Promoting Role of Bismuth on Hydrotalcite-Supported Platinum Catalysts in Aqueous Phase Oxidation of Glycerol to Dihydroxyacetone
Abstract
:1. Introduction
2. Results and Discussion
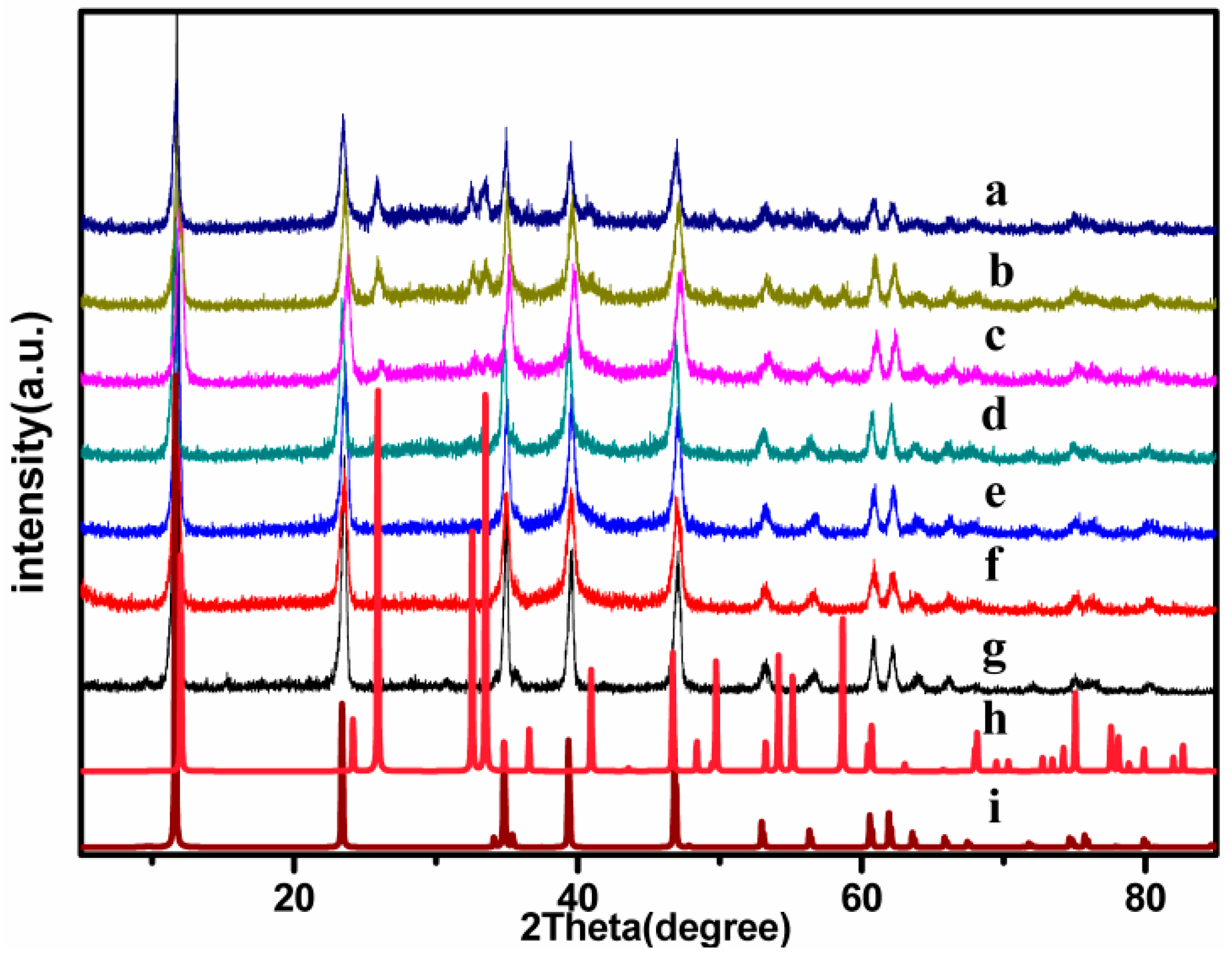
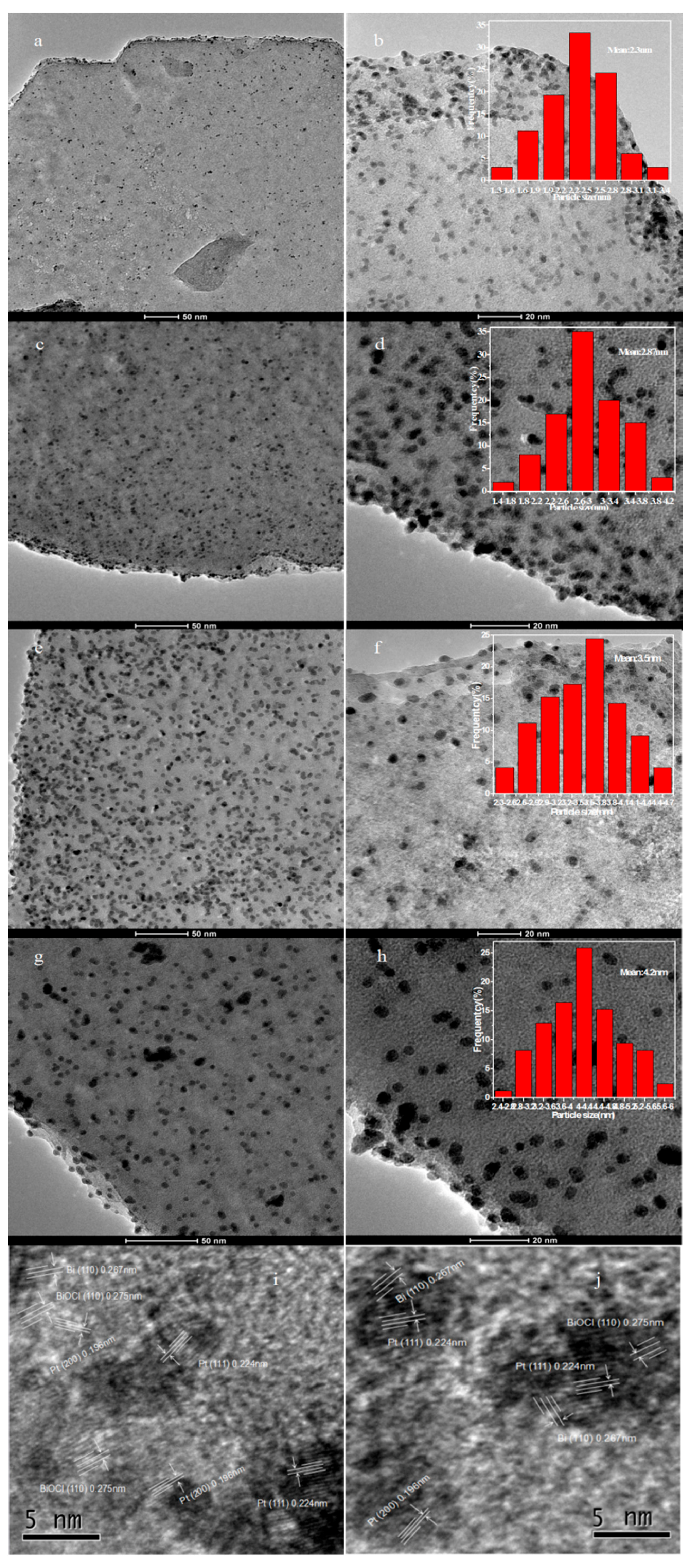
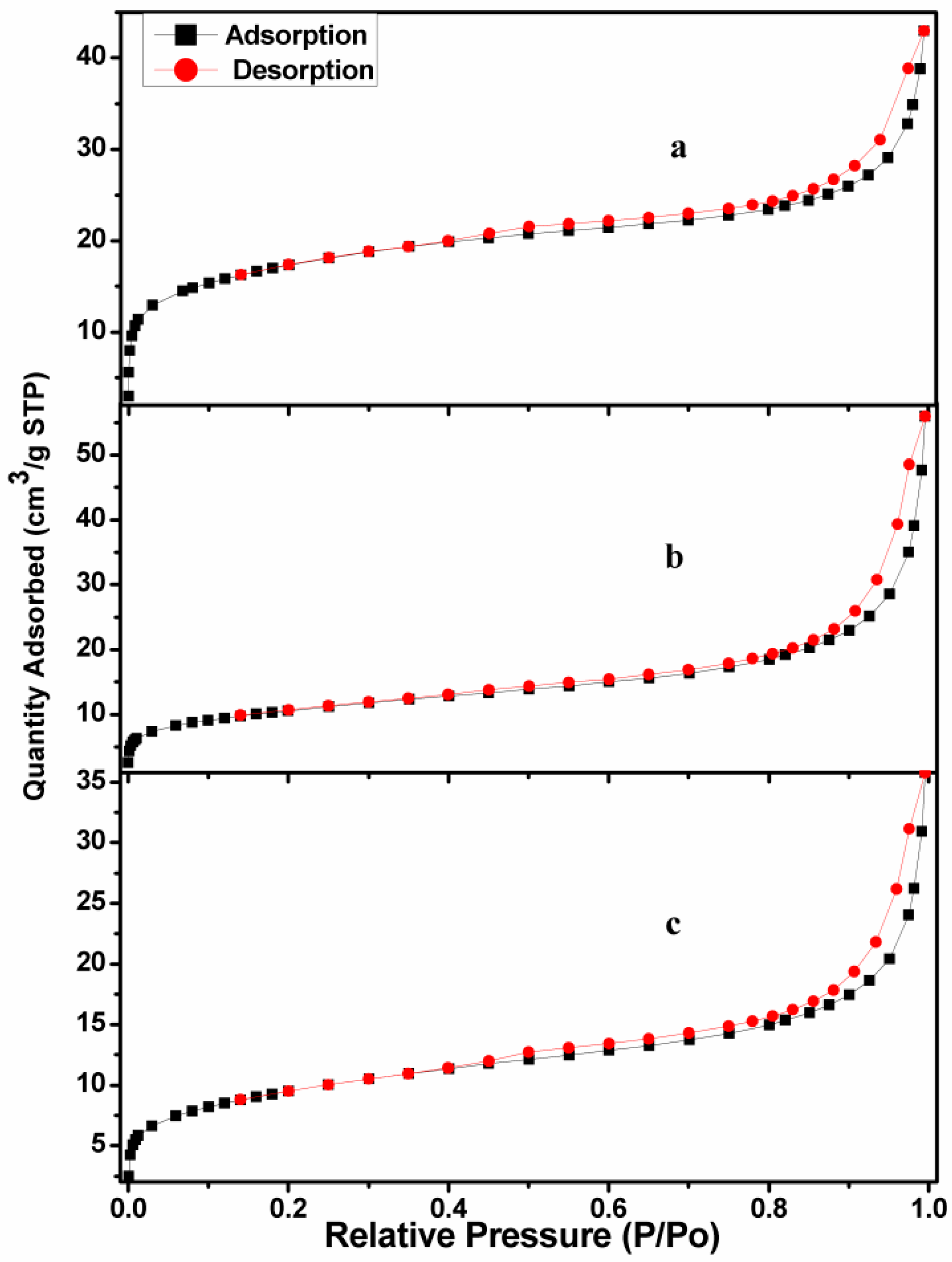
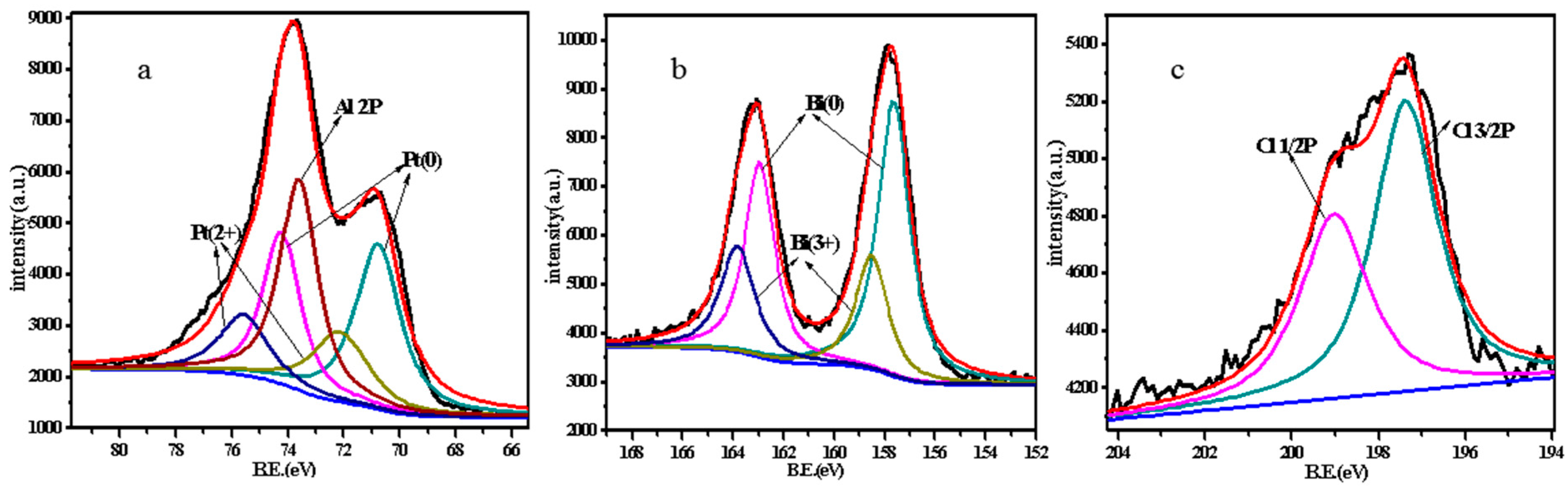
2.1. Catalyst Characterization
2.2. Catalytic Performance
2.2.1. Effect of Bi Content
2.2.2. Effect of Reaction Temperature
2.2.3. Effect of Reaction Time
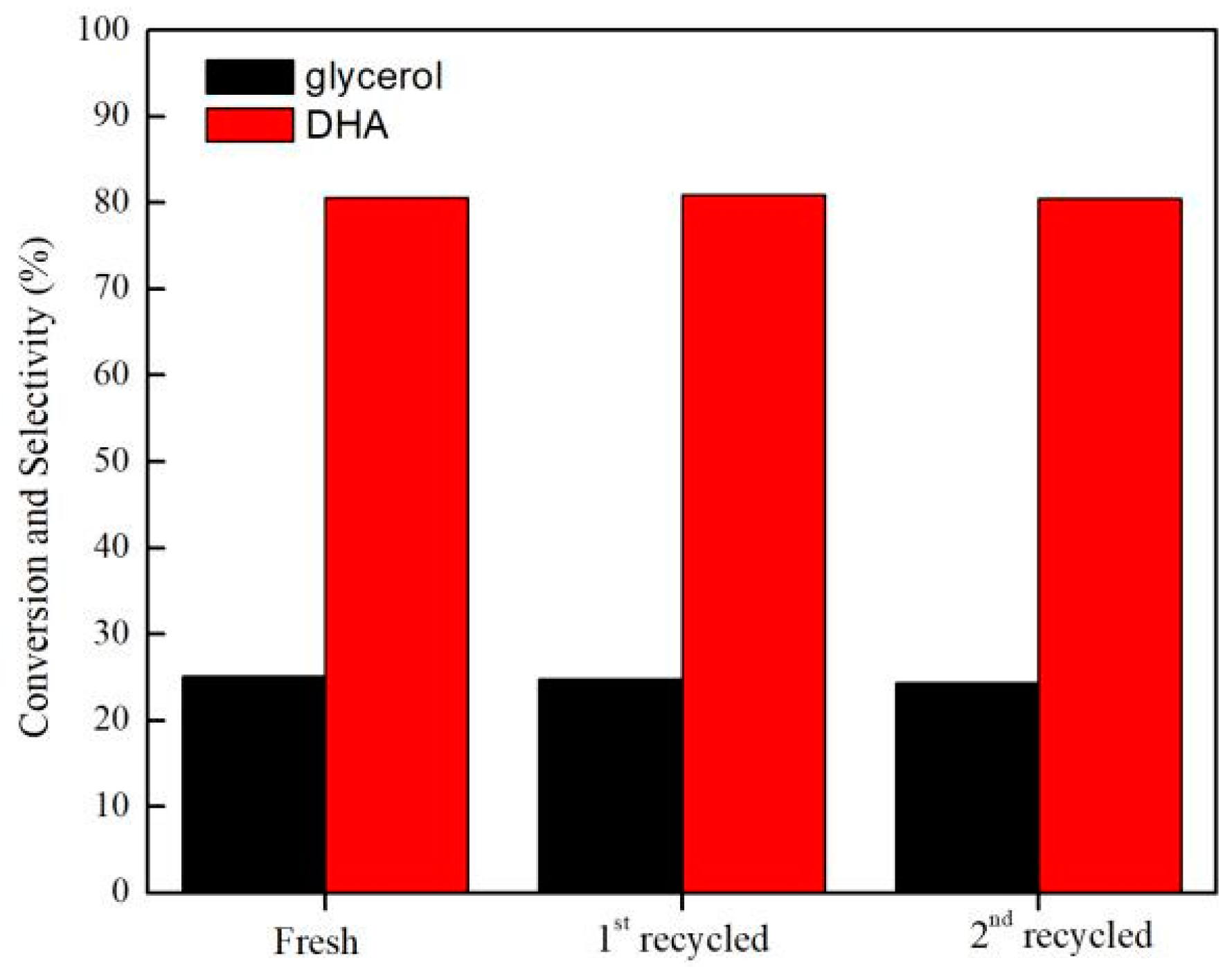
2.2.4. Catalyst Recycling
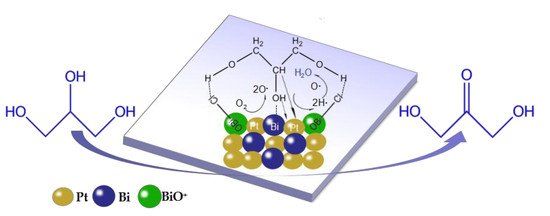
2.2.5. Glycerol Selective Oxidation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of Hydrotalcite (HT)
3.2.2. Metal Loading
3.3. Characterization
3.4. Catalytic Testing
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Brandner, A.; Lehnert, K.; Bienholz, A.; Lucas, M.; Claus, P. Production of biomass-derived chemicals and energy: Chemocatalytic conversions of glycerol. Top. Catal. 2009, 52, 278–287. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol oxidation using gold-containing catalysts. Acc. Chem. Res. 2015, 48, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Gao, J.; Sun, H.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Appl. Catal. B 2011, 106, 423–432. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Upare, P.P.; Le, N.T.; Hwang, Y.K.; Hwang, D.W.; Lee, U.H.; Chang, J.S. Facile synthesis of CeO2-supported gold nanoparticle catalysts for selective oxidation of glycerol into lactic acid. Appl. Catal. A Gen. 2013, 468, 260–268. [Google Scholar] [CrossRef]
- Demirel-Gülen, S.; Lucas, M.; Claus, P. Liquid phase oxidation of glycerol over carbon supported gold catalysts. Catal. Today 2005, 102, 166–172. [Google Scholar] [CrossRef]
- Villa, A.; Gaiassi, A.; Rossetti, I.; Bianchi, C.L.; van Benthem, K.; Veith, G.M.; Prati, L. Au on MgAl2O4 spinels: The effect of support surface properties in glycerol oxidation. J. Catal. 2010, 275, 108–116. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, S.; Li, H.; Ren, Y.; Liu, H. Efficient synthesis of lactic acid by aerobic oxidation of glycerol on Au–Pt/TiO2 catalysts. Chem. Eur. J. 2010, 16, 7368–7371. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Du, Y.; Li, C.; Yang, J.; Yang, G. Insight into effect of acid/base nature of supports on selectivity of glycerol oxidation over supported Au-Pt bimetallic catalysts. Appl. Catal. B 2015, 164, 334–343. [Google Scholar] [CrossRef]
- Brett, G.L.; He, Q.; Hammond, C.; Miedziak, P.J.; Dimitratos, N.; Sankar, M.; Knight, D.W. Selective Oxidation of Glycerol by Highly Active Bimetallic Catalysts at Ambient Temperature under Base-Free Conditions. Angew. Chem. Int. Ed. 2011, 50, 10136–10139. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Sun, K.Q.; Xu, B.Q. Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base. ACS Catal. 2014, 4, 2226–2230. [Google Scholar] [CrossRef]
- Kimura, H.; Tsuto, K.; Wakisaka, T.; Kazumi, Y.; Inaya, Y. Selective oxidation of glycerol on a platinum-bismuth catalyst. Appl. Catal. A Gen. 1993, 96, 217–228. [Google Scholar] [CrossRef]
- Kimura, H. Selective oxidation of glycerol on a platinum-bismuth catalyst by using a fixed bed reactor. Appl. Catal. A Gen. 1993, 105, 147–158. [Google Scholar] [CrossRef]
- Nie, R.; Liang, D.; Shen, L.; Gao, J.; Chen, P.; Hou, Z. Selective oxidation of glycerol with oxygen in base-free solution over MWCNTs supported PtSb alloy nanoparticles. Appl. Catal. B 2012, 127, 212–220. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting role of bismuth and antimony on Pt catalysts for the selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2016, 335, 95–104. [Google Scholar] [CrossRef]
- Xiao, Y.; Greeley, J.; Varma, A.; Zhao, Z.J.; Xiao, G. An experimental and theoretical study of glycerol oxidation to 1,3-dihydroxyacetone over bimetallic Pt-Bi catalysts. AlChE J. 2017, 63, 705–715. [Google Scholar] [CrossRef]
- Hu, W.; Knight, D.; Lowry, B.; Varma, A. Selective oxidation of glycerol to dihydroxyacetone over Pt–Bi/C catalyst: Optimization of catalyst and reaction conditions. Ind. Eng. Chem. Res. 2010, 49, 10876–10882. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Recent advances for layered double hydroxides (LDHs) materials as catalysts applied in green aqueous media. Catal. Today 2015, 247, 163–169. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, M.; Wei, M.; Duan, X. Advances in efficient electrocatalysts based on layered double hydroxides and their derivatives. J. Energy Chem. 2017, 26, 1094–1106. [Google Scholar] [CrossRef]
- Tsuji, A.; Rao, K.T.V.; Nishimura, S.; Takagaki, A.; Ebitani, K. Selective Oxidation of Glycerol by Using a Hydrotalcite-Supported Platinum Catalyst under Atmospheric Oxygen Pressure in Water. ChemSusChem 2011, 4, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Tongsakul, D.; Nishimura, S.; Thammacharoen, C.; Ekgasit, S.; Ebitani, K. Hydrotalcite-supported platinum nanoparticles prepared by a green synthesis method for selective oxidation of glycerol in water using molecular oxygen. Ind. Eng. Chem. Res. 2012, 51, 16182–16187. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Wang, F.; Ding, K.; Zhang, F.; Liu, X.; Xue, Y. Base-free selective oxidation of glycerol with 3% H2O2 catalyzed by sulphonato-salen-chromium(III) intercalated LDH. Catal. Commun. 2012, 28, 73–76. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Li, Y. Promoting effect of WOx on selective hydrogenolysis of glycerol to 1,3-propanediol over bifunctional Pt-WOx/Al2O3 catalysts. J. Mol. Catal. A 2015, 398, 391–398. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, B.; Liu, L.; Dong, J. Platinum Supported on WO3-Doped Aluminosilicate: A Highly Efficient Catalyst for Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. Ind. Eng. Chem. Res. 2017, 56, 11065–11074. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Intramolecular selective hydrogenation of cinnamaldehyde over CeO2–ZrO2-supported Pt catalysts. J. Catal. 2012, 285, 31–40. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Liu, Y.; Zhang, L. Photocatalytic properties BiOCl and Bi2O3nanofibers prepared by electrospinning. Scr. Mater. 2008, 59, 332–335. [Google Scholar] [CrossRef]
- Kwon, Y.; Birdja, Y.; Spanos, I.; Rodriguez, P.; Koper, M.T. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2012, 2, 759–764. [Google Scholar] [CrossRef]
- Mondelli, C.; Ferri, D.; Grunwaldt, J.D.; Krumeich, F.; Mangold, S.; Psaro, R.; Baiker, A. Combined liquid-phase ATR-IR and XAS study of the Bi-promotion in the aerobic oxidation of benzyl alcohol over Pd/Al2O3. J. Catal. 2007, 252, 77–87. [Google Scholar] [CrossRef]
- Roy, I.; Bhattacharyya, A.; Sarkar, G.; Saha, N.R.; Rana, D.; Ghosh, P.P.; Palit, M.; Das, A.R.; Chattopadhyay, D. In situ synthesis of a reduced graphene oxide/cuprous oxide nanocomposite: A reusable catalyst. RSC Adv. 2014, 4, 52044–52052. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, Z.; Dai, Y.; Jia, X.; Yu, H.; Yang, Y. Promoting role of bismuth on carbon nanotube supported platinum catalysts in aqueous phase aerobic oxidation of benzyl alcohol. Appl. Catal. B 2016, 181, 118–126. [Google Scholar] [CrossRef]
- Adachi-Pagano, M.; Forano, C.; Besse, J.-P. Synthesis of Al-rich hydrotalcite-like compounds by using the urea hydrolysis reaction—Control of size and morphology. J. Mater. Chem. 2003, 13, 1988–1993. [Google Scholar] [CrossRef]

| Pt–xBi/HT | Conversion (%) | Product Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OA | TA | HYPAC | GLYAC | GLYALD | GLYCAC | DHA | AA | ||
| Pt/HT | 12.4 | 0.2 | 0.4 | 0.0 | 11.8 | 38.8 | 5.7 | 38.3 | 4.8 |
| Pt–1Bi/HT | 21.5 | 2.6 | 2.1 | 5.5 | 9.3 | 11.4 | 0.8 | 68.0 | 0.3 |
| Pt–3Bi/HT | 21.0 | 2.0 | 1.8 | 5.4 | 8.4 | 11.8 | 0.7 | 69.6 | 0.3 |
| Pt–5Bi/HT | 27.3 | 1.9 | 2.1 | 5.4 | 7.9 | 11.9 | 1.0 | 69.7 | 0.1 |
| Pt–7Bi/HT | 25.1 | 1.1 | 2.0 | 2.0 | 2.9 | 10.6 | 0.7 | 80.6 | 0.1 |
| Pt–9Bi/HT | 19.5 | 1.8 | 1.6 | 4.0 | 7.5 | 11.4 | 0.7 | 73.0 | 0.0 |
| Temperature (°C) | Conversion (%) | Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OA | TA | HYPAC | GLYAC | GLYALD | GLYCAC | DHA | AA | ||
| 30 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 | 14.9 | 2.9 | 0.7 | 3.6 | 5.8 | 21.3 | 0.5 | 65.2 | 0.0 |
| 70 | 25.1 | 1.1 | 2.0 | 2.0 | 2.9 | 10.6 | 0.7 | 80.6 | 0.1 |
| 90 | 35.6 | 1.8 | 2.5 | 1.8 | 3.5 | 10.5 | 1.0 | 78.7 | 0.2 |
| Time (h) | Conversion (%) | Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OA | TA | HYPAC | GLYAC | GLYALD | GLYCAC | DHA | AA | ||
| 4 | 18.2 | 1.3 | 1.1 | 4.6 | 0.0 | 22.8 | 0.3 | 69.8 | 0.3 |
| 6 | 25.1 | 1.1 | 2.0 | 2.0 | 2.9 | 10.6 | 0.7 | 80.6 | 0.1 |
| 8 | 31.4 | 5.3 | 4.0 | 6.3 | 8.5 | 8.5 | 1.1 | 66.2 | 0.1 |
| 12 | 42.5 | 6.0 | 5.0 | 7.1 | 10.2 | 8.0 | 1.2 | 62.4 | 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.; Wang, Z.; Liang, Y.; Xu, H.; Liu, L.; Dong, J. Promoting Role of Bismuth on Hydrotalcite-Supported Platinum Catalysts in Aqueous Phase Oxidation of Glycerol to Dihydroxyacetone. Catalysts 2018, 8, 20. https://doi.org/10.3390/catal8010020
Xue W, Wang Z, Liang Y, Xu H, Liu L, Dong J. Promoting Role of Bismuth on Hydrotalcite-Supported Platinum Catalysts in Aqueous Phase Oxidation of Glycerol to Dihydroxyacetone. Catalysts. 2018; 8(1):20. https://doi.org/10.3390/catal8010020
Chicago/Turabian StyleXue, Wenjie, Zenglong Wang, Yu Liang, Hong Xu, Lei Liu, and Jinxiang Dong. 2018. "Promoting Role of Bismuth on Hydrotalcite-Supported Platinum Catalysts in Aqueous Phase Oxidation of Glycerol to Dihydroxyacetone" Catalysts 8, no. 1: 20. https://doi.org/10.3390/catal8010020