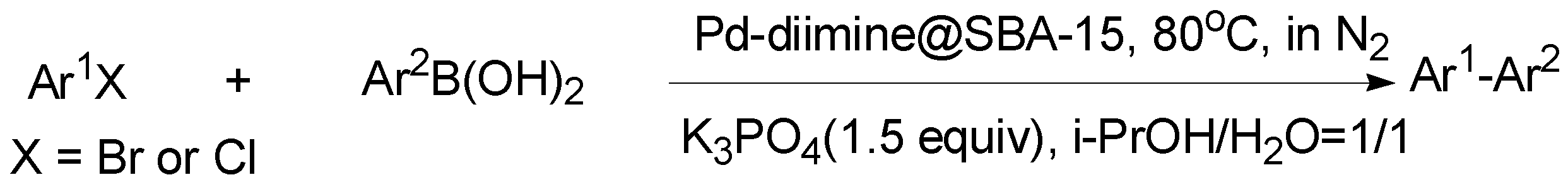Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction
Abstract
:1. Introduction
2. Results and Discussions
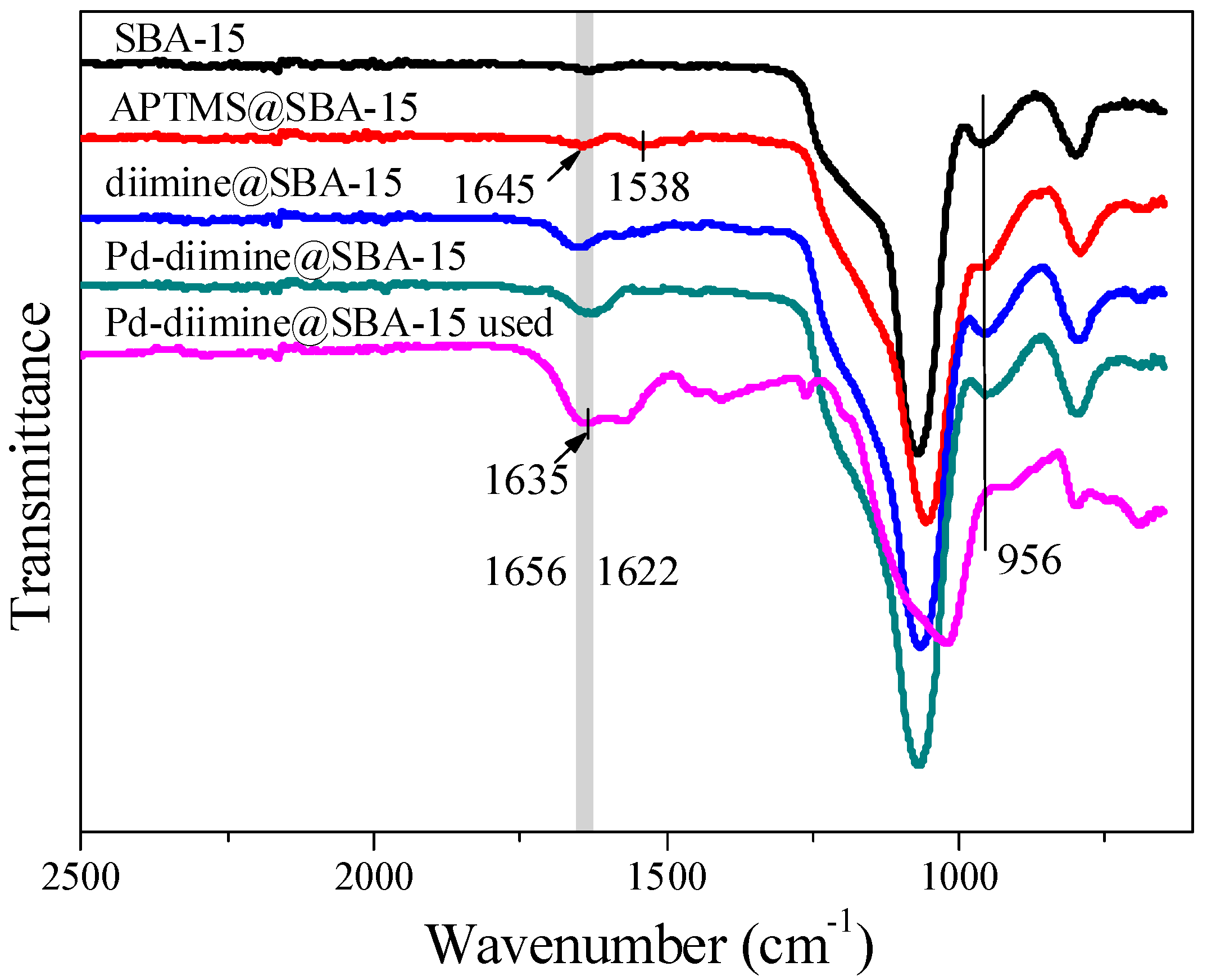
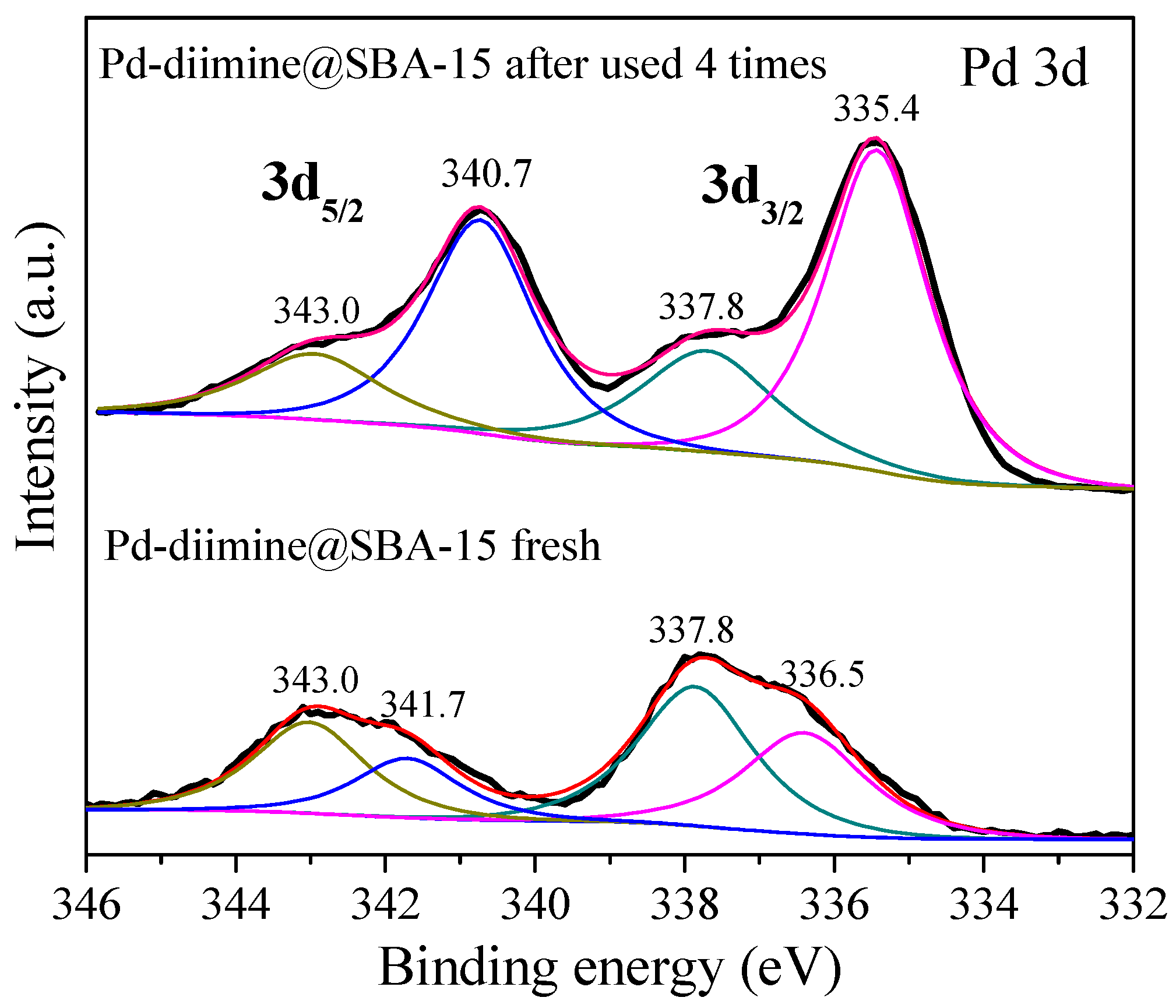
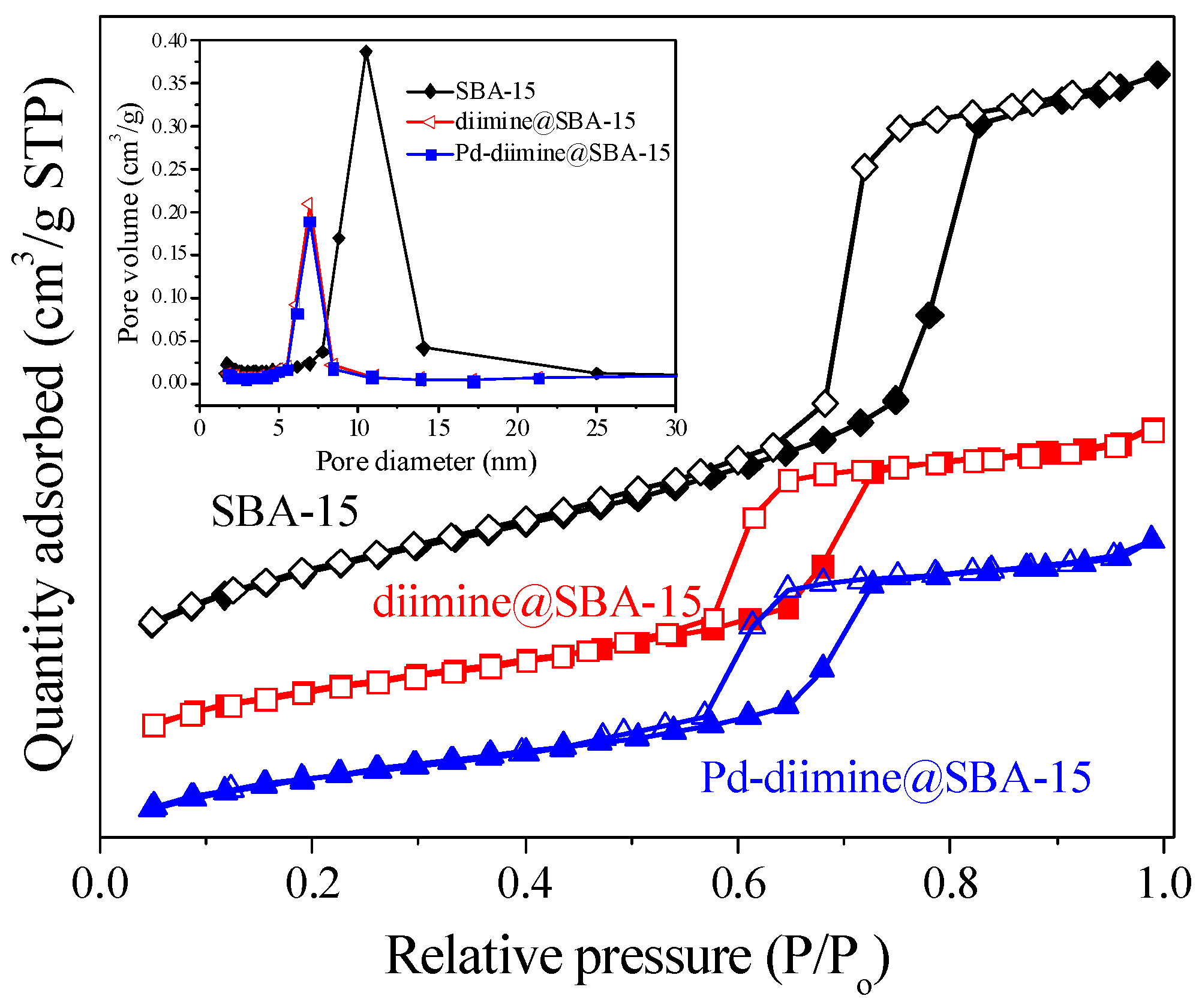
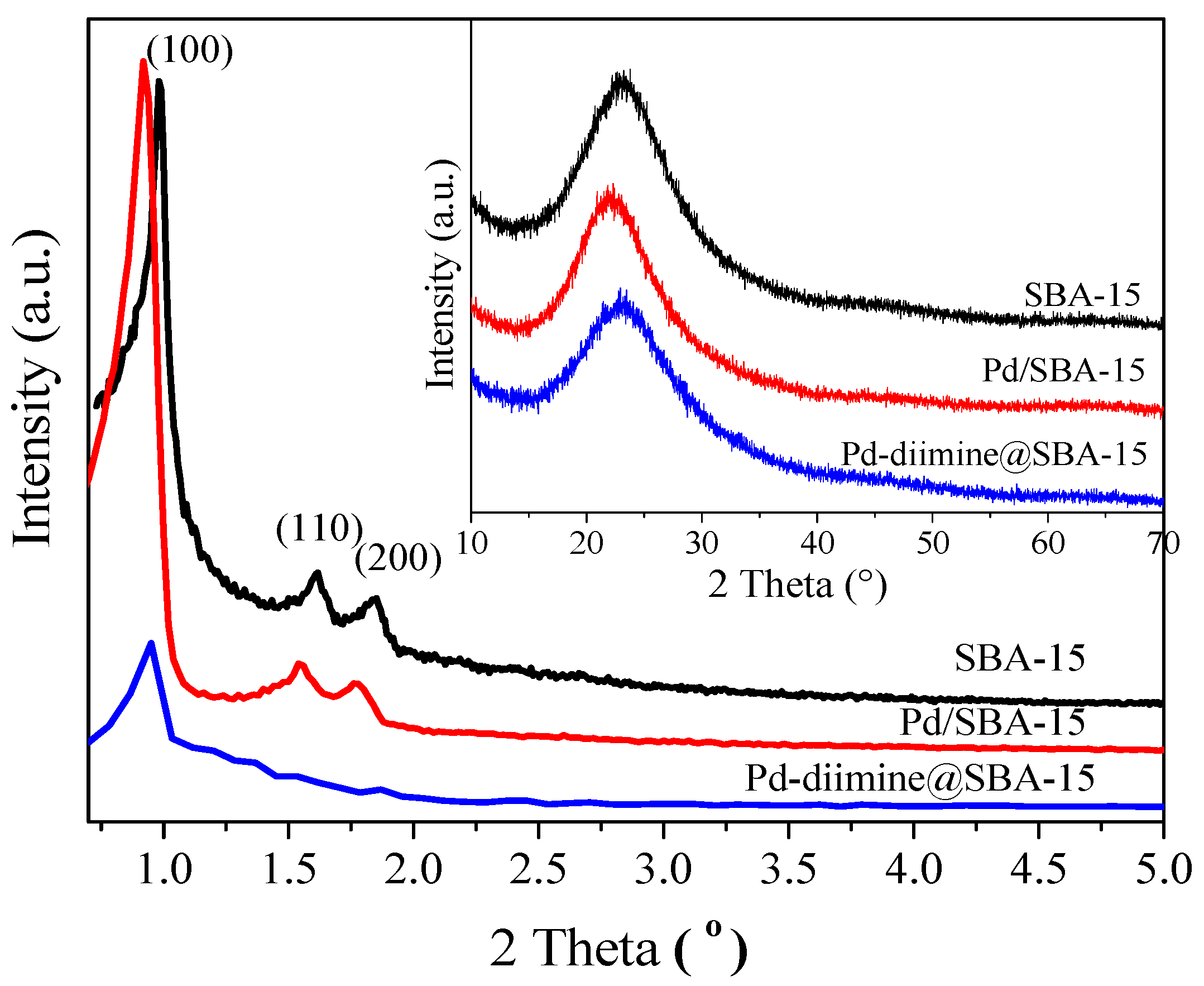
2.1. Structure and Textural Properties of Samples
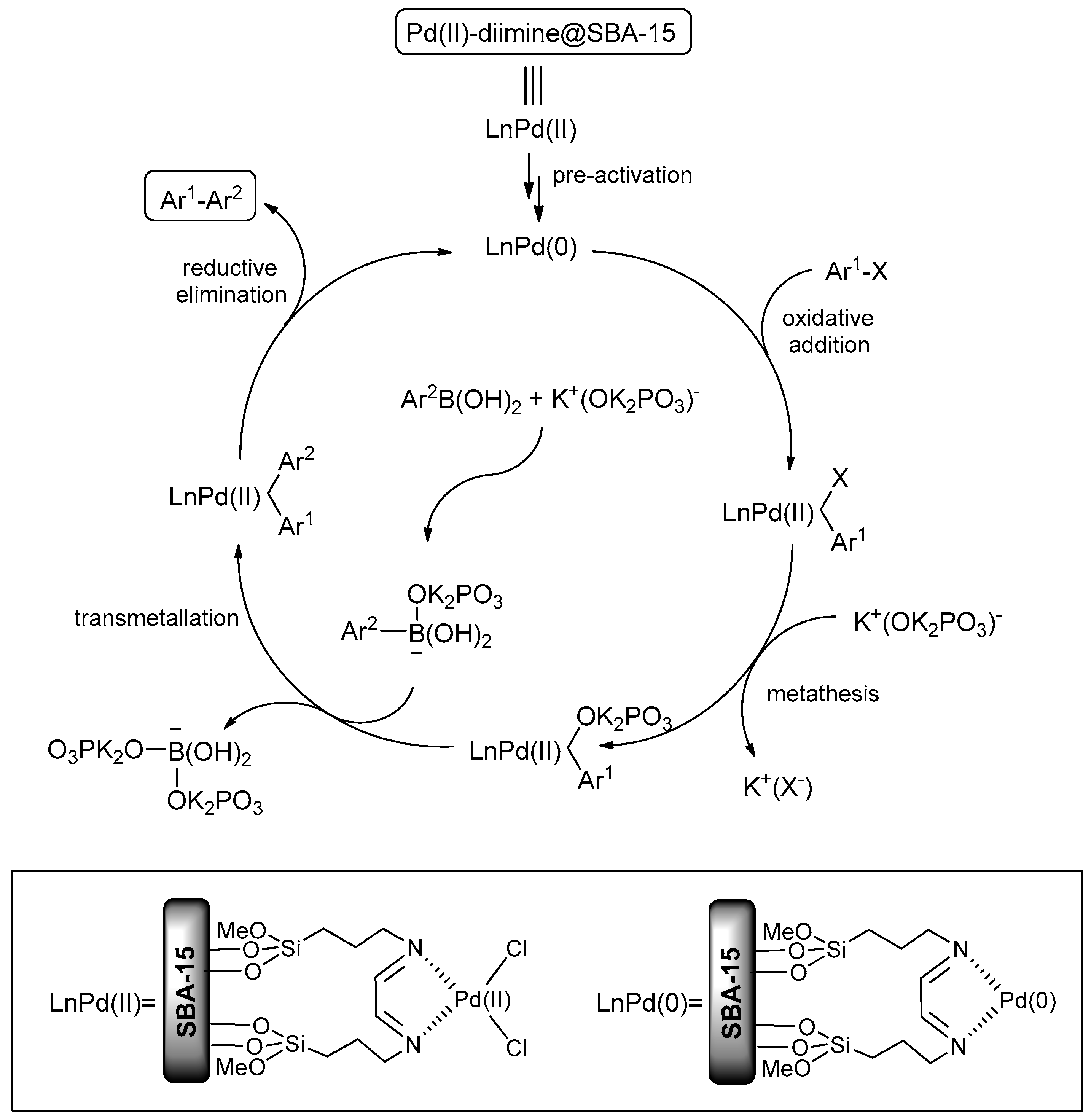
2.2. Catalytic Activities of Pd-Diimine@SBA-15 for Suzuki Coupling Reactions
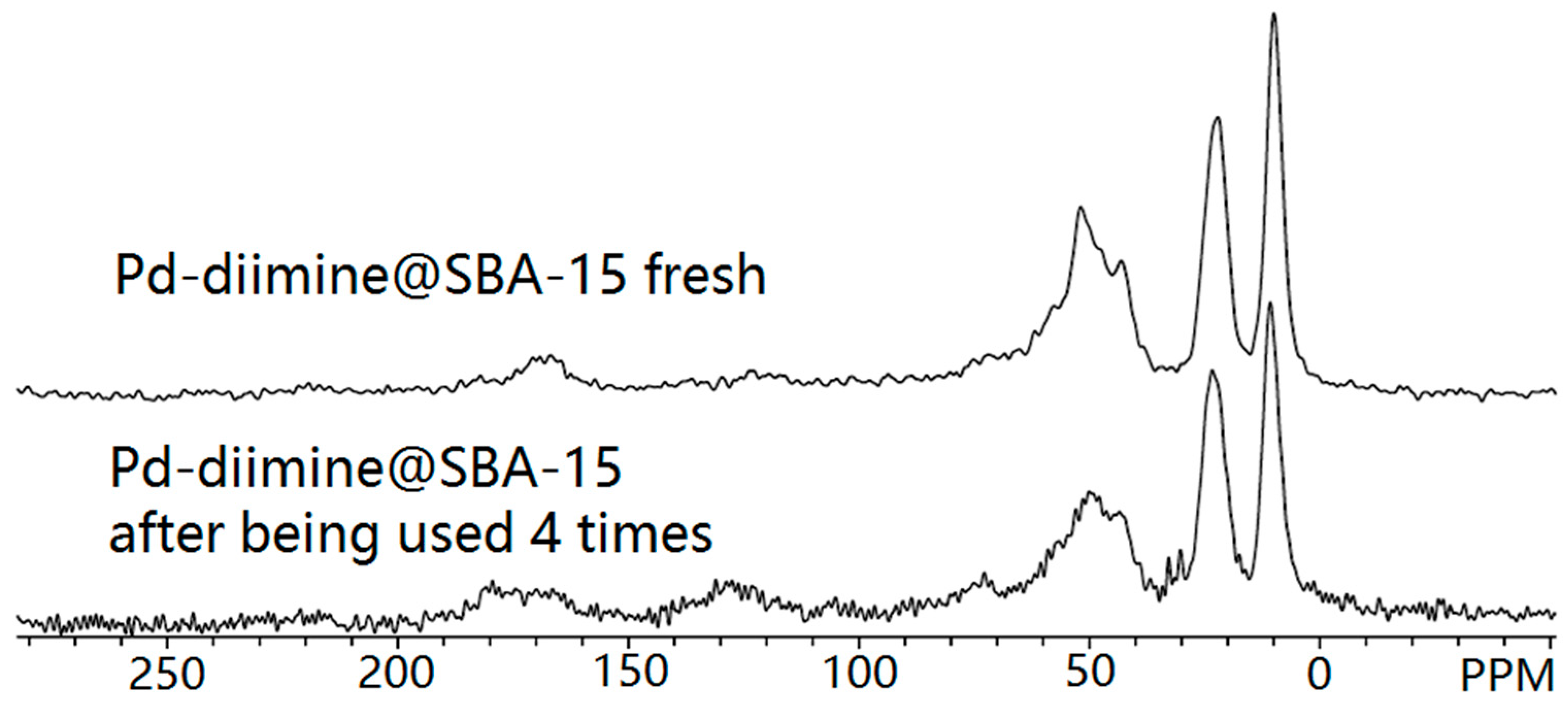
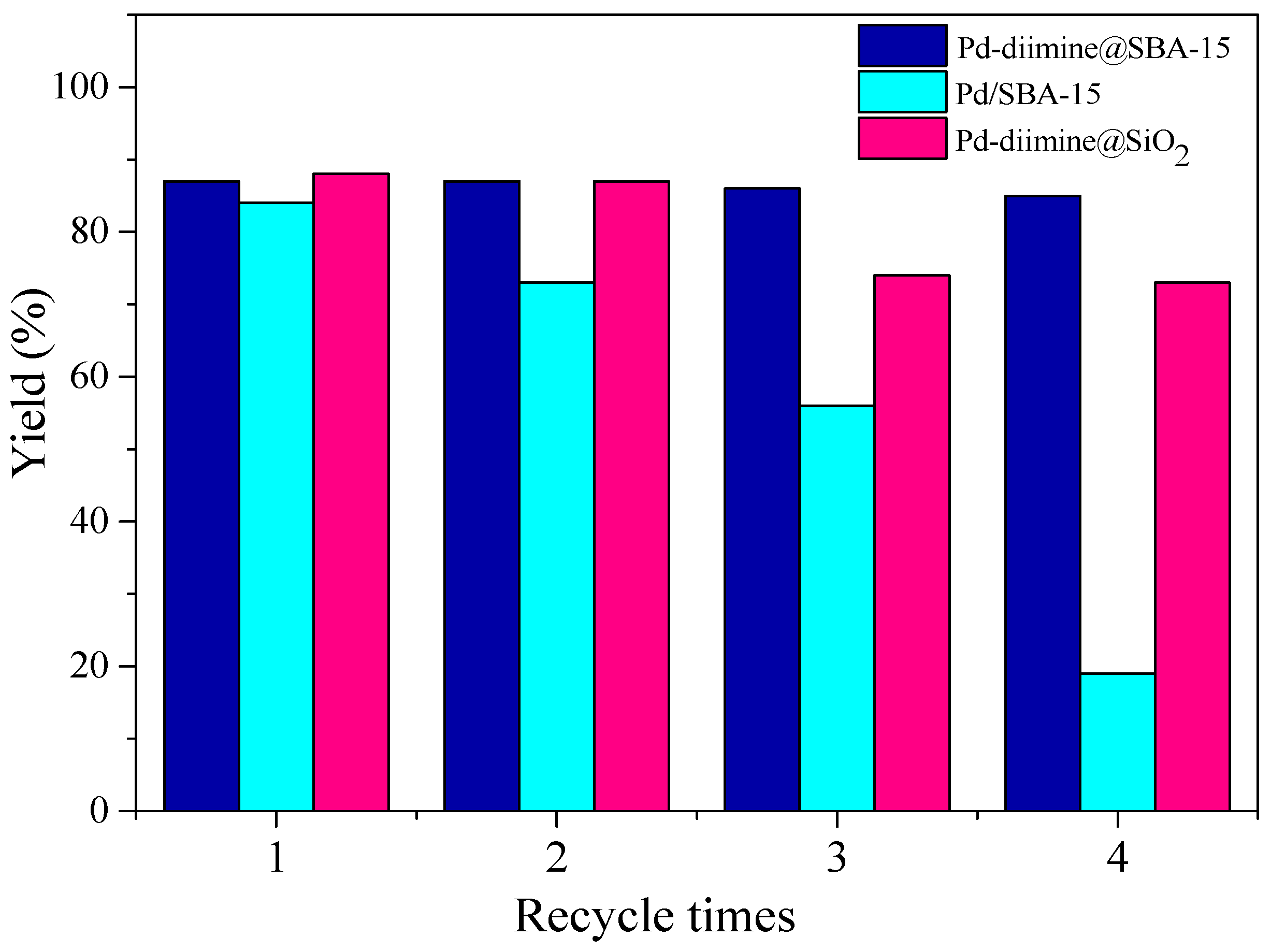
2.3. Repeated Use of the Pd-Diimine@SBA-15 Catalyst
3. Experimental Section
3.1. Synthesis of Materials
3.2. Characterization of Sample
3.3. Suzuki Coupling Reactions of Aryl Halides over Supported Pd Catalyst
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S.P. Cross-coupling and dehalogenation reactions catalyzed by (N-heterocyclic carbene) Pd (allyl) Cl complexes. J. Org. Chem. 2004, 69, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.A.C.; Morgon, N.H.; Ujaque, G.; Maseras, F. Computational characterization of the role of the base in the Suzuki-Miyauracross-Coupling Reaction. J. Am. Chem. Soc. 2005, 127, 9298–9307. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Lutz, M.; Spek, A.L.; Koten, G.V.; KleinGebbink, R.J.M. Bimetallic η6,η1-and PCP-pincer Ruthenium Palladium complexes: Synthesis, structure, and catalytic activity. Organometallics 2010, 29, 1157–1167. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Coordination singularities of a bis(p-xylyl)bis(benzimidazolylidene) ligand and the bis-iridium and rhodium-related complexes. Organometallics 2013, 32, 1112–1120. [Google Scholar] [CrossRef]
- Shen, A.; Ni, C.; Cao, Y.C.; Zhou, H.; Song, G.H.; Ye, X.F. Novel monoligated imine-Pd–NHC complexes: Extremely active pre-catalysts for Suzuki-Miyaura coupling of aryl chlorides. Tetrahedron Lett. 2014, 55, 3278–3282. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, Selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar]
- Narayanan, R.; El-Sayed, M.A. Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. J. Am. Soc. Chem. 2003, 125, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Effect of colloidal catalysis on the nanoparticle size distribution: Dendrimer-Pd vs. PVP-Pd nanoparticles catalyzing the Suzuki coupling reaction. J. Phys. Chem. B 2004, 108, 8572–8580. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Bahramian, B.; Jajarmi, S. Suzuki, Heck, and copper-free Sonogashira reactions catalyzed by 4-amino-5-methyl-3-thio-1, 2, 4-triazole-functionalized polystyrene resin-supported Pd (II) under aerobic conditions in water. J. Org. Chem. 2013, 724, 206–212. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Shokouhimehr, M.; Lee, Y.S. Polymer-Supported N-Heterocyclic Carbene-Palladium Complex for Heterogeneous Suzuki Cross-Coupling Reaction. J. Org. Chem. 2005, 70, 6714–6720. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Kim, J.H.; Lee, Y.S. Heterogeneous Heck Reaction Catalyzed by Recyclable Polymer-Supported N-Heterocyclic Carbene-Palladium Complex. Synlett 2006, 37, 618–620. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J. Mol. Catal. A Chem. 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Jun, S.W.; Shokouhimehr, M.; Lee, D.J.; Jang, Y.; Park, J.; Hyeon, T. One-pot synthesis of magnetically recyclable mesoporous silica supported acid–base catalysts for tandem reactions. Chem. Commun. 2013, 49, 7821–7823. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium Nanocatalysts Confined in Mesoporous Silica for Heterogeneous Reduction of Nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Liotta, L.F.; Salvo, A.M.P.; Giacalone, F.; Parola, V.L.; Aprile, C.; Noto, R. Multi-Layered, covalently supported ionic liquid phase (mlc-SILP) as highly cross-linked support for recyclable palladium catalysts for the Suzuki reaction in aqueous medium. Adv. Synth. Catal. 2011, 353, 2119–2130. [Google Scholar] [CrossRef]
- Wang, P.Y.; Wang, Z.Y.; Li, J.G.; Bai, Y.X. Preparation, characterization, and catalytic characteristic of Pdnanoparticles encapsulated in mesoporoussilica. Microp. Mesop. Mater. 2008, 116, 400–405. [Google Scholar] [CrossRef]
- Ma, C.Y.; Dou, B.J.; Li, J.J.; Cheng, J.; Hu, Q.; Hao, Z.P.; Qiao, S.Z. Catalytic oxidation of benzyl alcohol on Au or Au-Pdnanoparticles confined in mesoporoussilica. Appl. Catal. B Environ. 2009, 92, 202–208. [Google Scholar] [CrossRef]
- Mubofu, E.B.; Clark, J.H.; Macquarrie, D.J. A novel Suzuki reaction system based on a supported palladium catalyst. Green Chem. 2001, 3, 23–25. [Google Scholar] [CrossRef]
- Paul, S.; Clark, J.H. Highly active and reusable heterogeneous catalyst for the Suzuki reaction: Synthesis of biaryls and polyaryls. Green Chem. 2003, 5, 635–638. [Google Scholar] [CrossRef]
- Sarmah, C.; Sahu, D.; Das, P. Anchoring palladium acetate onto imine-functionalized silica gel through coordinative attachment: An effective recyclable catalyst for the Suzuki-Miyaura reaction in aqueous-isopropanol. Catal. Today 2012, 198, 197–203. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Ledebt, A.; Mammeri, F.; Herbst, F.; Carbonnier, B.; Ben Romdhane, H.; Delamar, M.; Chehimi, M.M. Hairy carbon nanotube@nano-Pd heterostructures: Design, characterization, and application in Suzuki C-C coupling reaction. Langmuir 2010, 26, 16115–16121. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.Q.; Ou, S.; Zou, C.; Wu, C.D. Assembly and post-modification of a metal–organic nanotube for highly efficient catalysis. J. Am. Chem. Soc. 2012, 134, 19851–19857. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Yin, J.W.; Chai, W.; Liang, C.; Shen, J.; Zhang, F. Multifunctional mesoporous silica supported palladium nanoparticles as efficient and reusable for water-medium Ullmann reaction. New J. Chem. 2012, 36, 1378–1384. [Google Scholar] [CrossRef]
- Yang, H.Q.; Han, X.J.; Li, G.; Wang, Y.W. N-Heterocyclic carbenepalladium complex supported on ionic liquid-modified SBA-16: An efficient and highly recyclable catalyst for the Suzuki and Heck reactions. Green Chem. 2009, 11, 1184–1193. [Google Scholar] [CrossRef]
- Yang, H.Q.; Han, X.J.; Li, G.; Ma, Z.C.; Hao, Y.J. Mesoporous ethane-silicas functionalized with a Bulky N-Heterocyclic carbene for Suzuki-Miyaura coupling of aryl chlorides and benzyl chlorides. J. Phys. Chem. C 2010, 114, 22221–22229. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Hemmati, S.; Veisi, H. Pd Immobilized on amidioxime- functionalized mesoporous SBA-15: A novel and highly active heterogeneous catalyst for Suzuki-Miyauracoupling reactions. J. Mol. Catal. A Chem. 2014, 393, 240–247. [Google Scholar] [CrossRef]
- Crudden, C.M.; Sateesh, M.; Lewis, R. Mercaptopropyl-modified mesoporoussilica: A remarkable support for the preparation of a reusable, heterogeneous palladium catalyst for coupling reactions. J. Am. Chem. Soc. 2005, 127, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.D.; MacQuarrie, S.; McEleney, K.; Crudden, C.M. Mesoporous Silica-Supported Pd Catalysts: An Investigation into Structure, Activity, Leaching and Heterogeneity. J. Catal. 2007, 252, 97–109. [Google Scholar] [CrossRef]
- Veisi, H.; Hamelian, M.; Hemmati, S. Palladium anchored to SBA-15 functionalized with melamine-pyridine groups as anovel and efficient heterogeneous nanocatalyst for Suzuki-Miyaura coupling reactions. J. Mol. Catal. A Chem. 2014, 395, 25–33. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, P.; Yus, M. Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. [Google Scholar] [CrossRef]
- Bass, J.D.; Solovyov, A.; Pascall, A.J.; Katz, A. Acid-basebifunctional and dielectric outer-sphere effects in heterogeneous catalysis: A comparative investigation of model primary amine catalysts. J. Am. Chem. Soc. 2006, 128, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gao, Q.; Yu, H.; Chen, Y.; Deng, F. Intensively competitive adsorption for heavy metal ions by PAMAM-SBA-15 and EDTA-PAMAM-SBA-15 inorganic–organic hybrid materials. Microp. Mesop. Mater. 2007, 103, 316–324. [Google Scholar] [CrossRef]
- Poel, H.V.D.; Koten, G.V.; Vrieze, K. Novel bonding modes of α-diimines. Synthesis and characterization of [MCl2L(α-diimine)] and [MCl2(α-diimine)n] (M = Pd, Pt; L = phosphine, arsine; n = 1, 2) containing σ,σ-N,N’, σ-N, or σ-N ↔ σ-N’ bonded α-diimines. Inorg. Chem. 1980, 19, 1145–1151. [Google Scholar] [CrossRef]
- Zhang, G.H.; Wang, P.Y.; Wei, X.F. Palladium supported on functionalized mesoporous silica as an efficient catalyst for Suzuki—Miyauracoupling reaction. Catal. Lett. 2013, 143, 1188–1194. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Hampsey, J.E.; Jiang, N.; Li, C.J.; Lu, Y.F. Surfactant-Templated organic functionalized mesoporous silica with phosphinoligands. Chem. Mater. 2005, 17, 1561–1569. [Google Scholar] [CrossRef]
- Shi, X.J.; Ji, S.F.; Wang, K.; Li, C.Y. Oxidative Dehydrogenation of Ethane with CO2 over Novel Cr/SBA-15/Al2O3/FeCrAl Monolithic Catalysts. Energy Fuels 2008, 22, 3631–3638. [Google Scholar] [CrossRef]
- Navarro, O.; Marion, N.; Oonishi, Y.; Kelly, R.A.; Nolan, S.P. Suzuki-Miyaura, α-Ketone Arylation and Dehalogenation Reactions Catalyzed by a Versatile N-Heterocyclic Carbene-Palladacycle Complex. J. Org. Chem. 2006, 71, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, H.; Yu, Y.; Cao, C.Y.; Song, W.G. One-Pot multistep cascade reactions over multifunctional nanocomposites with Pd nanoparticles supported on amine-modified mesoporous silica. Chem. Asian J. 2013, 8, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Taladriz-Blanco, P.; Hervés, P.; Pérez-Juste, J. Supported Pd Nanoparticles for Carbon-Carbon Coupling Reactions. Top. Catal. 2013, 56, 1154–1170. [Google Scholar]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Melero, J.A.; Stucky, G.D.; Grieken, R.V.; Morales, G. Direct syntheses of ordered SBA-15 mesoporous materials containing arenesulfonic acid groups. J. Mater. Chem. 2002, 12, 1664–1670. [Google Scholar] [CrossRef]
- Isfahani, A.L.; Mohammadpoor-Baltork, I.; Mirkhani, V.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Kia, R. Palladium nanoparticles immobilized on nano-Silica triazinedendritic polymer (Pdnp-nSTDP): An efficient and reusable catalyst for Suzuki-Miyauracross-coupling and Heck reactions. Adv. Synth. Catal. 2013, 355, 957–972. [Google Scholar] [CrossRef]

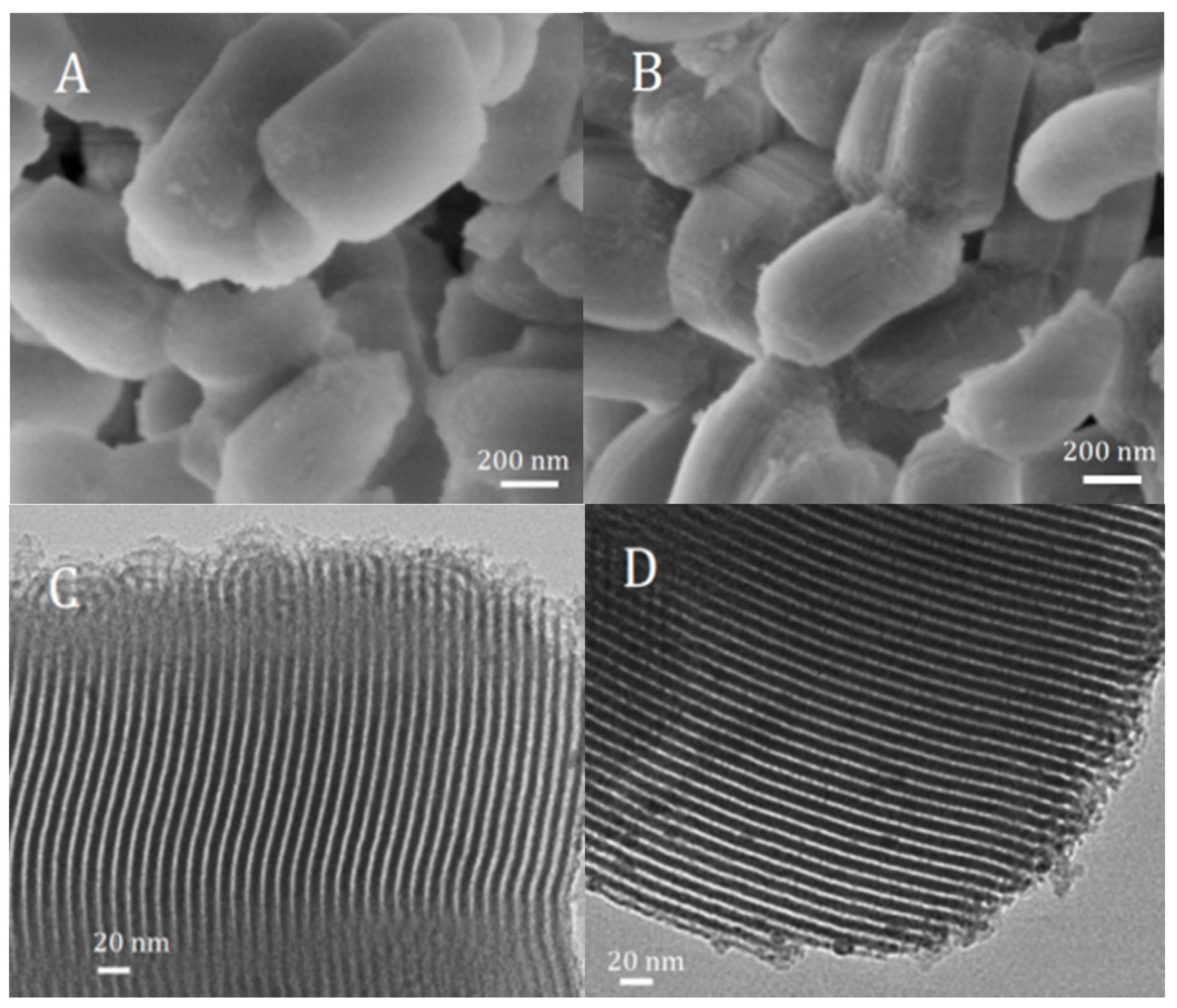

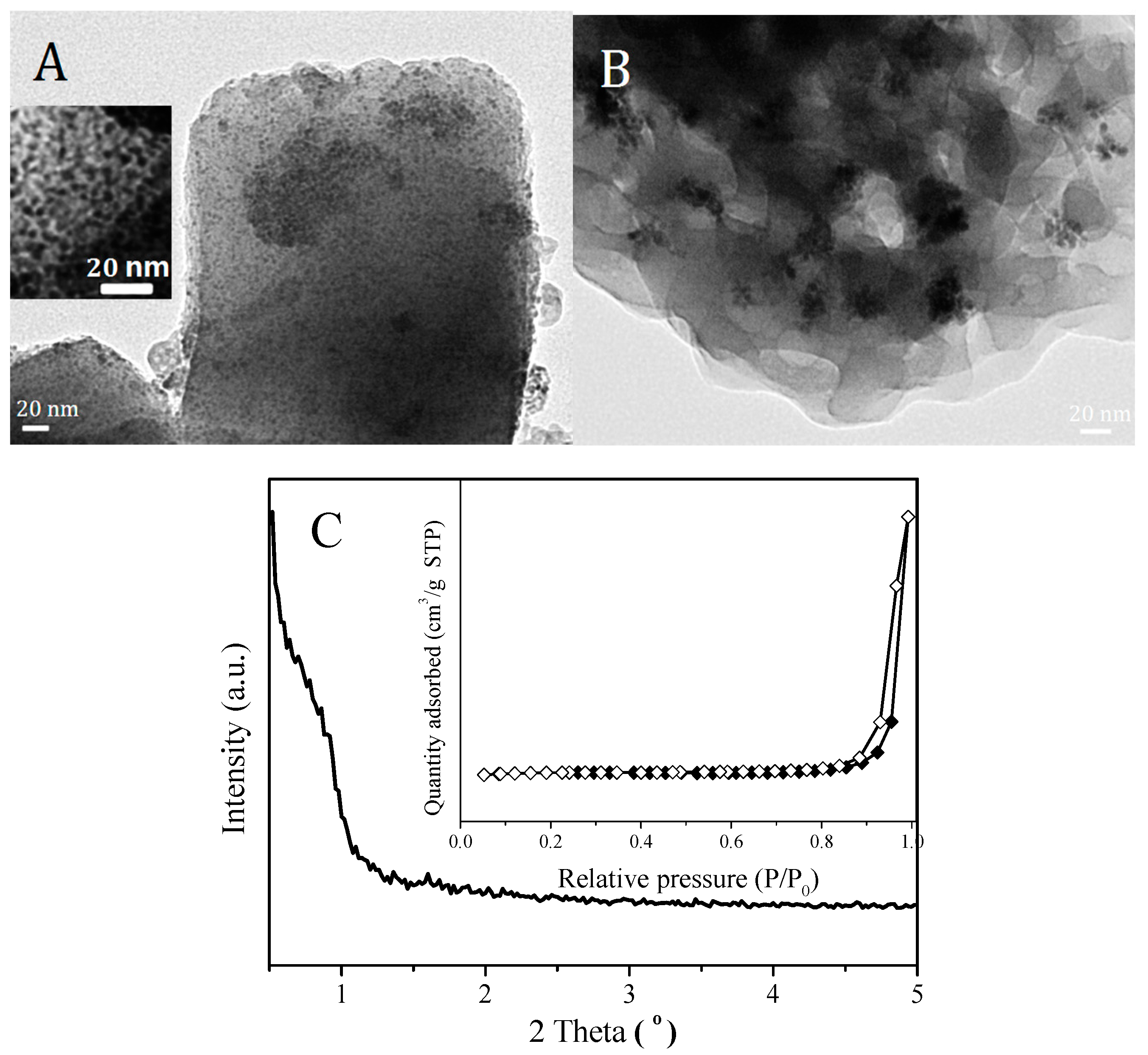
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | |
|---|---|---|---|---|
| Average | Most Probable | |||
| SBA-15 | 641 | 0.96 | 6.35 | 10.5 |
| diimine@SBA-15 | 398 | 0.53 | 5.58 | 6.90 |
| Pd-diimine@SBA-15 | 351 | 0.48 | 5.50 | 6.90 |
| Entry | Catalyst | Base | Time (h) | Conversion (%) | Selectivity (%) | Yield (%) (b) |
|---|---|---|---|---|---|---|
| 1 | SBA-15 | K2CO3 | 12 | 0 | 0 | 0 |
| 2 | PdCl2 (0.05 mol% Pd) | K2CO3 | 12 | 85 | 100 | 85 |
| 3 | Pd-diimine@SBA-15 | K2CO3 | 24 | 84 | 100 | 84 |
| 4 | Pd-diimine@SBA-15 | K3PO4 | 12 | 88 | 100 | 88 |
| 5 | Pd-diimine@SBA-15 | KOH | 4 | 91 | 100 | 91 |
| Entry | Aryl X | Boronic acid | Time (h) | Conversion (%) | Selectivity (%) | Yield (%) (b) |
|---|---|---|---|---|---|---|
| 1 | 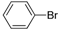 | 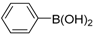 | 12 | 87 | 100 | 87 (87) |
| 2 | 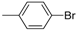 |  | 12 | 88 | 100 | 88 (84) |
| 3 |  |  | 8 | 91 | 100 | 91 (87) |
| 4 | 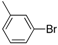 |  | 8 | 91 | 100 | 91 (88) |
| 5 |  |  | 6 | 87 | 100 | 87 (79) |
| 6 |  |  | 10 | 84 | 100 | 84 (77) |
| 7 | 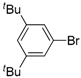 |  | 3 | 88 | 100 | 88 (81) |
| 8 |  |  | 4 | 99 | 100 | 99 (96) |
| 9 | 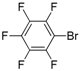 |  | 12 | 83 | 33 | 27 |
| 10 | 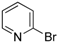 |  | 4 | 13 | 100 | 13 |
| 11 |  |  | 4 | 2 | 100 | 2 (c) |
| 12 | 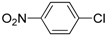 |  | 4 | 20 | 100 | 20 (c) |
| Catalyst | Cycle Times | Yield (b) (%) | Pd Leaching (ppm) | Pd leaching/Pd Loading (%) |
|---|---|---|---|---|
| Pd-diimine@SBA-15 (5.8wt % Pd) | 1 | 87 | 3.7 | 1.6 |
| 2 | 87 | 3.0 | 1.3 | |
| 3 | 86 | 2.2 | 0.9 | |
| 4 | 85 | 1.9 | 0.8 | |
| Pd/SBA-15 (4.7wt % Pd) | 1 | 84 | 23 | 12.2 |
| 2 | 73 | 13 | 6.9 | |
| 3 | 56 | 23 | 12.2 | |
| 4 | 19 | 9.1 | 4.8 | |
| Pd-diimine@SiO2 (5.2wt % Pd) | 1 | 88 | - | - |
| 2 | 87 | - | - | |
| 3 | 74 | - | - | |
| 4 | 73 | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Shen, A.; Cao, Y.; Lu, G. Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction. Catalysts 2016, 6, 181. https://doi.org/10.3390/catal6120181
Yu J, Shen A, Cao Y, Lu G. Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction. Catalysts. 2016; 6(12):181. https://doi.org/10.3390/catal6120181
Chicago/Turabian StyleYu, Jiahuan, An Shen, Yucai Cao, and Guanzhong Lu. 2016. "Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction" Catalysts 6, no. 12: 181. https://doi.org/10.3390/catal6120181
APA StyleYu, J., Shen, A., Cao, Y., & Lu, G. (2016). Preparation of Pd-Diimine@SBA-15 and Its Catalytic Performance for the Suzuki Coupling Reaction. Catalysts, 6(12), 181. https://doi.org/10.3390/catal6120181