From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nested PCR and Direct DNA Sequencing Can Detect Minor HPV Variants
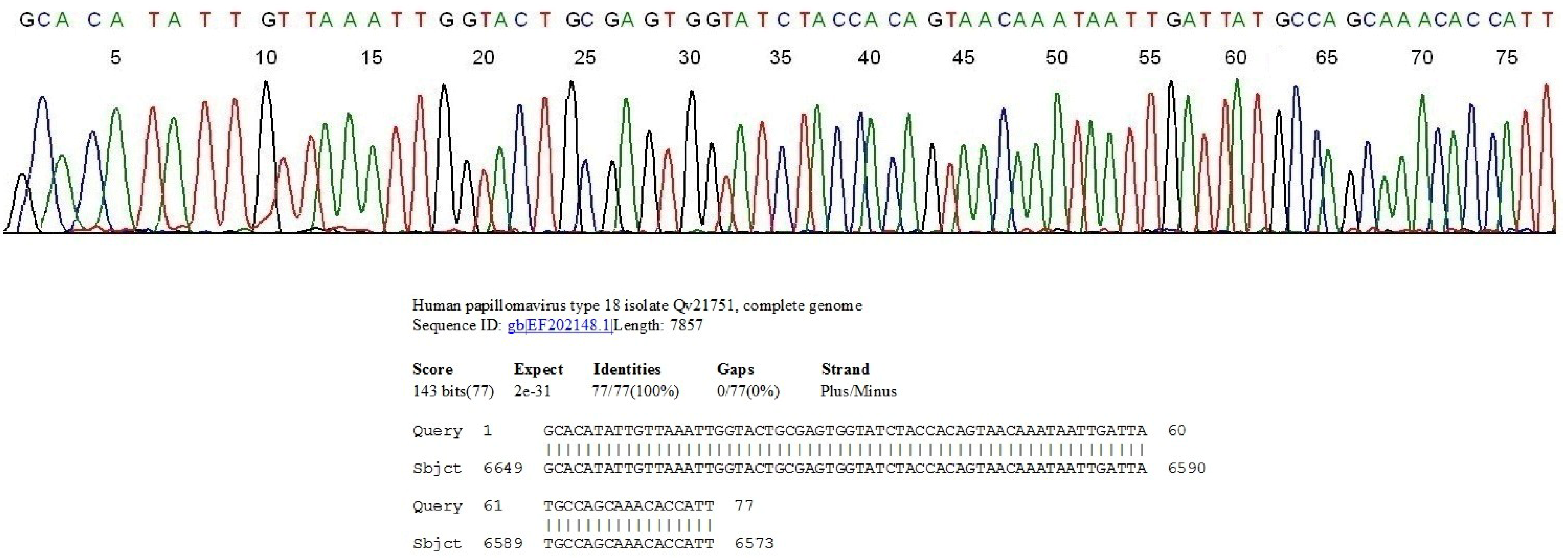
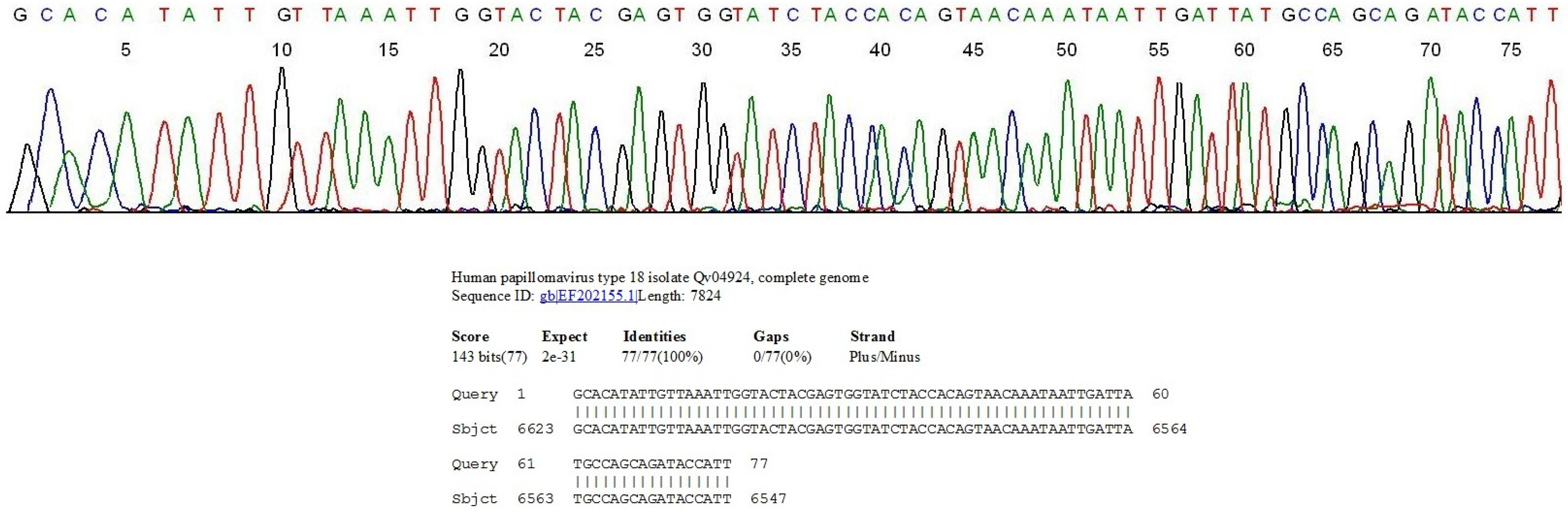
2.2. HPV Genotyping and Cytopathology in Samples Collected in Private Practice
| HPV Genotype | No. PAP-Negative | No. PAP-ASCUS | No. PAP-LSIL | No. PAP-HSIL(%) | [(PAP− cases)/(HPV+ cases)] = (%) |
|---|---|---|---|---|---|
| 6 | 17 | 7 | 6 | 0 | 17/30 (56.7) |
| 11 | 1 | 1 | 1 | 0 | 1/3 (33.3) |
| 16 | 55 | 16 | 20 | 22(19.5) | 55/113 (48.7) |
| 18 | 19 | 6 | 11 | 1(2.7) | 19/37 (51.4) |
| 26 | 0 | 0 | 3 | 0 | 0/3 (0) |
| 31 | 15 | 11 | 7 | 5(13.2) | 15/38 (39.5) |
| 32 | 5 | 0 | 0 | 0 | 5/5 (100) |
| 33 | 10 | 4 | 2 | 3(15.8) | 10/19 (52.6) |
| 35 | 13 | 2 | 6 | 2(8.7) | 13/23 (56.5) |
| 39 | 17 | 4 | 8 | 0 | 17/29 (58.6) |
| 40 | 3 | 2 | 0 | 0 | 3/5 (60.0) |
| 44 | 6 | 3 | 0 | 0 | 6/9 (33.3) |
| 45 | 22 | 5 | 3 | 1(3.2) | 22/31 (71.0) |
| 51 | 3 | 0 | 3 | 0 | 3/6 (50.0) |
| 52 | 31 | 7 | 12 | 4(7.4) | 31/54 (57.4) |
| 53 | 5 | 1 | 4 | 0 | 5/10 (50.0) |
| 54 | 40 | 2 | 2 | 0 | 40/44 (90.9) |
| 55 | 4 | 2 | 0 | 0 | 4/6 (66.7) |
| 56 | 9 | 4 | 5 | 0 | 9/18 (50.0) |
| 58 | 15 | 4 | 4 | 0 | 15/23 (65.2) |
| 59 | 18 | 3 | 8 | 1 (3.3) | 18/30 (60.0) |
| 61 | 19 | 4 | 1 | 0 | 19/24 (79.2) |
| 62 | 19 | 0 | 2 | 0 | 19/21 (90.5) |
| 66 | 2 | 3 | 15 | 0 | 2/20 (10.0) |
| 67 | 5 | 3 | 1 | 0 | 5/9 (55.6) |
| 68 | 2 | 0 | 1 | 0 | 2/3 (66.7) |
| 69 | 2 | 1 | 1 | 0 | 2/4 (50.0) |
| 70 | 11 | 0 | 2 | 0 | 11/13 (84.6) |
| 71 | 2 | 0 | 0 | 0 | 2/2 (100) |
| 72 | 17 | 0 | 0 | 0 | 17/17 (100) |
| 73 | 15 | 6 | 6 | 0 | 15/27 (55.6) |
| 74 | 1 | 1 | 0 | 0 | 1/2 (50.0) |
| 81 | 16 | 3 | 7 | 0 | 16/26 (61.5) |
| 83 | 2 | 1 | 0 | 0 | 2/3 (66.7) |
| 84 | 12 | 0 | 0 | 0 | 12/12 (100) |
| 86 | 1 | 0 | 1 | 0 | 1/2 (50.0) |
| 87 | 1 | 1 | 1 | 0 | 1/3 (33.3) |
| 89 | 1 | 0 | 1 | 0 | 1/2 (50.0) |
| 90 | 1 | 0 | 0 | 0 | 1/1 (100) |
| 91 | 1 | 0 | 0 | 0 | 1/1 (100) |
| Mixed | 18 | 17 | 5 | 2 (4.8) | 18/42 (42.9) |
| HPV+ cases Subtotal | 456 | 124 | 149 | 41 | 456/770 (59.2) |
| HPV− cases (PAP− %) | 7545 | 146 | 0 | 0 | 7545/7691 (98.1%) (<LSIL 100%) |
| Total No. | 8001 | 270 | 149 | 41 | 8461 |
2.3. Correlation of HPV Genotyping, HSIL Cytology and Histologic Findings
| HPV Genotype | Four-Quadrant Biopsies | |||||
|---|---|---|---|---|---|---|
| Negative | CIN1 | CIN2 | CIN3 | No Biopsies * | Total | |
| 16 | 0 | 4 | 4 | 11 | 3 | 22 |
| 18 | 0 | 1 | 0 | 0 | 0 | 1 |
| 31 | 1 | 2 | 1 | 1 | 0 | 5 |
| 33 | 1 | 0 | 1 | 1 | 0 | 3 |
| 35 | 0 | 0 | 1 | 1 | 0 | 2 |
| 45 | 0 | 1 | 0 | 0 | 0 | 1 |
| 52 | 0 | 0 | 2 | 2 | 0 | 4 |
| 59 | 0 | 0 | 1 | 0 | 0 | 1 |
| Mixed | 1 | 1 | 0 | 0 | 0 | 2 |
| Total | 3 | 9 | 10 | 16 | 3 | 41 |
2.4. HPV Genotype Persistence and Cytohistopathologic Dynamics
| HPV Type | Initial Cytology Classification | Follow-up Cytology | Biopsy Result | Patients TOTAL | |||||
|---|---|---|---|---|---|---|---|---|---|
| NEG | ASC | LSIL | NC | REG | PRO | CIN3 | <CIN3 | ||
| 6 | 8 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 |
| 11 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 16 | 42 | 14 | 7 | 34 | 6 | 23 | 8 | 17 | 63 |
| 18 | 8 | 4 | 2 | 5 | 6 | 3 | 0 | 2 | 14 |
| 31 | 8 | 0 | 1 | 2 | 1 | 6 | 1 | 2 | 9 |
| 33 | 2 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 4 |
| 35 | 3 | 2 | 4 | 3 | 5 | 1 | 0 | 1 | 9 |
| 39 | 5 | 2 | 0 | 2 | 2 | 3 | 0 | 2 | 7 |
| 45 | 10 | 0 | 2 | 6 | 0 | 6 | 3 | 5 | 12 |
| 51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 52 | 14 | 3 | 1 | 11 | 0 | 7 | 3 | 4 | 18 |
| 53 | 1 | 0 | 3 | 2 | 0 | 2 | 0 | 2 | 4 |
| 54 | 18 | 2 | 2 | 18 | 3 | 1 | 0 | 2 | 22 |
| 55 | 3 | 2 | 0 | 3 | 1 | 1 | 0 | 0 | 5 |
| 56 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 58 | 1 | 1 | 3 | 2 | 2 | 1 | 0 | 2 | 5 |
| 59 | 9 | 1 | 2 | 8 | 2 | 2 | 0 | 1 | 12 |
| 61 | 6 | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 6 |
| 62 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 3 |
| 66 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 2 |
| 68 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 70 | 8 | 0 | 0 | 6 | 0 | 2 | 0 | 2 | 8 |
| 72 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| 73 | 2 | 0 | 4 | 4 | 1 | 1 | 0 | 1 | 6 |
| 81 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| 83 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 84 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Total | 162 | 33 | 34 | 136 | 30 | 63 | 15 | 44 | 229 |
3. Experimental Section
3.1. Initial Sample Processing
3.2. Primary PCR with MY09 and MY11 Degenerate Primers
3.3. Heminested PCR with GP6 and MY11 Consensus General Primers
3.4. Direct DNA Sequencing for HPV Genotyping
3.5. Negative and Positive Controls
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wallin, K.L.; Wiklund, F.; Angstrom, T.; Bergman, F.; Stendahl, U.; Wadell, G.; Hallmans, G.; Dillner, J. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N. Engl. J. Med. 1999, 341, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; van den Brule, A.J.; Paull, G.; Svare, E.I.; Sherman, M.E.; Thomsen, B.L.; Suntum, M.; Bock, J.E.; Poll, P.A.; Meijers, C.J. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: Population based prospective follow up study. BMJ 2002, 325, 572–676. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, K.S.; Cubie, H.A.; Whitley, M.W.; Gilkison, G.; Arends, M.J.; Graham, C.; McGoogan, E. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. J. Clin. Pathol. 2005, 58, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Brummer, O.; Hollwitz, B.; Bohmer, G.; Kuhnle, H.; Petry, K.U. Human papillomavirus-type persistence patterns predict the clinical outcome of cervical intraepithelial neoplasia. Gynecol. Oncol. 2006, 102, 517–522. [Google Scholar] [CrossRef] [PubMed]
- WHO/ICO. 2010. WHO/ICO information centre on human papilloma virus (HPV) and cervical cancer, 2011. Available online: http://www.who.int/hpvcentre/en/ (accessed on 2 May 2014).
- Guzick, D.S. Efficacy of screening for cervical cancer: A review. Am. J. Public Health 1978, 68, 125–134. [Google Scholar] [CrossRef] [PubMed]
- CDC. Genital HPV Infection-Fact Sheet, Page last updated: 20 March 2014. Available online: http://www.cdc.gov/std/hpv/stdfact-hpv.htm (accessed on 2 May 2014).
- CDC. Cervical Cancer Statistics, Page last updated: 20 December 2012. Available online: http://www.cdc.gov/cancer/cervical/statistics/ (accessed on 2 May 2014).
- Janerich, D.T.; Hadjimichael, O.; Schwartz, P.E.; Lowell, D.M.; Meigs, J.W.; Merino, M.J.; Flannery, J.T.; Polednak, A.P. The screening histories of women with invasive cervical cancer, Connecticut. Am. J. Public Health 1995, 85, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Wentzensen, N. From human papillomavirus to cervical cancer. Obstet. Gynecol. 2010, 116, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Masure, M.; Levert, M.; Putaud, I.; Mangeonjean, C.; Lorenzato, M.; Nazeyrollas, P.; Gabriel, R.; Quereux, C.; Birembaut, P. Human papillomavirus detection by the hybrid capture II assay: A reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn. Mol. Pathol. 2000, 9, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Lorincz, A.T.; Mielzynska-Lohnas, I.; Scott, D.R.; Glass, A.G.; Sherman, M.E.; Schussler, J.E.; Schiffman, M. Results of human papillomavirus DNA testing with hybrid capture 2 assay are reproducible. J. Clin. Microbiol. 2002, 40, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Mach, H.; Volkin, D.B.; Troutman, R.D.; Wang, B.; Luo, Z.; Jansen, K.U.; Shi, L. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J. Pharm. Sci. 2006, 95, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.T. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine 2007, 25, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Edwards, R.P.; Zepp, F.; Carletti, I.; Dessy, F.J.; Trofa, A.F.; Schuind, A.; et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Human Vaccines 2009, 5, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lazcano-Ponce, E.; Villa, L.; Nolan, T.; Marchant, C.; Radley, D.; Golm, G.; McCarroll, K.; Yu, J.; Esser, M.T.; et al. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J. Infect. Dis. 2007, 196, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.L.; Ault, K.A.; Giuliano, A.R.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Brown, D.R.; Ferenczy, A.; Harper, D.M.; Koutsky, L.A.; et al. Immunologic responses following administration of a vaccine targeting human papillo-mavirus types 6, 11, 16, and 18. Vaccine 2006, 24, 5571–5583. [Google Scholar] [CrossRef] [PubMed]
- The Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar]
- VRBPAC Background Document Gardasil™ HPV Quadrivalent Vaccine. VRBPAC Meeting; 18 May 2006. Available online: http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006–4222B3.pdf (accessed on 2 May 2014).
- Trimble, C.L.; Piantadosi, S.; Gravitt, P.; Ronnett, B.; Pizer, E.; Elko, A.; Wilgus, B.; Yutzy, W.; Daniel, R.; Shah, K.; et al. Spontaneous regression of high-grade cervical dysplasia: Effects of human papillomavirus type and HLA phenotype. Clin. Cancer Res. 2005, 11, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Stoler, M.H.; Solomon, D.; Schiffman, M. The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: An ALTS report. Am. J. Clin. Pathol. 2007, 127, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.B.; Ma, Y.; Wibbelsman, C.; Darragh, T.M.; Powers, A.; Farhat, S.; Shiboski, S. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet. Gynecol. 2010, 116, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- CDC. Human Papillomavirus (HPV)-Associated Cancers, Page last updated: 13 August 2012. Available online: http://www.cdc.gov/cancer/hpv/statistics/age.htm (accessed on 2 May 2014).
- Beller, U.; Abu-Rustum, N.R. Cervical cancers after human papillomavirus vaccination. Obstet. Gynecol. 2009, 113, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Geraets, D.; Alemany, L.; Guimera, N.; de Sanjose, S.; de Koning, M.; Molijn, A.; Jenkins, D.; Bosch, X.; Quint, W. on behalf of the RIS HPV TT study group. Detection of rare and possibly carcinogenic human papillomavirus genotypes as single infections in invasive cervical cancer. J. Pathol. 2012, 228, 534–543. [Google Scholar]
- American Cancer Society. Cervical Cancer, Last Revised: 31 January 2014. Available online: http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-prevention (accessed on 2 May 2014).
- NCI FactSheet: Human Papillomavirus (HPV) Vaccines. Last Reviewed: 29 December 2011. Available online: http://www.cancer.gov/cancertopics/factsheet/prevention/HPV-vaccine (accessed on 2 May 2014).
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E.R. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar] [CrossRef] [PubMed]
- FDA NEWS RELEASE. FDA approves first human papillomavirus test for primary cervical cancer screening. 24 April 2014. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm (accessed on 2 May 2014). [Google Scholar]
- Tsai, T.C.; Chen, S.L. The biochemical and biological functions of human papilloma virus type16 E5 protein. Arch. Virol. 2003, 148, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zerfass, K.; Schulze, A.; Spitkovsky, D.; Friedman, V.; Henglein, B.; Jansen-Dürr, P. Sequential activation of cyclin E and cyclin A gene expression by human papilloma virus type 16 E7 through sequences necessary for transformation. J. Virol. 1995, 69, 6389–6399. [Google Scholar] [PubMed]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; zur Hausen, H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Barsotti, C.; Cosio, S.; Domenici, L.; Riccardo Genazzani, A. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: A review of the literature. Gynecol. Endocrinol. 2011, 27, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.K.; Lichtenstein, P.; Gyllensten, U.B. Heritability of cervical tumours. Int. J. Cancer 2000, 88, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Bratti, M.C.; Rodriguez, A.C.; Herrero, R.; Burk, R.D.; Porras, C.; González, P.; Sherman, M.E.; Wacholder, S.; Lan, Z.E.; et al. Common variants in immune and DNA repair genes and risk for human papillomavirus persistence and progression to cervical cancer. J. Infect. Dis. 2009, 199, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.W.; Chun, T.; Sul, D.; Hwang, K.W.; Kang, H.S.; Lee, D.J.; Han, I.K. Strategies against human papillomavirus infection and cervical cancer. J. Microbiol. 2004, 42, 255–266. [Google Scholar] [PubMed]
- Lee, S.H. Guidelines for the use of molecular tests for the detection and genotyping of human papilloma virus from clinical specimens. Methods Mol. Biol. 2012, 903, 65–101. [Google Scholar] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Delius, H.; Halpern, A.L.; Bernard, H.U. Analysis of genomic sequences of 95 papillomavirus types: Uniting typing, phylogeny, and taxonomy. J. Virol. 1995, 69, 3074–3083. [Google Scholar] [PubMed]
- Vernon, S.D.; Unger, E.R.; Williams, D. Comparison of human papillomavirus detection and typing by cycle sequencing, line blotting, and hybrid capture. J. Clin. Microbiol. 2000, 38, 651–655. [Google Scholar] [PubMed]
- Johnson, T.; Bryder, K.; Corbet, S.; Fomsgaard, A. Routine genotyping of human papillomavirus samples in Denmark. APMIS 2003, 111, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Speich, N.; Schmitt, C.; Bollmann, R.; Bollmann, M. Human papillomavirus (HPV) study of 2916 cytological samples by PCR and DNA sequencing: Genotype spectrum of patients from the west German area. J. Med. Microbiol. 2004, 53, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Meisels, A.; Fortin, R. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 1976, 20, 505–509. [Google Scholar] [PubMed]
- Wright, T.C., Jr.; Stoler, M.H.; Sharma, A.; Zhang, G.; Behrens, C.; Wright, T.L. ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am. J. Clin. Pathol. 2011, 136, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [PubMed]
- Jacobs, M.V.; Walboomers, J.M.; Snijders, P.J.; Voorhorst, F.J.; Verheijen, R.H.; Fransen-Daalmeijer, N.; Meijer, C.J. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: The age-related patterns for high-risk and low-risk types. Int. J. Cancer 2000, 87, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Hildesheim, A.; Bratti, C.; Sherman, M.E.; Hutchinson, M.; Morales, J.; Balmaceda, I.; Greenberg, M.D.; Alfaro, M.; Burk, R.D.; et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J. Natl. Cancer Inst. 2000, 92, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Molano, M.; Posso, H.; Weiderpass, E.; van den Brule, A.J.; Ronderos, M.; Franceschi, S.; Meijer, C.J.; Arslan, A.; Munoz, N. HPV Study Group HPV Study Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br. J. Cancer 2002, 87, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.C.; Pereira, J.S.; Prado, J.C.; Villa, L.L.; Rohan, T.E.; Franco, E.L. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J. Infect. Dis. 2001, 184, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, S.A.; van Velzen, D.; Korporaal, H.; Kok, P.L.; Boon, M.E. Transience of cervical HPV infection in sexually active, young women with normal cervicovaginal cytology. Br. J. Cancer 1995, 72, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Shew, M.L.; Qadadri, B.; Neptune, N.; Vargas, M.; Tu, W.; Juliar, B.E.; Breen, T.E.; Fortenberry, J.D. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J. Infect. Dis. 2005, 191, 182–192. [Google Scholar] [PubMed]
- Cuschieri, K.S.; Cubie, H.A.; Whitley, M.W.; Seagar, A.L.; Arends, M.J.; Moore, C.; Gilkisson, G.; McGoogan, E. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J. Clin. Pathol. 2004, 57, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Wensveen, C.W.; Kagie, M.J.; Nagelkerke, N.J.; Veldhuizen, R.W.; Trimbos, J.B. Can viral load, semi-quantitatively evaluated, of human papillomavirus predict cytological or histological outcome in women with atypical squamous or glandular cells of undetermined significance cytology? Eur. J. Gynaecol. Oncol. 2005, 26, 393–397. [Google Scholar]
- Sherman, M.E.; Wang, S.S.; Wheeler, C.M.; Rich, L.; Gravitt, P.E.; Tarone, R.; Schiffman, M. Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: Dominant impact of surrounding low-grade lesions. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1038–1044. [Google Scholar]
- Sherman, M.E.; Wang, S.S.; Tarone, R.; Rich, L.; Schiffman, M. Histopathologic extent of cervical intraepithelial neoplasia 3 lesions in the atypical squamous cells of undetermined significance low-grade squamous intraepithelial lesion triage study: Implications for subject safety and lead-time bias. Cancer Epidemiol. Biomark. Prev. 2003, 12, 372–379. [Google Scholar]
- Xi, L.F.; Hughes, J.P.; Edelstein, Z.R.; Kiviat, N.B.; Koutsky, L.A.; Mao, C.; Ho, J.; Schiffman, M. Human Papillomavirus (HPV) type 16 and type 18 DNA Loads at Baseline and Persistence of Type-Specific Infection during a 2-year follow-up. J. Infect. Dis. 2009, 200, 1789–1797. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Criel, A.M.; Benoy, I.H.; Arbyn, M.; Vereecken, A.J.; Bogers, J.J. Changes in type-specific human papillomavirus load predict progression to cervical cancer. J. Cell. Mol. Med. 2012, 16, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Garner-Hamrick, P.A.; Fisher, C. HPV episomal copy number closely correlates with cell size in keratinocyte monolayer cultures. Virology 2002, 301, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Cubie, H.A.; Seagar, A.L.; McGoogan, E.; Whitehead, J.; Brass, A.; Arends, M.J.; Whitley, M.W. Rapid real time PCR to distinguish between high risk human papillomavirus types 16 and 18. Mol. Pathol. 2001, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kinney, W.; Stoler, M.H.; Castle, P.E. Special commentary: Patient safety and the next generation of HPV DNA tests. Am. J. Clin. Pathol. 2010, 134, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Eklund, C.; Forslund, O.; Wallin, K.L.; Zhou, T.; Dillner, J.; WHO Human Papillomavirus Laboratory Network. The 2010 global proficiency study of human papillomavirus genotyping in vaccinology. J. Clin. Microbiol. 2012, 50, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Gong, B.; Cai, X.; Yang, X.; Gan, X.; Tong, X.; Li, H.; Zhu, M.; Yang, F.; Zhou, H.; et al. Prevent cervical cancer by screening with reliable human papillomavirus detection and genotyping. Cancer Med. 2012, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for industry and food and drug administration staff–establishing the performance characteristics of in vitro diagnostic devices for the detection or detection and differentiation of human papillomaviruses. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm181509.htm (accessed on 2 May 2014).
- Lee, S.H.; Vigliotti, V.S.; Vigliotti, J.S.; Pappu, S. Routine human papillomavirus genotyping by DNA sequencing in community hospital laboratories. Infect. Agent. Cancer 2007, 2. [Google Scholar] [CrossRef]
- Lee, S.H.; Vigliotti, V.S.; Pappu, S. Human papillomavirus (HPV) infection among women in a representative rural and suburban population of the United States. Inter. J. Gynecol. Obstet. 2009, 105, 210–214. [Google Scholar] [CrossRef]
- Lee, S.H.; Vigliotti, V.S.; Vigliotti, J.S.; Pappu, S. Validation of human papillomavirus genotyping by signature DNA sequence analysis. BMC Clin. Pathol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, A. Screening for the prevention of cervical cancer in the era of human papillomavirus vaccination: An Australian perspective. Acta Cytol. 2011, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Poljak, M.; Kovanda, A.; Kocjan, B.J.; Seme, K.; Jancar, N.; Vrtacnik-Bokal, E. The Abbott RealTime High Risk HPV test: Comparative evaluation of analytical specificity and clinical sensitivity for cervical carcinoma and CIN 3 lesions with the Hybrid Capture 2 HPV DNA test. Acta Dermatovenerol. Alp. Panon. Adriat. 2009, 18, 94–103. [Google Scholar]
- Tjalma, W.A.; Fiander, A.; Reich, O.; Powell, N.; Nowakowski, A.M.; Kirschner, B.; Koiss, R.; O’Leary, J.; Joura, E.A.; Rosenlund, M.; et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer 2013, 132, 854–867. [Google Scholar] [CrossRef] [PubMed]
- De Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Lurchachaiwong, W.; Junyangdikul, P.; Termrungruanglert, W.; Payungporn, S.; Sampatanukul, P.; Tresukosol, D.; Niruthisard, S.; Trivijitsilp, P.; Karalak, A.; Swangvaree, S.; et al. Whole-genome sequence analysis of human papillomavirus type 18 from infected Thai women. Intervirology 2010, 53, 161–166. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.A.; Peters, L.A.; Aziz, M.F.; Siregar, B.; Cornain, S.; Vrede, M.A.; Jordanova, E.S.; Fleuren, G.J. Human papillomavirus type 18 variants: Histopathology and E6/E7 polymorphisms in three countries. Int. J. Cancer 2005, 114, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Kiviat, N.B.; Hildesheim, A.; Galloway, D.A.; Wheeler, C.M.; Ho, J.; Koutsky, L.A. Human Papillomavirus Type 16 and 18 Variants: Race-Related Distribution and Persistence. J. Natl. Cancer Inst. 2006, 98, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Detection of human papillomavirus (HPV) L1 gene DNA possibly bound to particulate aluminum adjuvant in the HPV vaccine Gardasil®. J. Inorg. Biochem. 2012, 117, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Berlin Grace, V.M. HPV type 18 is more oncopotent than HPV16 in uterine cervical carcinogenesis although HPV16 is the prevalent type in Chennai, India. Indian J. Cancer 2009, 46, 203–207. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetrics and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetricians-Gynecologists. Number 61, April 2005. Human papillomavirus. Obstet. Gynecol. 2005, 105, 905–918. [Google Scholar]
- Stoler, M.H. Advances in cervical screening technology. Mod. Pathol. 2000, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Stoler, M.H. HPV for cervical cancer screening: Is the era of the molecular pap smear upon us? J. Histochem. Cytochem. 2001, 49, 1197–1198. [Google Scholar] [CrossRef]
- Stout, N.K.; Goldhaber-Fiebert, J.D.; Ortendahl, J.D.; Goldie, S.J. Trade-offs in cervical cancer prevention: Balancing benefits and risks. Arch. Intern. Med. 2008, 168, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Meijer, C.J.; Berkhof, J.; Castle, P.E.; Hesselink, A.T.; Franco, E.L.; Ronco, G.; Arbyn, M.; Bosch, F.X.; Cuzick, J.; Dillner, J.; et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 2009, 124, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Stoler, M.H.; Castle, P.E.; Solomon, D.; Schiffman, M. American Society for Colposcopy and Cervical Pathology. The expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assays. Am. J. Clin. Pathol. 2007, 127, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Surwit, E.; Moon, T.E.; Childers, J.M.; Davis, J.R.; Dorr, R.T.; Johnson, C.S.; Alberts, D.S. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: A randomized trial. J. Natl. Cancer Inst. 1994, 86, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Keefe, K.A.; Schell, M.J.; Brewer, C.; McHale, M.; Brewster, W.; Chapman, J.A.; Rose, G.S.; McMeeken, D.S.; Lagerberg, W.; Peng, Y.M.; et al. A randomized, double blind, Phase III trial using oral beta-carotene supplementation for women with high-grade cervical intraepithelial neoplasia. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1029–1035. [Google Scholar]
- Follen, M.; Atkinson, E.N.; Schottenfeld, D.; Malpica, A.; West, L.; Lippman, S.; Zou, C.; Hittelman, W.N.; Lotan, R.; Hong, W.K. A randomized clinical trial of 4-hydroxyphenylretinamide for high-grade squamous intraepithelial lesions of the cervix. Clin. Cancer Res. 2001, 7, 3356–3365. [Google Scholar] [PubMed]
- Stoler, M.H.; Wright, T.C., Jr.; Sharma, A.; Apple, R.; Gutekunst, K.; Wright, T.L. ATHENA (Addressing THE Need for Advanced HPV Diagnostics) HPV Study Group. High-risk human papillomavirus testing in women with ASC-US cytology: Results from the ATHENA HPV study. Am J. Clin. Pathol. 2011, 135, 468–475. [Google Scholar] [CrossRef] [PubMed]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [PubMed]
- Cuzick, J.; Clavel, C.; Petry, K.U.; Meijer, C.J.; Hoyer, H.; Ratnam, S.; Szarewski, A.; Birembaut, P.; Kulasingam, S.; Sasieni, P.; et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int. J. Cancer 2006, 119, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Giorgi-Rossi, P.; Carozzi, F.; Dalla Palma, P.; del Mistro, A.; de Marco, L.; de Lillo, M.; Naldoni, C.; Pierotti, P.; Rizzolo, R.; et al. New Technologies for Cervical Cancer screening Working Group. Human papillomavirus testing and liquid-based cytology in primary screening of women younger than 35 years: Results at recruitment for a randomised controlled trial. Lancet Oncol. 2006, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Dillner, J.; Rebolj, M.; Birembaut, P.; Petry, K.U.; Szarewski, A.; Munk, C.; de Sanjose, S.; Naucler, P.; Lloveras, B.; Kjaer, S.; et al. Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European cohort study. BMJ 2008, 337. [Google Scholar] [CrossRef]
- Huang, S.; Afonina, I.; Miller, B.A.; Beckmann, A.M. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int. J. Cancer 1997, 70, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Q.; Yu, S.Z.; Qu, W.; Cruz, Y.; Burk, R.D. Human papillomavirus types 52 and 58. Int. J. Cancer 1998, 75, 484–585. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Jiang, G.; Cruz, Y.; Chang, C.J.; Ho, G.Y.; Klein, R.S.; Burk, R.D. PCR detection of human papillomavirus: Comparison between MY09/MY11 and GP5+/GP6+ primer systems. J. Clin. Microbiol. 1997, 35, 1304–1310. [Google Scholar] [PubMed]
- Evans, M.F.; Adamson, C.S.; Simmons-Arnold, L.; Cooper, K. Touchdown general primer (GP5+/GP6+) PCR and optimized sample DNA concentration support the sensitive detection of human papillomavirus. BMC Clin. Pathol. 2005, 5. [Google Scholar] [CrossRef]
- Liu, S.S.; Leung, R.C.; Chan, K.K.; Cheung, A.N.; Ngan, H.Y. Evaluation of a newly developed GenoArray human papillomavirus (HPV) genotyping assay and comparison with the Roche Linear Array HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Onyekwuluje, J.M.; Steinau, M.; Swan, D.C.; Unger, E.R. A real-time PCR assay for HPV52 detection and viral load quantification. Clin. Lab. 2012, 58, 61–66. [Google Scholar] [PubMed]
- Sandri, M.T.; Lentati, P.; Benini, E.; Dell’Orto, P.; Zorzino, L.; Carozzi, F.M.; Maisonneuve, P.; Passerini, R.; Salvatici, M.; Casadio, C.; et al. Comparison of the Digene HC2 assay and the Roche AMPLICOR human papillomavirus (HPV) test for detection of high-risk HPV genotypes in cervical samples. J. Clin. Microbiol. 2006, 44, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Garland, S.M.; Rudland, E.; Tan, J.; Quinn, M.A.; Tabrizi, S.N. Comparison of the Digene Hybrid Capture 2 assay and Roche AMPLICOR and LINEAR ARRAY human papillomavirus (HPV) tests in detecting high-risk HPV genotypes in specimens from women with previous abnormal Pap smear results. J. Clin. Microbiol. 2007, 45, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, E.; Choi, J.; Jeong, S.; Kim, H.S. Comparison of the Abbott Real Time High-Risk Human Papillomavirus (HPV), Roche Cobas HPV, and Hybrid Capture 2 assays to direct sequencing and genotyping of HPV DNA. J. Clin. Microbiol. 2012, 50, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Kurian, E.M.; Caporelli, M.L.; Baker, S.; Woda, B.; Cosar, E.F.; Hutchinson, L. Cervista HR and HPV 16/18 assays vs. hybrid capture 2 assay: Outcome comparison in women with negative cervical cytology. Am. J. Clin. Pathol. 2011, 136, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Lindsay, L.; Pimenta, J.M.; Poole, C.; Jenkins, D.; Smith, J.S. Persistent human papillomavirus infection and cervical neoplasia: A systematic review and meta-analysis. Am. J. Epidemiol. 2008, 168, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.A.; Castle, P.E.; Schiffman, M.; Gravitt, P.E. Evaluation of any or type-specific persistence of high-risk human papillomavirus for detecting cervical precancer. J. Clin. Microbiol. 2012, 50, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C.A. Human papillomavirus (HPV) vaccine policy and evidence-based medicine: Are they at odds? Ann. Med. 2013, 45, 182–193. [Google Scholar]
- Wilyman, J. HPV vaccination programs have not been shown to be cost-effective in countries with comprehensive Pap screening and surgery. Infect. Agent. Cancer 2013, 8. [Google Scholar] [CrossRef]
- Rothman, S.M.; Rothman, D.J. Marketing HPV Vaccine implications for adolescent health and medical professionalism. JAMA 2009, 302, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Gharizadeh, B.; Oggionni, M.; Zheng, B.; Akom, E.; Pourmand, N.; Ahmadian, A.; Wallin, K.L.; Nyrén, P. Type-specific multiple sequencing primers: A novel strategy for reliable and rapid genotyping of human papillomaviruses by pyrosequencing technology. J. Mol. Diagn. 2005, 7, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chinchai, T.; Chansaenroj, J.; Junyangdikul, P.; Swangvaree, S.; Karalak, A.; Niruthisard, S.; Poovorawan, Y. Comparison between direct sequencing and INNO-LiPA methods for HPV detection and genotyping in Thai Women. Asian Pac. J. Cancer Prev. 2011, 12, 989–994. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Vigliotti, J.S.; Vigliotti, V.S.; Jones, W. From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice. Cancers 2014, 6, 2072-2099. https://doi.org/10.3390/cancers6042072
Lee SH, Vigliotti JS, Vigliotti VS, Jones W. From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice. Cancers. 2014; 6(4):2072-2099. https://doi.org/10.3390/cancers6042072
Chicago/Turabian StyleLee, Sin Hang, Jessica S. Vigliotti, Veronica S. Vigliotti, and William Jones. 2014. "From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice" Cancers 6, no. 4: 2072-2099. https://doi.org/10.3390/cancers6042072
APA StyleLee, S. H., Vigliotti, J. S., Vigliotti, V. S., & Jones, W. (2014). From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice. Cancers, 6(4), 2072-2099. https://doi.org/10.3390/cancers6042072




