin, A.H.; Lynn Tomlinson, G.R. Biorenewable Naphtha Composition and Methods of Making Same. U.S. Patent 8581013 B2, 12 November 2013. [Google Scholar]
- Vermeiren, W.; Van Gyseghem, N. A process for the Production of Bio-Naphtha from Complex Mixtures of Natural Occurring Fats & Oils. WO 2011012439 A1, 3 February 2011. [Google Scholar]
- Bielansky, P.; Weinert, A.; Schönberger, C.; Reichhold, A. Catalytic conversion of vegetable oils in a continuous FCC pilot plant. Fuel Process. Technol. 2011, 92, 2305–2311. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Schaidle, J.A.; Ferrell, J.R., III; Wang, J.; Moens, L.; Hensley, J.E. Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: Catalyst development through the study of model compounds. Green Chem. 2014, 16, 454–490. [Google Scholar] [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef] [PubMed]
- Quattlebaum, W.M., Jr.; Toussaint, W.J. Process of Making Olefins. U.S. Patent 2407291 A, 10 September 1946. [Google Scholar]
- Toussant, W.J.; Dunn, J.T.; Jackson, D.R. Production of Butadiene from Alcohol. Ind. Eng. Chem. 1947, 39, 120–125. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [PubMed]
- Chieregato, A.; Ochoa, J.V.; Bandinelli, C.; Fornasari, G.; Cavani, F.; Mella, M. On the chemistry of ethanol on basic oxides: Revising mechanisms and intermediates in the lebedev and guerbet reactions. ChemSusChem 2015, 8, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.L.; Zeng, A.P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.B.; Anthony, P.B.; Robin, E.O.; Jun, S.P.P. Microorganisms for Producing Butadiene and Methods Related Thereto. WO 2012177710 A1, 27 December 2012. [Google Scholar]
- Zhang, D.; Barri, S.A.I.; Chadwick, D. N-Butanol to iso-butene in one-step over zeolite catalysts. Appl. Catal. A Gen. 2011, 403, 1–11. [Google Scholar] [CrossRef]
- Arpe, H.-J.; Weissermel, K. Industrial Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2010; ISBN 3527320024. [Google Scholar]
- Reppe, W.; Steinhofer, A.; Daumiller, G. Verfahren zur Herstellung von Diolefinen. DE 899350, 10 August 1953. [Google Scholar]
- Kopke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol Production by Acetogenic Bacteria, an Alternative Route to Chemical Synthesis, Using Industrial Waste Gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yamada, Y.; Sato, S. Selective dehydration of 2,3-butanediol to 3-buten-2-ol over ZrO2 modified with CaO. Appl. Catal. A Gen. 2014, 487, 226–233. [Google Scholar] [CrossRef]
- Mohammadi, M.; Najafpour, G.D.; Younesi, H.; Lahijani, P.; Uzir, M.H.; Mohamed, A.R. Bioconversion of synthesis gas to second generation biofuels: A review. Renew. Sustain. Energy Rev. 2011, 15, 4255–4273. [Google Scholar] [CrossRef]
- Choi, D.W.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 2010, 160, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Bredwell, M.D.; Srivastava, P.; Worden, R.M. Reactor Design Issues for Synthesis-Gas Fermentations. Biotechnol. Prog. 1999, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Madhukar, G.R.; Elmore, B.B.; Huckabay, H.K. Microbial conversion of synthesis gas components to useful fuels and chemicals, Microbial Conversion of Synthesis Gas Components to Useful Fuels and Chemicals Symposium. In Seventeenth Symposium on Biotechnology for Fuels and Chemicals; Wyman, C.E., Davison, B.H., Eds.; ABAB Symposium, Volume 57/58; Humana Press: Totowa, NJ, USA, 1996. [Google Scholar]



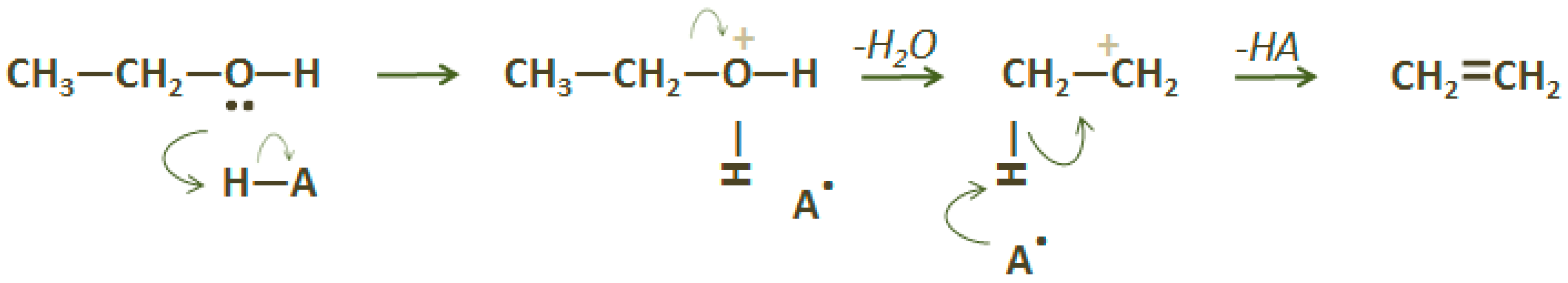
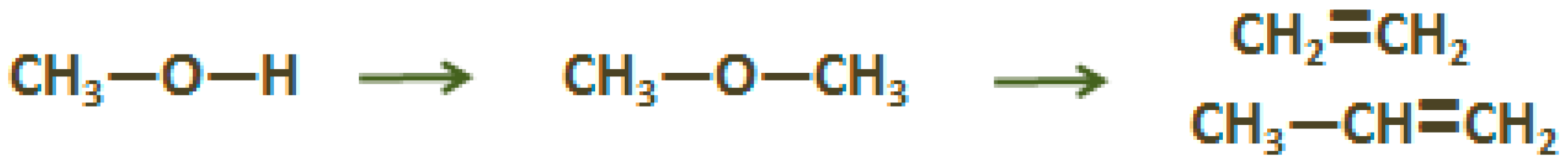
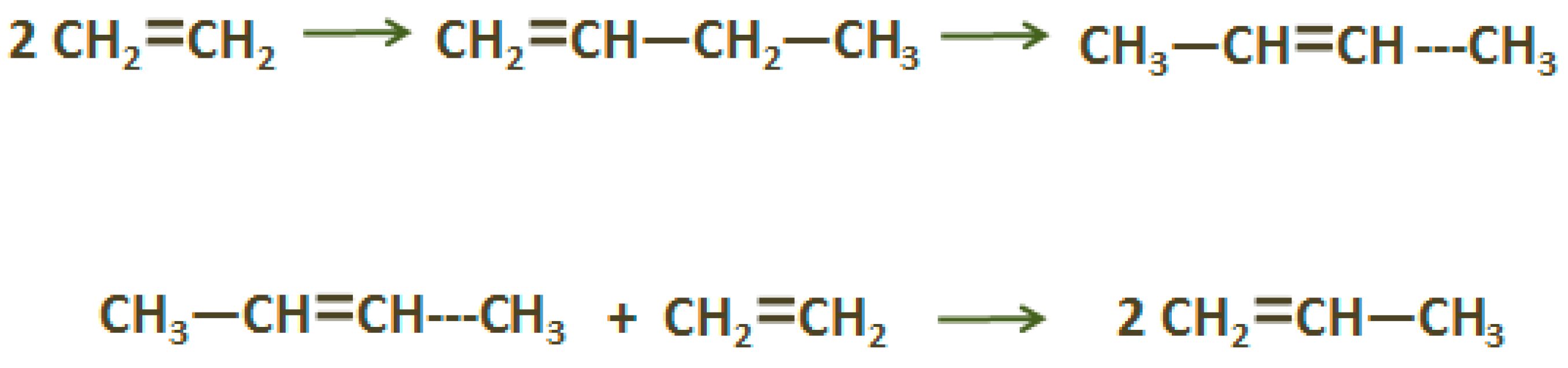


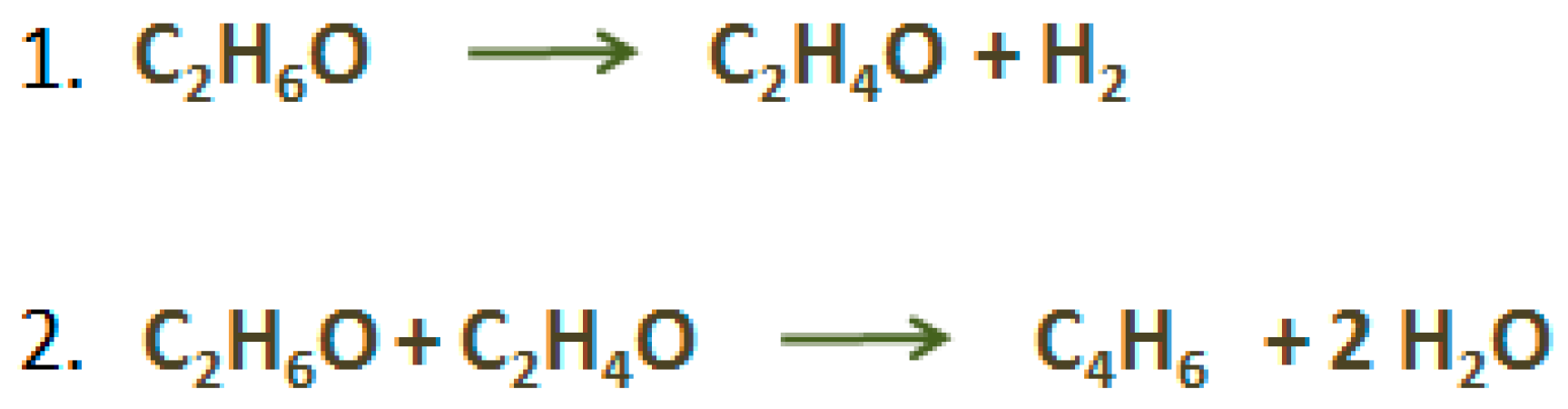
| Catalyst | Ethanol Conversion (%) | Selectivity to C2H4 (%) | Temperature (°C) | WHSV a/LHSV b (h−1) | Reference |
|---|---|---|---|---|---|
| Mn-SAPO-34 | 99.4 | 98.4 | 340 | 2.0 a | [35] |
| 0.5%La-2%P-HZSM-5 | 100.0 | 99.9 | 240–280 | 2.0 a | [47] |
| TPA-MCM-41 * | 98.0 | 99.9 | 300 | 2.9 a | [48] |
| SynDol ** | 99.0 | 96.8 | 450 | 26–234 b | [29,44,45,46] |
| STA-MCM-41 *** | 99.0 | 99.9 | 250 | 2.9 a | [49] |
| Process | Steam Cracking | Bio-Synthesis | Bio-Ethanol Dehydration | MTO | DMTO |
|---|---|---|---|---|---|
| Feedstock | HC (Naphtha) | ACC/SAM | Bio-ethanol | Bio-methanol | Bio-DME |
| Operating Conditions | 675–700 °C atmospheric pressure | Ambient, aerobic conditions | 180–500 °C | 300–500 °C, low pressure | 675–750 °C, low pressure |
| Advantages | Already installed technology | Selective sustainable production | Commercial application | Close to commercial application | |
| Disadvantages | Energy intense, environmental concerns, finite resources | Increased cost, low productivity | Limited bio-ethanol supply | Limited production of bio-feedstocks | |
| Yield to C2H4 | 31.3% | - | 99.9% | 41.5% | 45.0% |
| Catalyst | Glycerol Conversion (%) | Selectivity to C3H6 (%) | Temperature (°C) | WHSV (h−1) | Reference |
|---|---|---|---|---|---|
| Ir/ZrO2 & HZSM-5-30 | 100.0 | 85.0 | 250 | 1.0 | [89] |
| Fe–Mo/Black Carbon | 88.0 | 76.0 | 300 | - | [90] |
| WO3-Cu/Al2O3 & SiO2-Al2O3 | 100.0 | 84.8 | 250 | - | [91] |
| Fe/Mo | 100.0 | 90.0 | 300 | 5.4 | [92] |
| Process | Steam Cracking | FCC | Dehydrogenation | Metathesis | GTO |
|---|---|---|---|---|---|
| Feedstock | HC | HC | Propane | Bio-olefins | Glycerol |
| Operating Conditions | 750–900 °C, moderate pressure | 500–550 °C, moderate pressure | 500–700 °C, atmospheric pressure | 0–260 °C, moderate pressure | 250–400 °C, hydrogen pressure |
| Advantages | Already installed technology | Greener than Steam Cracking, Flexibility of operation | Already installed units | Already installed units | Sustainable production |
| Disadvantages | Energy intense, environmental concerns, finite resources | Yield depends on feedstock, catalyst deactivation, product recovery | Catalyst deactivation, endothermic reaction | Limited production of bio-feedstocks | Require hydrogen atmosphere, lab-scale |
| Yield to C3H6 | 18.0% | 25.0% | 85.0% | 90.0% | 90.0% |
| Process | Steam Cracking | Dehydrogenation | Lebedev/Ostromisslenski | Dehydration |
|---|---|---|---|---|
| Feedstock | Naphtha | Butane/Butenes | Bio-ethanol | Bio-butanediols |
| Operating Conditions | 750–900 °C, moderate pressure | 600–700/400–500 °C | 400–650 °C | 250–350 °C |
| Advantages | Installed technology | Well-established technology, on-purpose production | Bio-based, on-purpose production | Bio-based, on-purpose production |
| Disadvantages | Energy demanding, environmental concerns, finite resources, limited production | High endothermicity, catalyst deactivation | Catalyst deactivation, various by-products | Limited production of bio-feedstock, various by-products |
| Yield to C4H6 | 4.5% | 70.0%/71.8% | 72.0%/56.5% | up to 95.0% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacharopoulou, V.; Lemonidou, A.A. Olefins from Biomass Intermediates: A Review. Catalysts 2018, 8, 2. https://doi.org/10.3390/catal8010002
Zacharopoulou V, Lemonidou AA. Olefins from Biomass Intermediates: A Review. Catalysts. 2018; 8(1):2. https://doi.org/10.3390/catal8010002
Chicago/Turabian StyleZacharopoulou, Vasiliki, and Angeliki A. Lemonidou. 2018. "Olefins from Biomass Intermediates: A Review" Catalysts 8, no. 1: 2. https://doi.org/10.3390/catal8010002




