Catalytic Studies of Sodium Hydroxide and Carbon Monoxide Reaction
Abstract
:1. Introduction
2. Experimental Study
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Reaction Study
3. Results and Discussion
3.1. Characterization of Catalysts
3.1.1. Catalysts Crystallite Size
| Ball milling time (h) | Al2O3 Mixed nickel (Å) | Magnetite (Å) | Raney nickel (Å) |
|---|---|---|---|
| 0 | - | Micron size | 413 |
| 1 | 184 | 181 | - |
| 2 | 158 | 169 | 209 |
| 4 | 150 | - | 190 |
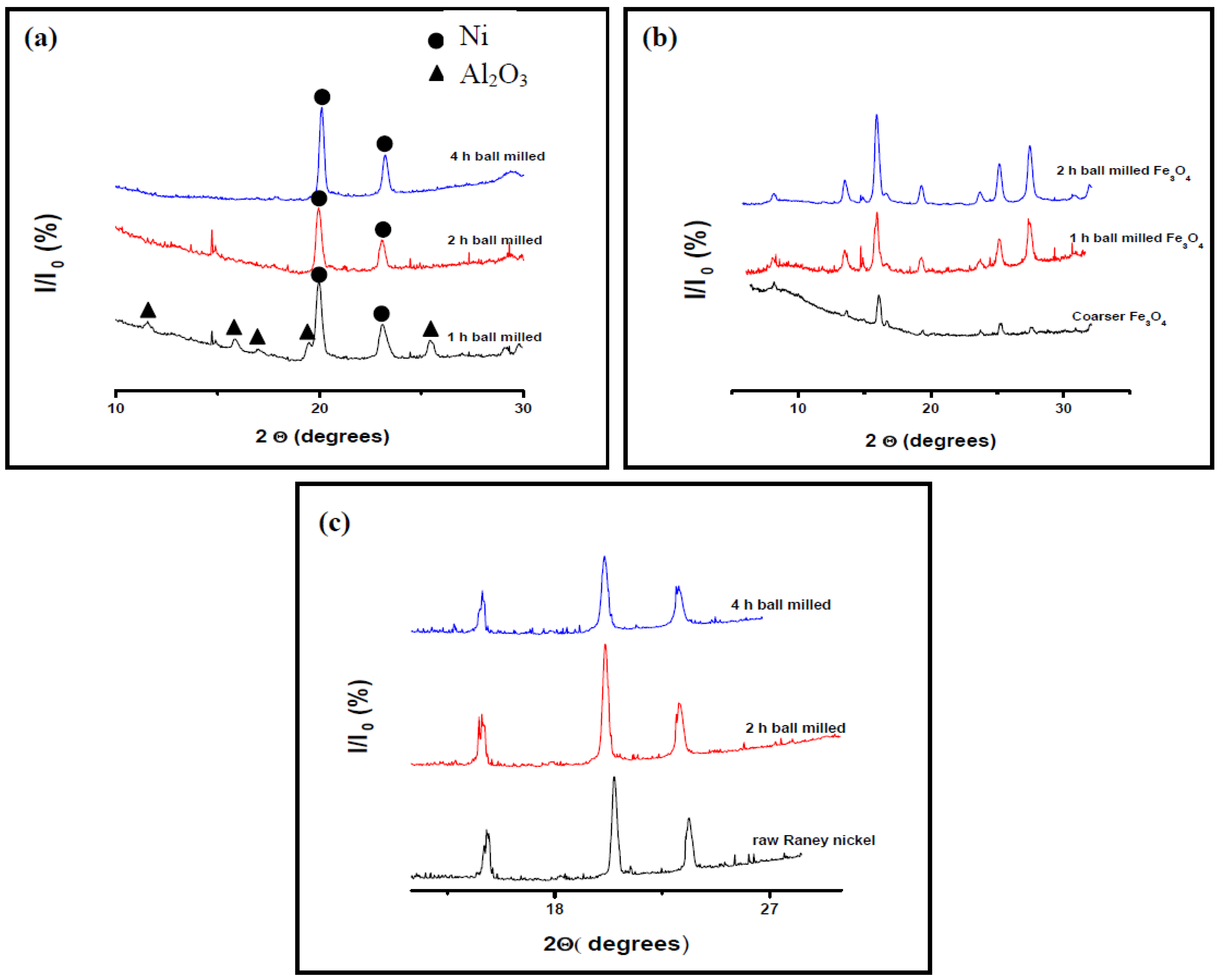
3.1.2. Morphology and Particle Size Analysis
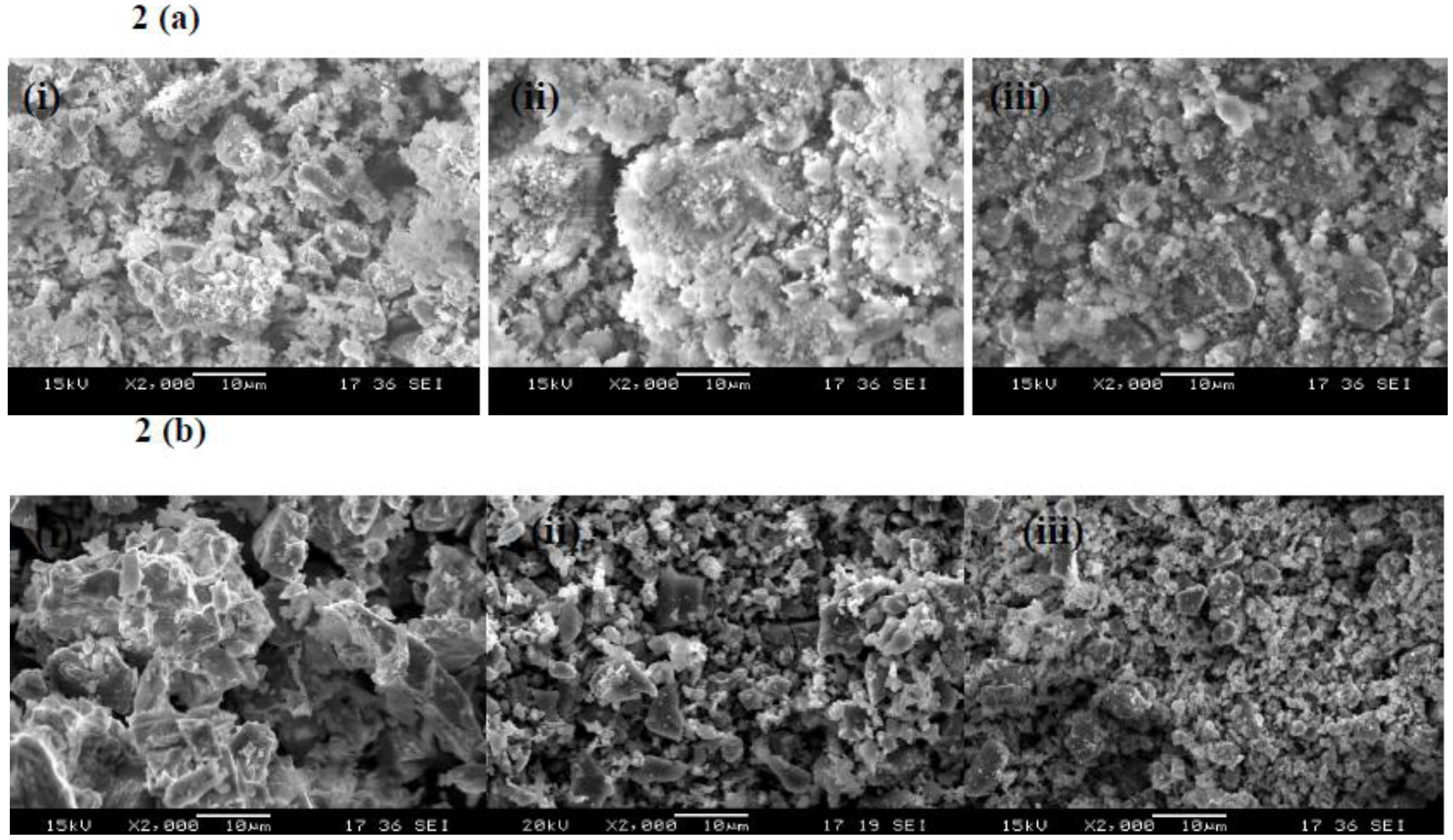

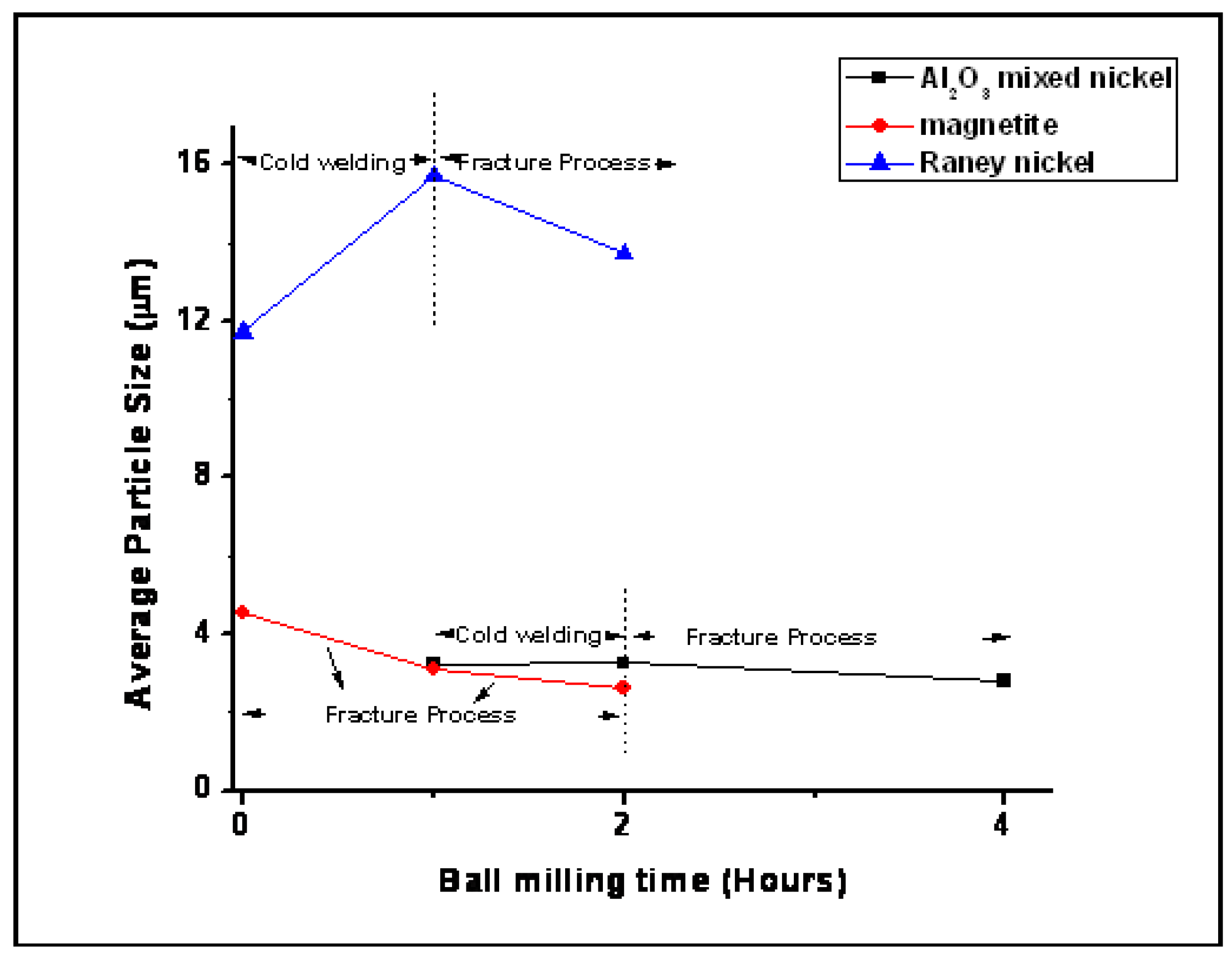
3.2. Reaction Results

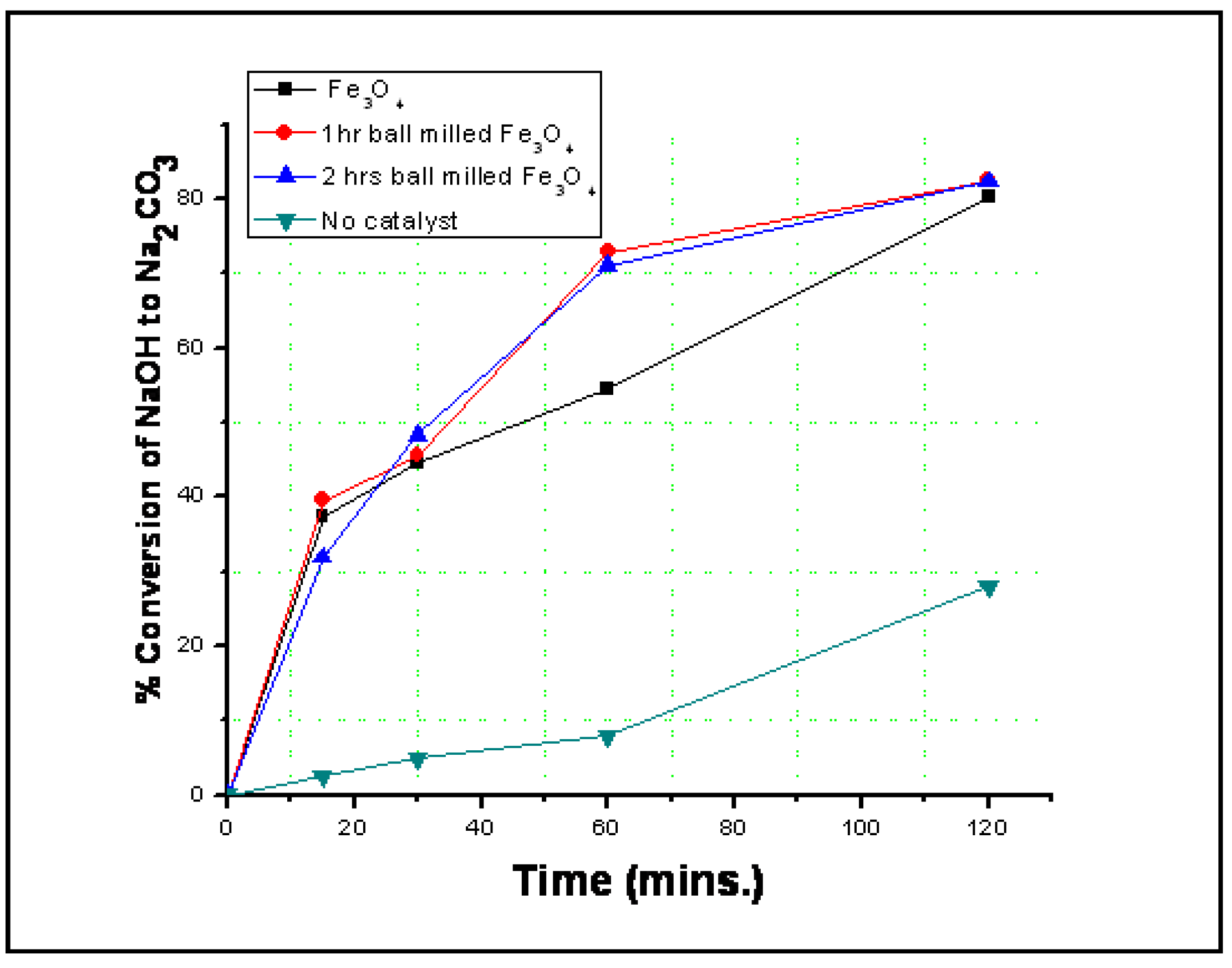
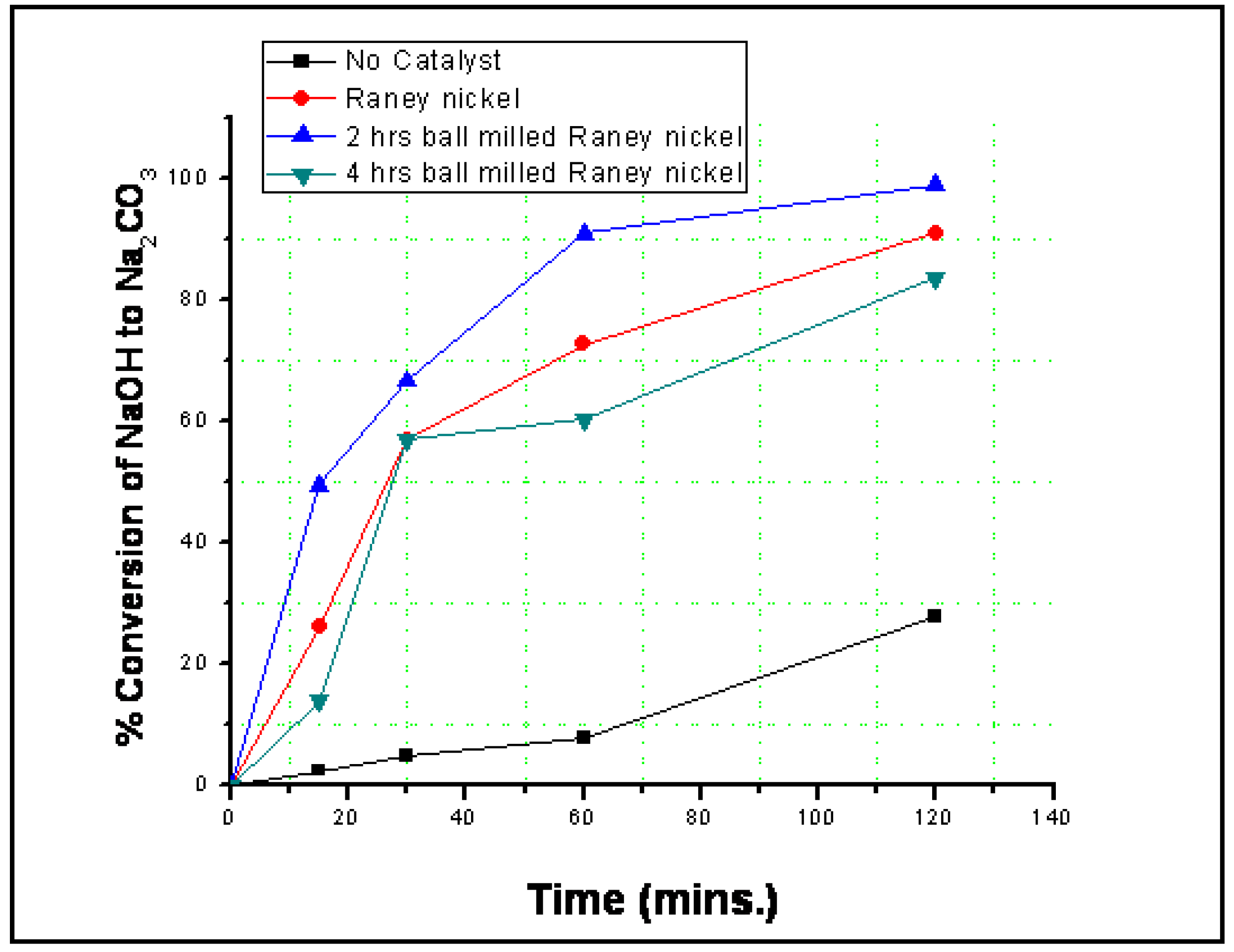
4. Conclusions
References
- Saxena, S.; Kumar, S.; Drozd, V.A. Modified steam-methane-reformation reaction for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 4366–4369. [Google Scholar] [CrossRef]
- Saxena, S.K.; Drozd, V.; Durygin, A. A fossil-fuel based recipe for clean energy. Int. J. Hydrog. Energy 2008, 33, 3625–3631. [Google Scholar] [CrossRef]
- Reichman, B.; Mays, W.; Strebe, J.; Fetcenko, M. Ovonic Renewable Hydrogen (ORH—low temperature hydrogen from renewable fuels. Int. J. Hydrog. Energy 2010, 35, 4918–4924. [Google Scholar] [CrossRef]
- Kamo, T.; Takaoka, K.; Otomo, J.; Takahashi, H. Effect of steam and sodium hydroxide for the production of hydrogen on gasification of dehydrochlrinated poly (vinyl chloride). Fuel 2006, 85, 1052–1059. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Role of sodium hydroxide in the production of hydrogen gas from the hydrothermal gasification of biomass. Int. J. Hydrog. Energy 2009, 34, 5645–5656. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Ahmad, S.; Aitani, A.; Rahman, F.; Al-Dawood, A.; Al-Muhaish, F. Decomposition of hydrocarbons to hydrogen and carbon. Appl. Catal. A 2009, 359, 1–24. [Google Scholar] [CrossRef]
- Muradov, N. Hydrogen via methane decomposition: An application for decarbonization of fossil fuels. Int. J. Hydrog. Energy 2001, 26, 1165–1175. [Google Scholar] [CrossRef]
- Drozd, V.; Saxena, S.K.; Garimella, S.V.; Durygin, A. Hydrogen release from a mixture of NaBH4 and Mg(OH)2. Int. J. Hydrog. Energy 2007, 32, 3370–3375. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrog. Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S.; Drozd, V.A. Modified method for production of Hydrogen from Methane. Int. J. Energy Res. 2012, 36, 1133–1138. [Google Scholar] [CrossRef]
- Muradova, N.Z.; Veziroglu, T.N. Green path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrog. Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Ishida, M.; Otsuka, K.; Takenaka, S.; Yamanaka, I. One-step production of CO- and CO2 -free hydrogen from biomass. J. Chem. Tech. Biotech. 2005, 80, 281–284. [Google Scholar] [CrossRef]
- Su, S.; Li, W.; Bai, Z.; Xiang, H.; Bai, J. Effects of ionic catalysis on hydrogen production by the steam gasification of cellulose. Int. J. Hydrog. Energy 2010, 35, 4459–4465. [Google Scholar] [CrossRef]
- Pistonesi, C.; Juan, A.; Irigoyen, B.; Amadeo, N. Theoretical and experimental study of methane steam reforming reactions over nickel catalyst. Appl. Surface Sci. 2007, 253, 4427–4437. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Hou, K.; Hughes, R. The kinetics of methane steam reforming over a Ni/α-Al2O catalyst. Chem. Eng. J. 2001, 82, 311–328. [Google Scholar] [CrossRef]
- Numaguchi, T.; Shoji, K.; Yoshida, S. Hydrogen effect on α-Al2O3 supported Ni catalyst for steam methane reforming reaction. Appl. Catal. A 1995, 133, 241–262. [Google Scholar] [CrossRef]
- Nozaki, T.; Muto, N.; Kado, S.; Okazaki, K. Dissociation of vibrationally excited methane on Ni catalyst Part 1. Application to methane steam reforming. Catal. Today 2004, 89, 57–65. [Google Scholar]
- Hauptmann, H.; Walter, W.F. The action of raney nickel on organic sulfur compounds. Chem. Rev. 1962, 62, 347–404. [Google Scholar] [CrossRef]
- Gerhard, E.; Helmut, K. Bulk Catalysts and supports. Prep. Solid Catal. 1997, 1, 30–34. [Google Scholar]
- Lund, C.R.F.; Kubsh, J.E.; Dumesic, J.A. Water-Gas Shift Over Magnetite-Based Catalysts: Nature of Active Sites for Adsorption and Catalysis. ACS Symp. Ser. 1985, 279, 313. [Google Scholar] [CrossRef]
- Rethwisch, D.G.; Phillips, J.; Chen, Y.; Hayden, T.F.; Dumesic, J.A. Water gas shift over magnetite particles supported on graphite: Effects of treatments in CO/CO2 and H2/H2O gas mixtures. J. Catal. 1985, 91, 167–180. [Google Scholar]
- Beeck, O. Catalysis and the Adsorption of Hydrogen on Metal Catalysts. Adv. Catal. 1950, 2, 151–195. [Google Scholar] [CrossRef]
- Trapnell, B.M.W. Specificity in catalysis by metals. Quart. Rev. 1954, 8, 404–421. [Google Scholar] [CrossRef]
- Gregg, S.J. The Surface Chemistry of Solids; Reinhold Pub. Corp.: New York, NY, USA, 1969; p. 233. [Google Scholar]
- Beeck, O. Catalysis—A challenge to the Physicist. Rev. Mod. Phys. 1945, 17, 61–71. [Google Scholar] [CrossRef]
- Couper, A.; Eley, D.D. The parahydrogen conversion of palladium-gold alloys. Discuss. Faraday Soc. 1950, 8, 172–184. [Google Scholar] [CrossRef]
- Dowden, D.A.; Reynolds, P.W. Some reactions over alloy catalysts. Discuss. Faraday Soc. 1950, 8, 184–190. [Google Scholar] [CrossRef]
- Hofer, E.M.; Hintermann, H.E. Correlation between catalytic activity of Raney nickel and its structure. Trans. Faraday Soc. 1964, 60, 1457–1465. [Google Scholar] [CrossRef]
- Suryanarayan, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Koch, C.C. Synthesis of nanostructured materials by mechanical milling: Problems and opportunities. Nanostruct. Mater. 1997, 9, 13–22. [Google Scholar] [CrossRef]
- Guerrero-Paz, J.; Jaramillo-Vigueras, D. Nanometric grain formation in ductile powders by low energy ball milling. Nanostruct. Mater. 1999, 11, 1123–1132. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Guo, J.T.; Jiang, T.D.; Wang, S.H. Investigation of defects in a mechanically alloyed nanocrystalline NiAl alloy by positron annihilation spectroscopy. J. Mater. Sci. Lett. 1998, 17, 137–139. [Google Scholar]
- Liang, G.; Huot, J.; Schulz, R. Hydrogen storage properties of the mechanically alloyed LaNi5-based materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Mohamed, F.A. A dislocation model for the minimum grain size obtainable by milling. Acta Mater. 2003, 51, 4107–4119. [Google Scholar] [CrossRef]
- Fecht, H.J. Nanostructure formation by mechanical attrition. Nanostruct. Mater. 1995, 6, 33–42. [Google Scholar] [CrossRef]
- Zhou, J.B.; Rao, K.P. Structure and morphology evolution during mechanical alloying Ti-Al-Si powder systems. J. Alloys Compd. 2004, 384, 125–130. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, Y.; Ma, L.; Shen, X.; Xu, S. Structure, morphology and electrocatalytic characteristics if nickel powders treated by mechanical milling. Int. J. Hydrog. Energy 2008, 33, 6351–6356. [Google Scholar] [CrossRef]
- Lee, S. Alternative Fuels; Taylors& Francis: Washington, DC, USA, 1996; p. 127. [Google Scholar]
- Lee, G.G.; Hashimoto, H.; Watanabe, R. Development of Particle Morphology during Dry Ball Milling of Cu Powder. Mater. Trans. JIM 1995, 36, 548–554. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kumar, S.; Drozd, V.; Saxena, S.K. Catalytic Studies of Sodium Hydroxide and Carbon Monoxide Reaction. Catalysts 2012, 2, 532-543. https://doi.org/10.3390/catal2040532
Kumar S, Drozd V, Saxena SK. Catalytic Studies of Sodium Hydroxide and Carbon Monoxide Reaction. Catalysts. 2012; 2(4):532-543. https://doi.org/10.3390/catal2040532
Chicago/Turabian StyleKumar, Sushant, Vadym Drozd, and Surendra K. Saxena. 2012. "Catalytic Studies of Sodium Hydroxide and Carbon Monoxide Reaction" Catalysts 2, no. 4: 532-543. https://doi.org/10.3390/catal2040532
APA StyleKumar, S., Drozd, V., & Saxena, S. K. (2012). Catalytic Studies of Sodium Hydroxide and Carbon Monoxide Reaction. Catalysts, 2(4), 532-543. https://doi.org/10.3390/catal2040532




