Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention
Abstract
:1. Introduction
2. Accumulation of Bioactive Compounds in Tomato
3. Chemoprotective Characteristics of Tomato Bioactive Compounds
3.1. Carotenoids
3.2. Polyphenols
4. Breeding Strategies for the Improvement of Carotenoid and Polyphenol Content in Tomato
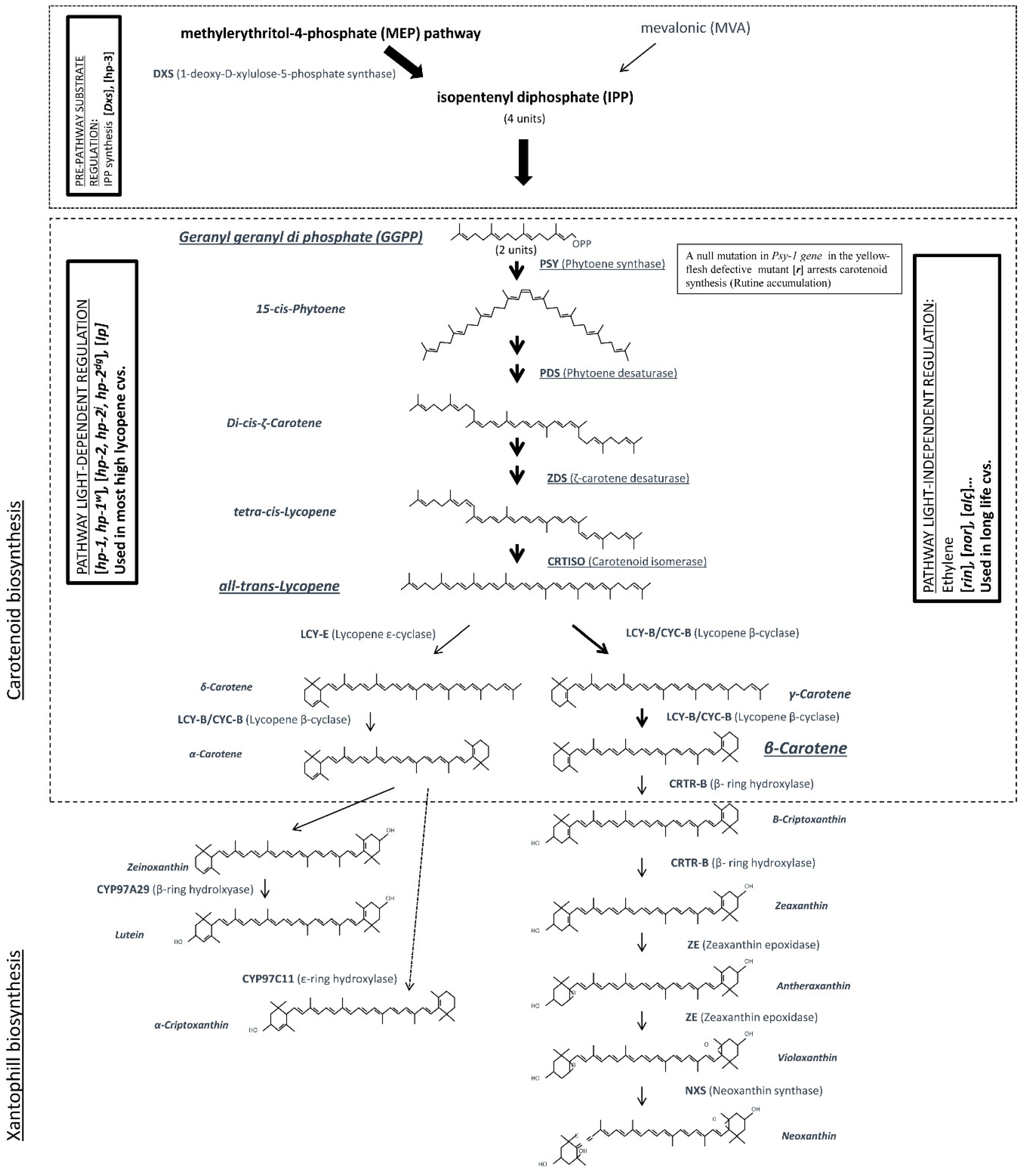
4.1. Carotenoids
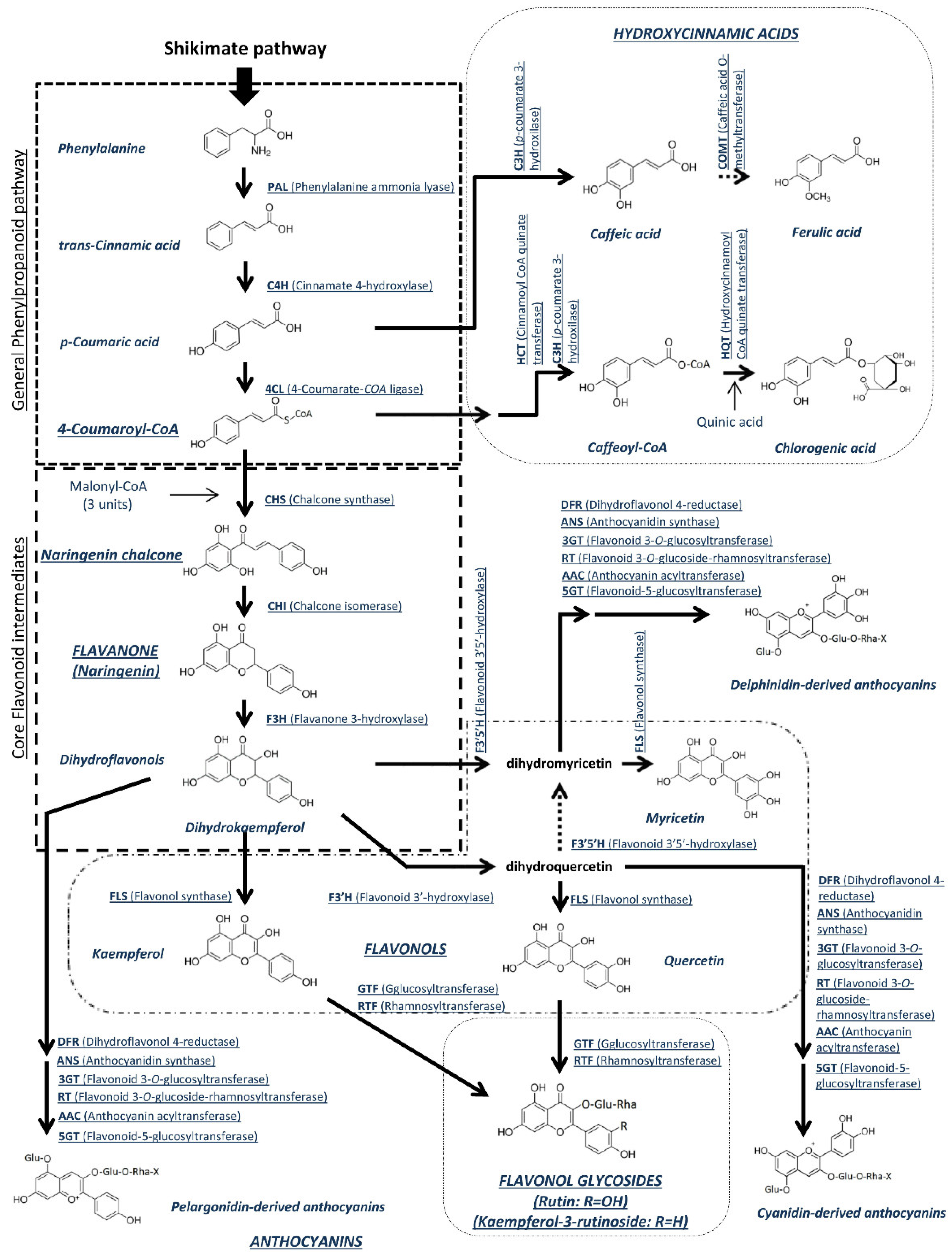
4.2. Polyphenols
4.3. Joint Accumulation of Carotenoids and Polyphenols
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruhn, C.M.; Feldman, N.; Garlitz, C.; Harwood, J.; Ivans, E.; Marshall, M.; Riley, A.; Thurber, D.; Williamson, E. Consumer perceptions of quality: Apricots, cantaloupes, peaches, pears, strawberries, and tomatoes. J. Food Qual. 1991, 14, 187–195. [Google Scholar] [CrossRef]
- Cappellano, K.L. Influencing food choices: Nutrition labeling, health claims, and front-of-the-package labeling. Nutr. Today 2009, 44, 269–273. [Google Scholar] [CrossRef]
- Goldman, I.L. Molecular breeding of healthy vegetables. EMBO Rep. 2011, 12, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Chandrasekara, A.; Zhong, Y. Bioactive phytochemicals in vegetables. In Handbook of Vegetables and Vegetable Processing; Sinha, N.K., Hui, Y.H., Evranuz, E.Ö., Siddiq, M., Ahmed, J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 125–158. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873. [Google Scholar] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402–1406. [Google Scholar]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.M.; Gillespie, C.; Kimmons, J.E.; Seymour, J.D.; Serdula, M.K. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Available online: http://www.cdc.gov/pcd/issues/2008/apr/07_0049.htm (accessed on 1 May 2016).
- Vattem, D.A.; Ghaedian, R.; Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005, 14, 120–130. [Google Scholar] [PubMed]
- Boileau, T.W.; Liao, Z.; Kim, S.; Lemeshow, S.; Erdman, J.W.; Clinton, S.K. Prostate Carcinogenesis in N-methyl-N-nitrosourea (NMU)-Testosterone-Treated Rats Fed Tomato Powder, Lycopene, or Energy-Restricted Diets. J. Natl. Cancer Inst. 2003, 95, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; DeGroff, V.L.; Clinton, S.K. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J. Nutr. 2003, 133, 2367–2376. [Google Scholar] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479–3485. [Google Scholar]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [PubMed]
- Fielding, J.M.; Rowley, K.G.; Cooper, P.; O’Dea, K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac. J. Clin. Nutr. 2005, 14, 131–136. [Google Scholar] [PubMed]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. food and drug administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 2007, 99, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst. 2009, 101, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.; Anand, S. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Mignone, L.I.; Giovannucci, E.; Newcomb, P.A.; Titus-Ernstoff, L.; Trentham-Dietz, A.; Hampton, J.M.; Willett, W.C.; Egan, K.M. Dietary carotenoids and the risk of invasive breast cancer. Int. J. Cancer 2009, 124, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Dietary compared with blood concentrations of carotenoids and breast cancer risk : A systematic review and meta-analysis of prospective. Am. J. Clin. Nutr. 2012, 2007, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sic. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Leiva-Brondo, M.; Valcárcel, M.; Cortés-Olmos, C.; Roselló, S.; Cebolla-Cornejo, J.; Nuez, F. Exploring alternative germplasm for the development of stable high vitamin C content in tomato varieties. Sci. Hortic. 2012, 133, 84–88. [Google Scholar] [CrossRef]
- Standfuss, J.; Terwisscha van Scheltinga, A.C.; Lamborghini, M.; Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Galpaz, N.; Galpaz, N.; Ronen, G.; Ronen, G.; Khalfa, Z.; Khalfa, Z.; Zamir, D.; Zamir, D.; Hirschberg, J.; Hirschberg, J. A Chromoplast-Specific Carotenoid Biosynthesis Pathway Is Revealed by Cloning of the Tomato white-flower Locus. Plant Cell 2006, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.N.; Hobson, G.E.; McGlasson, W.B. The constituents of tomato fruit—the influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The flavonoids of tomatoes. J. Agric. Food Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Fray, R.G.; Grierson, D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S. Investigation of Raman chemical imaging for detection of lycopene changes in tomatoes during postharvest ripening. J. Food Eng. 2011, 107, 277–288. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chen, S.L.; Wang, C.K. Contribution of tomato phenolics to antioxidation and down-regulation of blood lipids. J. Agric. Food Chem. 2007, 55, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Valcárcel, M.; Herrero-Martínez, J.M.; Cebolla-Cornejo, J.; Roselló, S. Fast simultaneous determination of prominent polyphenols in vegetables and fruits by reversed phase liquid chromatography using a fused-core column. Food Chem. 2015, 169, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; Ric De Vos, C.H.; van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Colliver, S.; Bovy, A.; Collins, G.; Muir, S.; Robinson, S.; De Vos, C.H.; Verhoeyen, M.E. Improving the nutritional content of tomatoes through reprogramming their flavonoid biosynthetic pathway. Phytochem. Rev. 2002, 1, 113–123. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Mes, P.J.; Boches, P.; Myers, J.R.; Durst, R. Characterization of tomatoes expressing anthocyanin in the fruit. J. Am. Soc. Hortic. Sci. 2008, 133, 262–269. [Google Scholar]
- Sapir, M.; Oren-Shamir, M.; Ovadia, R.; Reuveni, M.; Evenor, D.; Tadmor, Y.; Nahon, S.; Shlomo, H.; Chen, L.; Meir, A.; et al. Molecular aspects of Anthocyanin fruit tomato in relation to high pigment-1. J. Hered. 2008, 99, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of Tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Lean, M.E.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Khachik, F.; Goli, M.B.; Beecher, G.R.; Holden, J.; Lusby, W.R.; Tenorio, M.D.; Barrera, M.R. Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J. Agric. Food Chem. 1992, 40, 390–398. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Takkouche, B.; Caamaño-isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 340–345. [Google Scholar] [PubMed]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nutr. 2005, 134, 1226–1230. [Google Scholar]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Wu, K.; Erdman, J.W.; Schwartz, S.J.; Platz, E.A.; Leitzmann, M.; Clinton, S.K.; Degroff, V. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Hung, J.C.; Heber, D.; Go, V.L.; Reuter, V.E.; Cordon-Cardo, C.; Scher, H.I.; Marshall, J.R.; Zhang, Z.F. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 749–756. [Google Scholar] [PubMed]
- Kristal, A.R.; Till, C.; Platz, E.A.; Song, X.; King, I.B.; Neuhouser, M.L.; Ambrosone, C.B.; Thompson, I.M. Serum lycopene concentration and prostate cancer risk: Results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014, 106, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Yen, Y.T.; Huang, C.S.; Hu, M.L. Growth inhibitory efficacy of lycopene and beta-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol. Nutr. Food Res. 2011, 55, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kirsh, V.A.; Mayne, S.T.; Peters, U.; Chatterjee, N.; Leitzmann, M.F.; Dixon, L.B.; Urban, D.A.; Crawford, E.D.; Hayes, R.B. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 92–98. [Google Scholar] [CrossRef] [PubMed]
- AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Available online: http://www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (accessed on 1 May 2016).
- Kuckuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 861–868. [Google Scholar]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs. orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003, 92, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Stacewicz-Sapunatzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; van Breemen, R.; Ashton, D.; Bowen, P.E. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J. Natl. Cancer Inst. 2001, 93, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, N.K.; Saxena, S.; Singh, U.P.; Goyal, N.K.; Arora, R.P. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol. Oncol. Semin. Orig. Investig. 2005, 23, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Burch, P.; Hillman, D.; Vanyo, J.M.; Dakhil, S.; Nikcevich, D.; Rowland, K.; Morton, R.; Flynn, P.J.; Young, C.; et al. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: Results of a phase II study from the north central cancer treatment group. Urology 2007, 69, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.E.; Hall, M.C.; Borden, L.S.; Miller, A.A.; Hu, J.J.; Lee, W.R.; Stindt, D.; D’Agostino, R.; Lovato, J.; Harmon, M.; et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology 2006, 67, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Bunker, C.; McDonald, A.; Evans, R.; de la Rosa, N.; Boumosleh, J.; Patrick, A. A randomized trial of lycopene supplementation in tobago Men with high prostate cancer risk. Nutr. Cancer 2007, 57, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Barnett, M.J.; Kristal, A.R.; Ambrosone, C.B.; King, I.B.; Thornquist, M.; Goodman, G.G. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Xing, M.; Yu, L.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pacific J. cancer Prev. 2012, 14, 1911–1918. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Mayne, S.T.; Swanson, C.A. Nutrition and lung cancer. Cancer Causes Control 1996, 7, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, O.; Albanes, D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Albanes, D.; Heinonen, O.P.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Rautalahti, M.; Hartman, A.M.; Palmgren, J.; Freedman, L.S.; Haapakoski, J.; et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996, 88, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin a on Lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Russell, R. Procarcinogenic and anticarcinogenic effects of beta-carotene. Nutr. Rev. 1999, 57, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Groshong, S.D.; Husgafvel-Pursiainen, K.; Genova, E.; Lucia, M.S.; Wolff, H.; Virtamo, J.; Albanes, D. Effects of beta-carotene supplementation on molecular markers of lung carcinogenesis in male smokers. Cancer Prev. Res. 2010, 3, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Sampson, J.N.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Virtamo, J.; Albanes, D. Metabolomic profile of response to supplementation with beta-carotene in the alpha-tocopherol, beta-carotene cancer prevention Study. Am. J. Clin. Nutr. 2013, 98, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Hendrickson, S.J.; Brinton, L.A.; Buring, J.E.; Campos, H.; Dai, Q.; Dorgan, J.F.; Franke, A.A.; Gao, Y.T.; Goodman, M.T.; et al. Circulating carotenoids and risk of breast cancer: Pooled analysis of eight prospective studies. J. Natl. Cancer Inst. 2012, 104, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; De Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-De Jong, J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P.; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life after Cancer Epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.F.; Gail, M.; Li, G.Y.; et al. Nutrition intervention trials in linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Meng, Q.; Zhai, S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin. Nutr. 2015, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; De Luca, C.; Kostyuk, V.; Pastore, S. Plant polyphenols and tumors: From mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009, 16, 3943–3965. [Google Scholar] [CrossRef] [PubMed]
- Loa, J.; Chow, P.; Zhang, K. Studies of structure–activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control 1999, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case-control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Spertini, L.; Parpinel, M.; Gnagnarella, P.; Lagiou, P.; Negri, E.; Franceschi, S.; Montella, M.; Peterson, J.; Dwyer, J.; et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomark. Prev. 2005, 14, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Negri, E.; Talamini, R.; Bosetti, C.; Parpinel, M.; Gnagnarella, P.; Franceschi, S.; Dal Maso, L.; Montella, M.; Giacosa, A.; et al. Flavonoids and colorectal cancer in italy. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Cruz–Correa, M.; Shoskes, D.; Sanchez, P.; Zhao, R.; Hylind, L.; Wexner, D.; Giardiello, F. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Review: Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Järvinen, R.; Seppänen, R.; Hellövaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.; Virtamo, J.; Korhonen, P.; Albanes, D.; Pietinen, P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control 2001, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Rossi, M.; Lagiou, A.; Tzonou, A.; La Vecchia, C.; Trichopoulos, D. Flavonoid intake and liver cancer: A case-control study in Greece. Cancer Causes Control 2008, 19, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, C.; Ma, Y.; Weng, S.; Tang, N.; Wu, S.; Ji, B.-C.; Ma, C.-Y.; Ko, Y.-C.; Funayama, S.; et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. In Vivo 2012, 26, 971–978. [Google Scholar] [PubMed]
- Rajendra Prasad, N.; Karthikeyan, A.; Karthikeyan, S.; Venkata Reddy, B. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Frydoonfar, H.R.; McGrath, D.R.; Spigelman, A.D. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Color. Dis. 2003, 5, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Patel, Y.M. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation. Breast Cancer Res. Treat. 2004, 85, 103–110. [Google Scholar] [CrossRef] [PubMed]
- So, F.; Guthrie, N.; Chambers, A.; Moussa, M.; Carroll, K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer 1996, 26, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Enfissi, E.M.A.; Halket, J.M.; Truesdale, M.R.; Yu, D.; Gerrish, C.; Bramley, P.M. Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 2007, 19, 3194–3211. [Google Scholar] [CrossRef] [PubMed]
- Pecker, I.; Chamovitz, D.; Linden, H.; Sandmann, G.; Hirschberg, J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc. Natl. Acad. Sci. USA 1992, 89, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to beta-carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Cohen, M.; Zamir, D.; Hirschberg, J. Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon cyclase is down- regulated during ripening and is elevated in the mutant Delta. Plant J. 1999, 17, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stommel, J.R. RAPD and AFLP tagging and mapping of Beta (B) and Beta modifier (MoB), two genes which influence beta-carotene accumulation in fruit of tomato (Lycopersicon esculentum Mill.). Theor. Appl. Genet. 2000, 100, 368–375. [Google Scholar] [CrossRef]
- Kasahara, H. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-Deoxy-D-Xylulose-5-Phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Lois, L.M.; Rodríguez-Concepción, M.; Gallego, F.; Campos, N.; Boronat, A. Carotenoid biosynthesis during tomato fruit development: Regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J. 2000, 22, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Cebolla-Cornejo, J.; Roselló, S.; Nuez, F. Selection of tomato rich in nutritional terpenes. In Natural Products; Ramawat, K., Mérillon, J., Eds.; Springer-Verlag: Berlin, Germany; Heidelberg, Germany, 2013; pp. 2853–2881. [Google Scholar]
- Alba, R.; Cordonnier-Pratt, M.-M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; Van Tuinent, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef] [PubMed]
- Bino, R.J.; De Vos, C.H.R.; Lieberman, M.; Hall, R.D.; Bovy, A.; Jonker, H.H.; Tikunov, Y.; Lommen, A.; Moco, S.; Levin, I. The light-hyperresponsive high pigment-2dg mutation of tomato: Alterations in the fruit metabolome. New Phytol. 2005, 166, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.C.; Fenzi, F.; Ciliento, R.; Alfano, F.; Bowler, C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 1999, 11, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, R.E.; Kerckhoffs, L.H.; van Tuinen, A.; Koornneef, M. Photomorphogenic mutants of tomato. Plant Cell Environ. 1997, 20, 746–751. [Google Scholar] [CrossRef]
- Lavi, N.; Tadmor, Y.; Meir, A.; Bechar, A.; Oren-Shamir, M.; Ovadia, R.; Reuveni, M.; Nahon, S.; Shlomo, H.; Chen, L.; et al. Characterization of the intense pigment tomato genotype emphasizing targeted fruit metabolites and chloroplast biogenesis. J. Agric. Food Chem. 2009, 57, 4818–4826. [Google Scholar] [CrossRef] [PubMed]
- Tomes, M.L.; Quackenbush, F.W. Caro-red, a new provitamin a rich tomato. Econ. Bot. 1958, 12, 256–260. [Google Scholar] [CrossRef]
- Tigchelaar, C.C.; Tomes, M.L. “Caro-Rich” Tomato. Available online: http://hortsci.ashspublications.org/content/by/year (accessed on 1 May 2016).
- Georgiev, H.; Vulkova-Atchkova, Z.; Vladimirov, B.G.; Baralieva, D. Breeding determinate tomatoes with higt Beta-carotene content. Gr. Lozar. Nauk. 1981, 18, 28–31. [Google Scholar]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Stigliani, A.L.; Giorio, G.; D’Ambrosio, C. Characterization of P450 Carotenoid B- And E-hydroxylases of tomato and transcriptional regulation of xanthophyll biosynthesis in root, leaf, petal and fruit. Plant Cell Physiol. 2011, 52, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Stommel, J.R. Genetic enhancement of tomato fruit nutritive value. In Genetic Improvement of Solanaceous Crops (Volume 2); Razdan, M., Matoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 193–238. [Google Scholar]
- Rick, C.M. High soluble-solids content in large-fruited tomato lines derived from a wild green-fruited species. Hilgardia 1974, 42, 493–510. [Google Scholar] [CrossRef]
- Enfissi, E.M.; Fraser, P.D.; Lois, L.-M.; Boronat, A.; Schuch, W.; Bramley, P.M. Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol. J. 2005, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Fray, R.G.; Wallace, A.; Fraser, P.D.; Valero, D.; Hedden, P.; Bramley, P.M.; Grierson, D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995, 8, 693–701. [Google Scholar] [CrossRef]
- Römer, S.; Fraser, P.D.; Kiano, J.W.; Shipton, C.A.; Misawa, N.; Schuch, W.; Bramley, P.M. Elevation of the provitamin A content of transgenic tomato plants. Nat. Biotechnol. 2000, 18, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Rosati, C.; Aquilani, R.; Dharmapuri, S.; Pallara, P.; Marusic, C.; Tavazza, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000, 24, 413–419. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Giorio, G.; Marino, I.; Merendino, A.; Petrozza, A.; Salfi, L.; Stigliani, A.L.; Cellini, F. Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci. 2004, 166, 207–214. [Google Scholar] [CrossRef]
- Apel, W.; Bock, R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol. 2009, 151, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, B.; He, J.; Hao, Q.; Lu, X.; Wang, L. Regulation of carotenoid content in tomato by silencing of lycopene β/ε-cyclase genes. Plant Mol. Biol. Rep. 2011, 29, 117–124. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, W.; Zhang, J.; Xu, Q.; Deng, X. Effect of the citrus lycopene β-Cyclase transgene on carotenoid metabolism in transgenic tomato fruits. PLoS ONE 2012, 7, e32221. [Google Scholar] [CrossRef] [PubMed]
- Corona, V.; Aracri, B.; Kosturkova, G.; Bartley, G.E.; Pitto, L.; Giorgetti, L.; Scolnik, P.A.; Giuliano, G. Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J. 1996, 9, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Chinnusamy, V.; Bansal, K.C. Isolation and functional characterization of lycopene beta-cyclase (CYC-B) promoter from Solanum habrochaites. BMC Plant Biol. 2010, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hiwasa-Tanase, K.; Kuroda, H.; Hirai, T.; Aoki, K.; Takane, K.; Ezura, H. Novel promoters that induce specific transgene expression during the green to ripening stages of tomato fruit development. Plant Cell Rep. 2012, 31, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davuluri, G.R.; van Tuinen, A.; Fraser, P.D.; Manfredonia, A.; Newman, R.; Burgess, D.; Brummell, D.A.; King, S.R.; Palys, J.; Uhlig, J.; et al. Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat. Biotechnol. 2005, 23, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, B.; Zhang, M.; Wang, L.; Cui, M.; Wang, Q.; Leng, P. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and beta-carotene contents in tomato fruit. J. Exp. Bot. 2012, 63, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, Y.; Zhang, M.; Wang, L.; Ren, J.; Cui, M.; Wang, Y.; Ji, K.; Li, P.; Li, Q.; et al. Suppression of 9-cis-Epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol. 2012, 158, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Levin, I. Regulating phytonutrient levels in plants-toward modification of plant metabolism for human health. In Recent Advances in Plant Biotechnology; Kirakosyan, A., Kaufman, P.B., Eds.; Springer: New York, NY, USA, 2009; pp. 289–330. [Google Scholar]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Butelli, E.; Hill, L.; Parr, A.; Niggeweg, R.; Bailey, P.; Weisshaar, B.; Martin, C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008, 56, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Verhoeyen, M.E.; Bovy, A.; Collins, G.; Muir, S.; Robinson, S.; de Vos, C.H.; Colliver, S. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. J. Exp. Bot. 2002, 53, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; de Jesús Ornelas-Paz, J. Chemistry, stability, and biological actions of carotenoids. In Fruit and Vegetable Phytochemicals; de la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Wiley-Blackwell: Hoboken, IA, USA, 2010; pp. 177–222. [Google Scholar]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bovy, A.; de Vos, R.; Kemper, M.; Schijlen, E.; Pertejo, M.A.; Muir, S.; Collins, G.; Robinson, S.; Verhoeyen, M.; Hughes, S.; et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes. Plant Cell 2002, 14, 2509–2526. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.-R.; Molthoff, J.; de Vos, R.; Hekkert, B.T.; Orzaez, D.; Fernández-Moreno, J.-P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J.; et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010, 152, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Willits, M.G.; Kramer, C.M.; Prata, R.T.; de Luca, V.; Potter, B.G.; Steffens, J.C.; Graser, G. Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J. Agric. Food Chem. 2005, 53, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, E. Inheritance in tomatoes. Genetics 1925, 10, 305–317. [Google Scholar] [PubMed]
- Zhang, Y.; Butelli, E.; Alseekh, S.; Tohge, T.; Rallapalli, G.; Luo, J.; Kawar, P.G.; Hill, L.; Santino, A.; Fernie, A.R.; et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Misra, P.; Choudhary, D.; Yadav, R.; Goel, R.; Bhambhani, S.; Sanyal, I.; Trivedi, R.; Kumar Trivedi, P. AtMYB12 expression in tomato leads to large scale differential modulation in transcriptome and flavonoid content in leaf and fruit tissues. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, T.; Yang, C.; Li, H.; Yang, M.; Ijaz, R.; Ye, Z.; Zhang, Y. Transcriptome profiling of tomato fruit development reveals transcription factors associated with ascorbic acid, carotenoid and flavonoid biosynthesis. PLoS ONE 2015, 10, e0130885. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, D.; Lazzeri, V.; Calvenzani, V.; Dall’Asta, C.; Galaverna, G.; Tonelli, C.; Petroni, K.; Ranieri, A. Flavonoid profiling and biosynthetic gene expression in flesh and peel of two tomato genotypes grown under UV-B-depleted conditions during ripening. J. Agric. Food Chem. 2008, 56, 5905–5915. [Google Scholar] [CrossRef] [PubMed]
- Azari, R.; Tadmor, Y.; Meir, A.; Reuveni, M.; Evenor, D.; Nahon, S.; Shlomo, H.; Chen, L.; Levin, I. Light signaling genes and their manipulation towards modulation of phytonutrient content in tomato fruits. Biotechnol. Adv. 2010, 28, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mathews, H.; Clendennen, S.K.; Caldwell, C.G.; Liu, X.L.; Connors, K.; Matheis, N.; Schuster, D.K.; Menasco, D.J.; Wagoner, W.; Lightner, J.; et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 2003, 15, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; Reuveni, M.; Evenor, D.; Oren-Shamir, M.; Ovadia, R.; Sapir-Mir, M.; Bootbool-Man, A.; Nahon, S.; Shlomo, H.; Chen, L.; et al. ANTHOCYANIN1 from Solanum chilense is more efficient in accumulating anthocyanin metabolites than its Solanum lycopersicum counterpart in association with the ANTHOCYANIN FRUIT phenotype of tomato. Theor. Appl. Genet. 2012, 124, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, D.; Li, X.; Zhao, S.; Sui, N.; Meng, Q. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol. Biochem. 2015, 89, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Fantini, E.; Bassolino, L.; Povero, G.; Spelt, C.; Buti, S.; Giuliano, G.; Quattrocchio, F.; Koes, R.; Perata, P.; et al. Tomato R2R3-MYB proteins SlANT1 and SlAN2: Same protein activity, different roles. PLoS ONE 2015, 10, e0136365. [Google Scholar] [CrossRef] [PubMed]
- Rick, C.M.; Cisneros, P.; Chetelat, R.T.; DeVerna, J.W. Abg—a gene on chromosome 10 for purple fruit derived from S. lycopersicoides. Rep. Tomato Genet. Coop. 1994, 44, 29–30. [Google Scholar]
- Canady, M.A.; Ji, Y.; Chetelat, R.T. Homeologous recombination in Solanum lycopersicoides introgression lines of cultivated tomato. Genetics 2006, 174, 1775–1788. [Google Scholar] [CrossRef] [PubMed]
- Rick, C.M.; Reeves, A.F.; Zobel, R.W. Inheritance and linkage relations of four new mutants. Tomato Genet. Coop. Rep. 1968, 18, 34–35. [Google Scholar]
- Kerckhoffs, L.H.; De Groot, N.A.; van Tuinen, A.; Schreuder, M.E.; Nagatani, A.; Koornneef, M.; Kendrick, R.E. Physiological characterization of exaggerated-photoresponse mutants of tomato. J. Plant Physiol. 1997, 150, 578–587. [Google Scholar] [CrossRef]
- Povero, G.; Gonzali, S.; Bassolino, L.; Mazzucato, A.; Perata, P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J. Plant Physiol. 2011, 168, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Mcroberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Xu, J.; Rhodes, D.; Shen, Y.; Song, W.; Katz, B.; Tomich, J.; Wang, W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016, 202, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.C.; Shelton, B.A.; Howard, L.R.; Lee, S.; Vrebalov, J.; Giovannoni, J.J. The tomato high-pigment (hp) locus maps to chromosome 2 and influences plastome copy number and fruit quality. Theor. Appl. Genet. 1997, 95, 1069–1079. [Google Scholar] [CrossRef]
- Long, M.; Millar, D.J.; Kimura, Y.; Donovan, G.; Rees, J.; Fraser, P.D.; Bramley, P.M.; Bolwell, G.P. Metabolite profiling of carotenoid and phenolic pathways in mutant and transgenic lines of tomato: identification of a high antioxidant fruit line. Phytochemistry 2006, 67, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, D.; Graziani, G.; Lercari, B.; Fogliano, V.; Soldatini, G.F.; Ranieri, A. Changes in carotenoid and ascorbic acid contents in fruits of different tomato genotypes related to the depletion of UV-B radiation. J. Agric. Food Chem. 2005, 53, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- Sestari, I.; Zsögön, A.; Rehder, G.G.; Teixeira, L. de L.; Hassimotto, N.M.; Purgatto, E.; Benedito, V.A.; Pereira-Peres, L.E. Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Sci. Hortic. 2014, 175, 111–120. [Google Scholar] [CrossRef]
| Carotenoid | Concentration | Polyphenol | Concentration |
|---|---|---|---|
| Lycopene | 7.8–18.1 | Naringenin chalcone | 0.9–18.2 |
| Phytoene | 1.0–2.9 | Rutin | 0.5–4.5 |
| Phytofluene | 0.2–1.6 | Quercetin | 0.7–4.4 |
| β-Carotene | 0.1–1.2 | Chlorogenic acid | 1.4–3.3 |
| γ-Carotene | 0.05–0.3 | Caffeic Acid | 0.1–1.3 |
| δ-Carotene | 0–0.2 | Naringenin | 0–1.3 |
| Lutein | 0.09 | Kaempferol-3-rutinoside | 0–0.8 |
| Neurosporene | 0–0.03 | p-Coumaric acid | 0–0.6 |
| α-Carotene | 0–0.002 | Ferulic acid | 0.2–0.5 |
| Neoxanthin | - | Kaempferol | 0–0.2 |
| Violaxanthin | - | Myricetin | - |
| Anteraxanthin | - | Cyanidin | - |
| Zeaxanthin | - | Pelargonidin | - |
| Delphinidin | - |
| Altered Activity | Mutant/Gene | Fruit Colour | Details |
|---|---|---|---|
| Single step of biosynthesis pathway | r (yellow flesh) | Yellow fruits | Null mutation of PSY-1 gene arrests carotenoid synthesis and the yellow colour is due to rutin accumulation |
| t (tangerine) | Orange fruits | Defective CRTISO enzyme. Prolycopene (orange colour) is accumulated | |
| og (old gold) and ogc (old gold crimson) | Intense red fruits | Frameshift mutations originating defective CYC-B Lycopene is accumulated at the expense of β-carotene | |
| B (Beta) | Orange fruits | Mutation in the promoter of CYC-B gene (increased transcription). β -carotene increases at the expense of lycopene | |
| Del (Delta) | Orange fruits | LCY-E transcription is increased, and more δ-carotene is produced at the expense of lycopene | |
| hp-3 (high pigment 3) | Intense red fruits | Defective ZE mutant. Biosynthesis of ABA is decreased, resulting in increased plastid division and higher accumulation of carotenoids | |
| Regulation | hp-1, hp-1w (high pigment 1) | Intense red fruits | Mutation of DD1 homolog. Altered light regulation. Increased total carotenoids, vitamin C and polyphenols |
| hp-2, hp-2j, hp-2dg (high pigment 2) | Intense red fruits | Mutation of TDET1. Altered light signal-transduction machinery. Increased total carotenoids, vitamin C and quercetin | |
| Ip (Intense pigment) | Intense red fruits | Promotion of phytochrome signal amplification. Increased soluble solids and total carotenoids |
| Altered activity | Mutant/gene | Fruit colour | Details |
|---|---|---|---|
| Regulation | Y (yellow) | Pink fruits | Mutation of SlMYB12, transcription factor involved in the regulation of phenylpropanoid/flavonoid pathway. Lack of accumulation of naringenin chalcone in fruit peel |
| Aft (Anthocyanin fruit) | Purple fruits (external) | Probable regulation of flavonoid/anthocyanidin synthesis in fruits. Increased levels of flavonols, delphinidin, malvidin and petunidin | |
| Abg (Aubergine) | Purple fruits (external) | Possible allele of Aft. | |
| atv (atroviolacea) | Light purple fruits (external) | Possible role in the phytochrome phyB1 high irradiance response pathway. Strong accumulation of anthocyanins in vegetative parts. Small effect on fruit, but it has synergic effects with Aft | |
| hp-1, hp-1w (high pigment 1) | Intense red fruits | Mutation of DD1 homolog. Altered light regulation. Increased total carotenoids, vitamin C and polyphenols | |
| hp-2, hp-2j, hp-2dg (high pigment 2) | Intense red fruits | Mutation of TDET1. Altered light signal-transduction machinery. Increased total carotenoids, vitamin C and quercetin |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. https://doi.org/10.3390/cancers8060058
Martí R, Roselló S, Cebolla-Cornejo J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers. 2016; 8(6):58. https://doi.org/10.3390/cancers8060058
Chicago/Turabian StyleMartí, Raúl, Salvador Roselló, and Jaime Cebolla-Cornejo. 2016. "Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention" Cancers 8, no. 6: 58. https://doi.org/10.3390/cancers8060058






