Antibiotic Treatment in End-of-Life Cancer Patients—A Retrospective Observational Study at a Palliative Care Center in Sweden
Abstract
:1. Introduction
2. Methods
2.1. Study Cohort
2.2. Data Extraction
2.3. Statistical Analysis
2.4. Ethic Statement
3. Results
3.1. Demography, Antibiotics and Infections
3.2. Type of Cancer
3.3. Vitamin D and Effect of Antibiotics
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwak, Y.G.; Moon, C.; Kim, E.S.; Kim, B.N. Frequent Prescription of Antibiotics and High Burden of Antibiotic Resistance among Deceased Patients in General Medical Wards of Acute Care Hospitals in Korea. PLoS ONE 2016, 11, e0146852. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Karim, I.A.; Sammel, R.B.; Prange, M.A. Causes of death at autopsy in an inpatient hospice program. J. Palliat. Med. 2007, 10, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Chou, Y.C.; Hsu, P.S.; Tsai, S.T.; Hwang, S.J.; Wu, B.Y.; Lin, M.H.; Chen, T.W. Antibiotic prescription for fever episodes in hospice patients. Support. Care Cancer 2002, 10, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Kim, J.H.; Kim, D.W.; Im, S.A.; Kim, T.Y.; Heo, D.S.; Bang, Y.J.; Kim, N.K. Antibiotic use during the last days of life in cancer patients. Eur. J. Cancer Care 2006, 15, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Stiel, S.; Krumm, N.; Pestinger, M.; Lindena, G.; Nauck, F.; Ostgathe, C.; Radbruch, L.; Elsner, F. Antibiotics in palliative medicine—Results from a prospective epidemiological investigation from the HOPE survey. Support. Care Cancer 2012, 20, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Kenner, D.; Sali, A. Bacterial infections in terminally ill hospice patients. J. Pain Symptom Manag. 2000, 20, 326–334. [Google Scholar] [CrossRef]
- Cheng, B.H.; Sham, M.M.; Chan, K.Y.; Li, C.W.; Au, H.Y. Intensive palliative care for patients with hematological cancer dying in hospice: Analysis of the level of medical care in the final week of life. Am. J. Hosp. Palliat. Care 2015, 32, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.E.; Meier, C.A.; McKinney, C.M.; Pottinger, P.S. Antimicrobial Use in Patients on a Comfort Care Protocol: A Retrospective Cohort Study. J. Palliat. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Silveira, M.J.; Vitale, C.A.; Malani, P.N. Antimicrobial use at the end of life among hospitalized patients with advanced cancer. Am. J. Hosp. Palliat. Care. 2012, 29, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.H.; Albrecht, J.S.; Fromme, E.K.; Noble, B.N.; McGregor, J.C.; Comer, A.C.; Furuno, J.P. Antimicrobial use for symptom management in patients receiving hospice and palliative care: A systematic review. J. Palliat. Med. 2013, 16, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.Y.; Hu, W.Y.; Huang, H.L.; Yao, C.A.; Chen, C.Y. Prevailing ethical dilemmas in terminal care for patients with cancer in Taiwan. J. Clin. Oncol. 2009, 27, 3964–3968. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.J.; Fraser, T.G.; Davis, M.P.; Kodish, E. Anti-infective therapy at the end of life: Ethical decision-making in hospice-eligible patients. Bioethics 2005, 19, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.L.; Clarfield, A.M.; Moses, A.E. Ethical issues relating to the use of antimicrobial therapy in older adults. Clin Infect. Dis. 2001, 33, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Furuno, J.P.; Noble, B.N.; Fromme, E.K. Should we refrain from antibiotic use in hospice patients? Expert Rev. Anti. Infect. Ther. 2016, 14, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Sperneder, S.; Höijer, J.; Bergqvist, J.; Björkhem-Bergman, L. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients—Results from an observational study in sweden. PLoS ONE 2015, 10, e0128223. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Björkhem-Bergman, L. Vitamin D supplementation to patients with frequent respiratory tract infections: A post hoc analysis of a randomized and placebo-controlled trial. BMC Res. Notes 2015, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Bergman, P. Vitamin D and patients with palliative cancer. BMJ Support. Palliat. Care 2016, 6, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Juthani-Mehta, M.; Malani, P.N.; Mitchell, S.L. Antimicrobials at the End of Life: An Opportunity to Improve Palliative Care and Infection Management. JAMA 2015, 314, 2017–2018. [Google Scholar] [CrossRef] [PubMed]
- White, P.H.; Kuhlenschmidt, H.L.; Vancura, B.G.; Navari, R.M. Antimicrobial use in patients with advanced cancer receiving hospice care. J. Pain Symptom Manag. 2003, 25, 438–443. [Google Scholar] [CrossRef]
- Van Der Steen, J.T.; Pasman, H.R.; Ribbe, M.W.; van der Wal, G.; Onwuteaka-Philipsen, B.D. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand. J. Infect. Dis. 2009, 41, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Givens, J.L.; Jones, R.N.; Shaffer, M.L.; Kiely, D.K.; Mitchell, S.L. Survival and comfort after treatment of pneumonia in advanced dementia. Arch. Intern. Med. 2010, 170, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Ahronheim, J.C.; Morrison, R.S.; Baskin, S.A.; Morris, J.; Meier, D.E. Treatment of the dying in the acute care hospital. Advanced dementia and metastatic cancer. Arch. Intern. Med. 1996, 156, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate immune function by Toll-like receptors: Distinct responses in newborns and the elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Beleigoli, A.M.; Boersma, E.; Diniz Mde, F.; Vidigal, P.G.; Lima-Costa, M.F.; Ribeiro, A.L. C-reactive protein and B-type natriuretic peptide yield either a non-significant or a modest incremental value to traditional risk factors in predicting long-term overall mortality in older adults. PLoS ONE 2013, 8, e75809. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.S.; McGregor, J.C.; Fromme, E.K.; Bearden, D.T.; Furuno, J.P. A nationwide analysis of antibiotic use in hospice care in the final week of life. J. Pain Symptom Manag. 2013, 46, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, L.L.; Lau, D.T.; Shega, J.W. Medications that Older Adults in Hospice Care in the United States Take, 2007. J. Am. Geriatr. Soc. 2015, 63, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
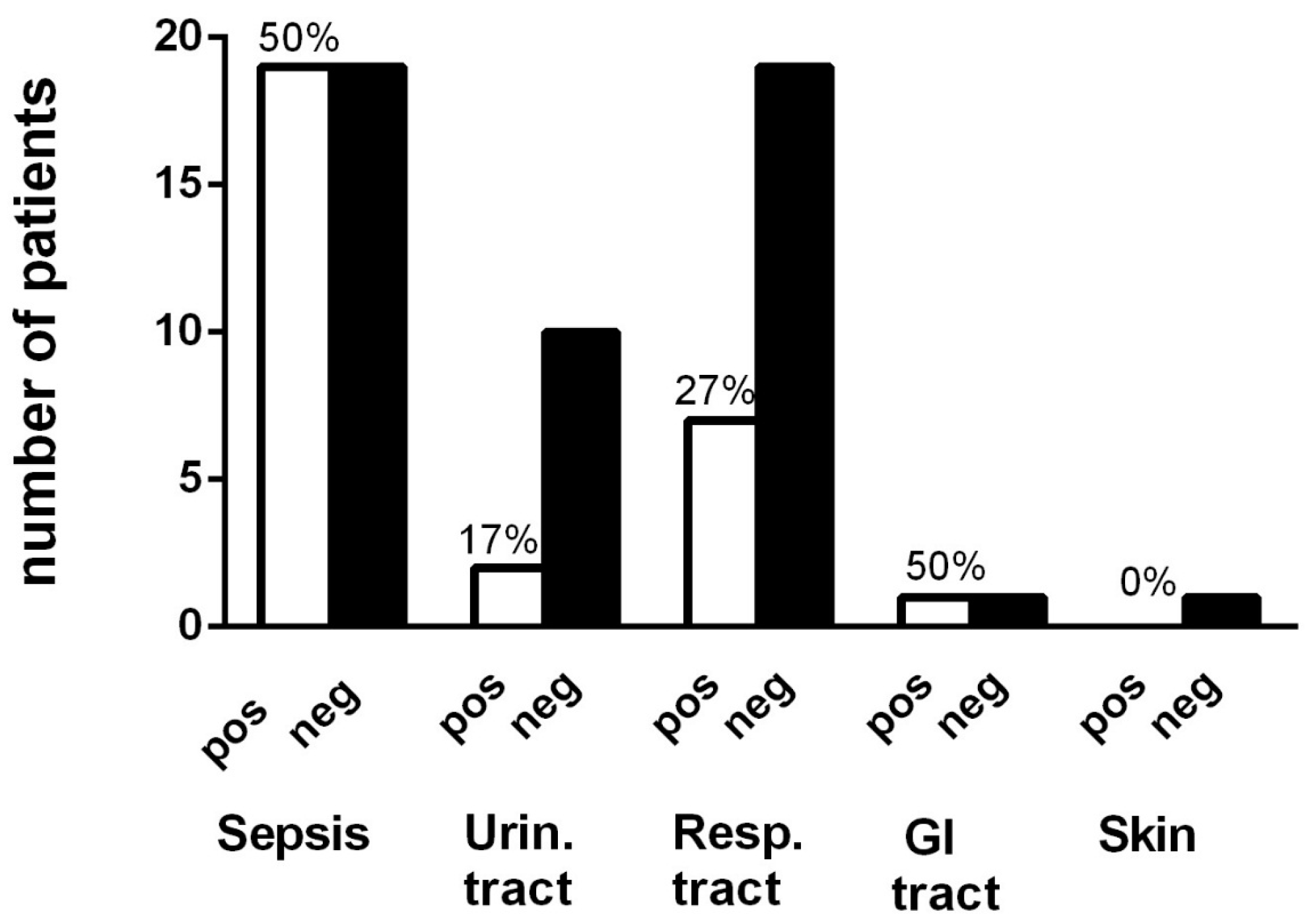
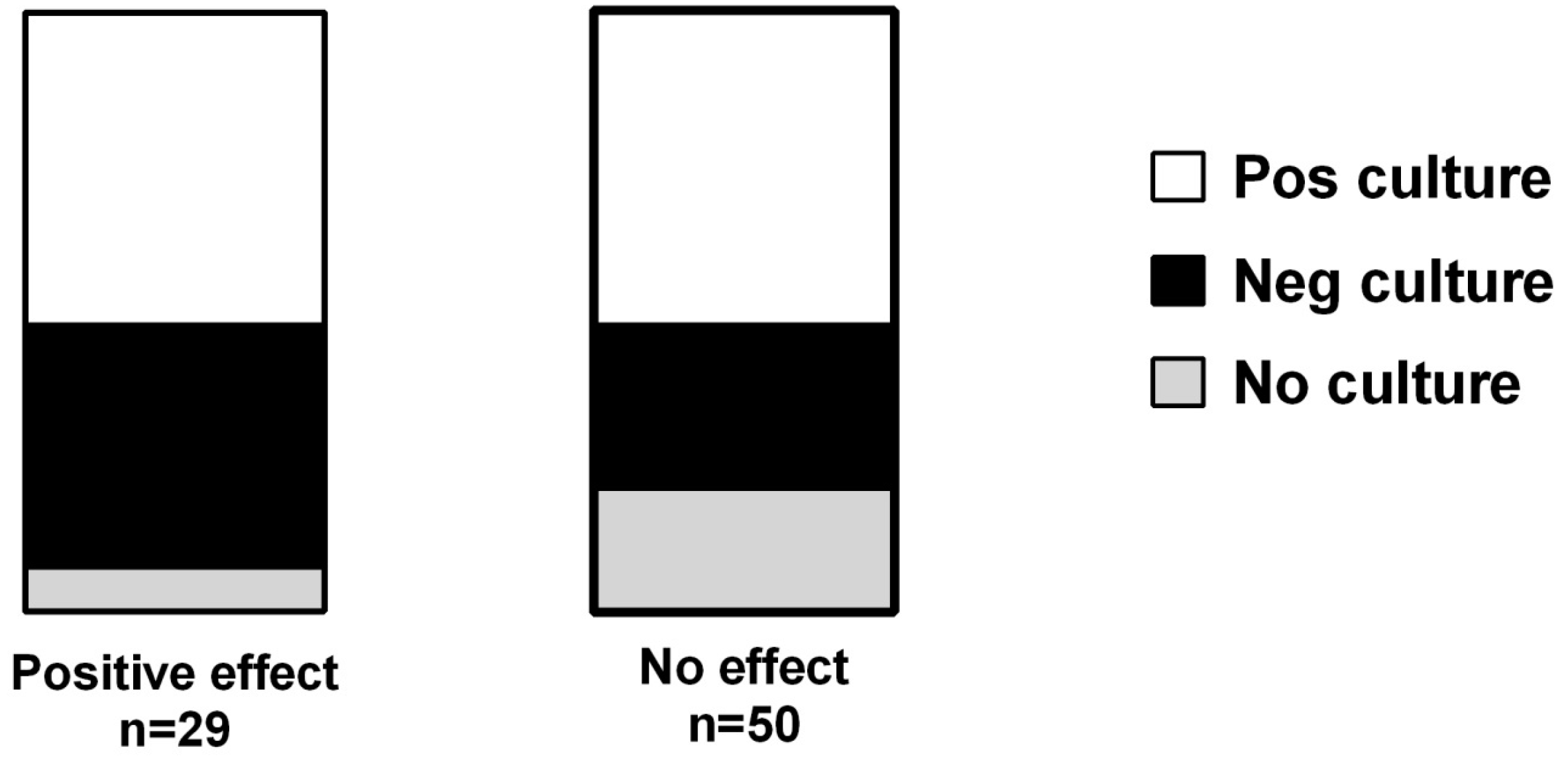
| Age Median (Range) | Men % (n) | CRP mg/L Median (Range) | Vit. D nmol/L Median (Range) | Albumin g/L Median (Range) | Adverse Event % (n) | Culture Taken % (n) | Pos Culture % (n) | |
|---|---|---|---|---|---|---|---|---|
| All (n = 160) | 71 (18–95) | 43% (69) | 61 (1–597) | 36 (8–133) | 24 (11–39) | NA | NA | NA |
| No Antibiotics (n = 81) | 72 (32–95) | 42% (34) | 44 * (1–283) | 39 (8–133) | 25 (16–39) | NA | NA | NA |
| Antibiotics (n = 79) | 68 (18–90) | 44% (35) | 124 * (1–597) | 33 (8–120) | 22 (11–37) | 3.8% (3) | 85% (67) | 52% (41) |
| Pos effect (n = 29) | 69 (36–90) | 34% (10) | 76 (7–319) | 37 (10–112) | 22 (14–31) | 3.4% (1) | 93% (27) | 52% (15) |
| No effect (n = 50) | 68 (18–86) | 50% (25) | 141 (1–597) | 32 (8–120) | 22 (11–37) | 4% (2) | 80% (40) | 52% (26) |
| Cultures | Pos Effect, n = 15 (15 Cultures) | No Effect, n = 26 (32 Cultures) |
|---|---|---|
| Blood | 7 S. epidermidis 3 Enterococcus species 1 Corynebacterium species 1 Klebsiella species 1 S. marscecens 1 | 8 S. epidermidis 4 Enterococcus species 1 Enterobacter species 1 S. aureus 1 Klebsiella species 1 |
| Urine | 4 E. coli 2 Klebsiella species 2 | 14 E. coli 7 Klebsiella species 1 S. aureus 1 Enterococcus species 3 Enterobacter species 1 Legionella species 1 |
| Skin | 0 | 5 S. aureus 4 E. faecialis 1 |
| Sputum | 1 Bacillus species 1 | 3 S. aureus 3 |
| Feces | 1 C. difficile 1 | 0 |
| Nasopharynx | 0 | 2 M. catharralis 1 S. aureus 1 |
| Synovial fluid | 1 S. aureus 1 | 0 |
| Pleura fluid | 1 S. epidermidis 1 | 0 |
| Type of Cancer | All (n = 160) | Antibiotics (n = 79) | Pos Effect (n = 29) | No Effect (n = 50) |
|---|---|---|---|---|
| Brain tumor | 4 | 1 | 0 | 1 100% |
| Breast cancer | 19 | 10 | 3 30% | 7 70% |
| Gynecological | 16 | 9 | 4 44% | 5 56% |
| GI-cancer | 29 | 13 | 5 38% | 8 62% |
| Gallbladder | 6 | 3 | 2 67% | 1 33% |
| Head-Neck cancer | 8 | 4 | 2 50% | 2 50% |
| Hematologic mal. | 8 | 3 | 2 67% | 1 33% |
| Kidney | 3 | 2 | 1 50% | 1 50% |
| Lung cancer | 32 | 19 | 6 32% | 13 68% |
| Malignant Melanoma | 3 | 0 | NA | NA |
| Mesotelioma | 2 | 1 | 1 100% | 0 |
| Pancreas | 14 | 6 | 2 33% | 4 67% |
| Prostate cancer | 13 | 8 | 1 12% | 7 88% |
| Other | 3 | 0 | NA | NA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helde-Frankling, M.; Bergqvist, J.; Bergman, P.; Björkhem-Bergman, L. Antibiotic Treatment in End-of-Life Cancer Patients—A Retrospective Observational Study at a Palliative Care Center in Sweden. Cancers 2016, 8, 84. https://doi.org/10.3390/cancers8090084
Helde-Frankling M, Bergqvist J, Bergman P, Björkhem-Bergman L. Antibiotic Treatment in End-of-Life Cancer Patients—A Retrospective Observational Study at a Palliative Care Center in Sweden. Cancers. 2016; 8(9):84. https://doi.org/10.3390/cancers8090084
Chicago/Turabian StyleHelde-Frankling, Maria, Jenny Bergqvist, Peter Bergman, and Linda Björkhem-Bergman. 2016. "Antibiotic Treatment in End-of-Life Cancer Patients—A Retrospective Observational Study at a Palliative Care Center in Sweden" Cancers 8, no. 9: 84. https://doi.org/10.3390/cancers8090084





