Molecular Imaging and Therapy of Merkel Cell Carcinoma
Abstract
:1. Introduction
2. Molecular Imaging and Therapy of Merkel Cell Carcinoma
2.1. Potential Agents
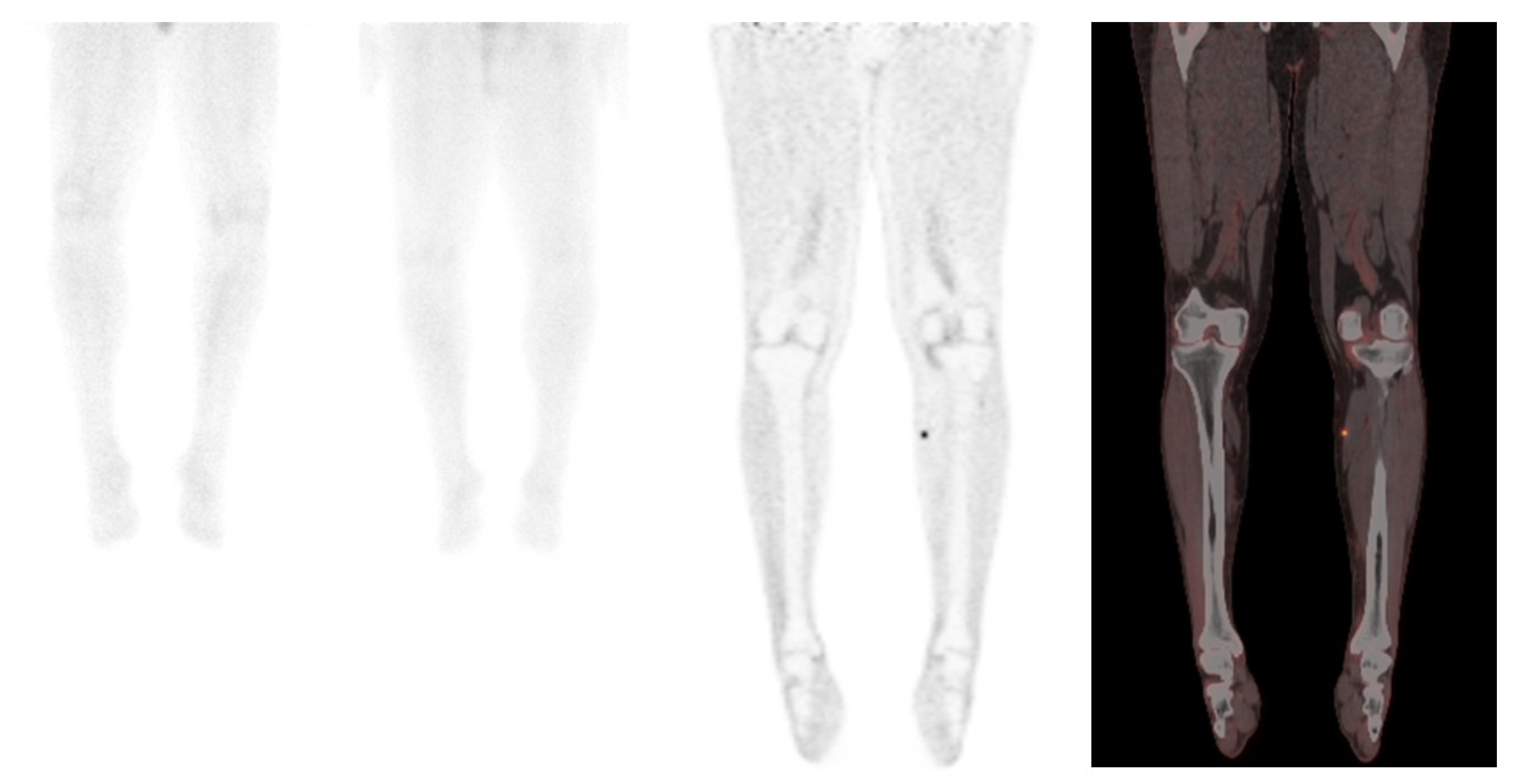

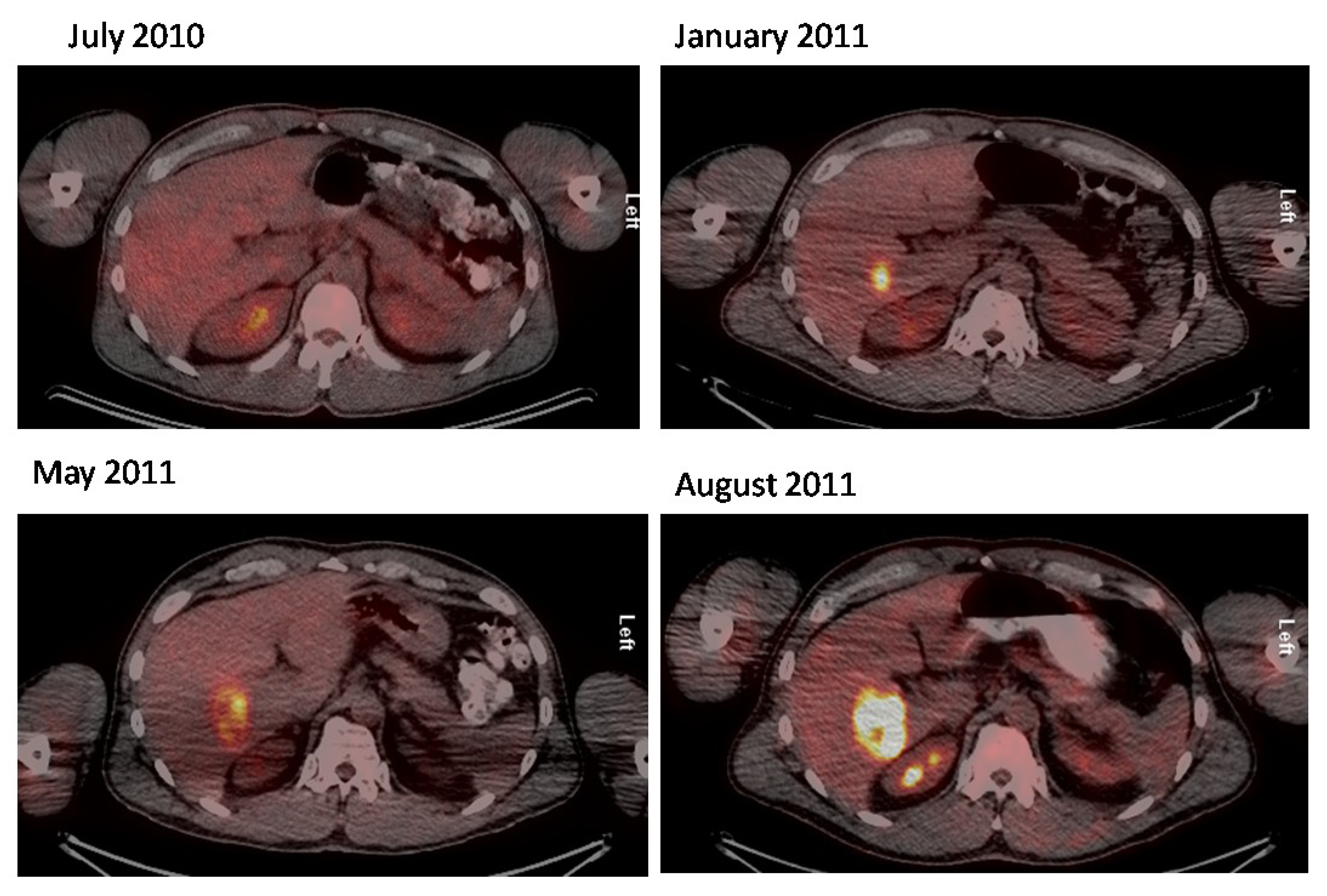

2.2. 18F-FDG PET/CT in Merkel Cell Carcinoma
| Author (number of patients) | SRS sensitivity | SRS specificity | FDG sensitivity | FDG specificity |
|---|---|---|---|---|
| [18] n = 20 | 78% | 96% | NA | NA |
| [28] n = 21 | NA | NA | 94% | 100% |
| [27] n = 11 | NA | NA | 92% | 100% |
| [19] n = 16 | NA | NA | 85.7% | 96.2% |
3. Sentinel Node Biopsy in Merkel Cell Carcinoma
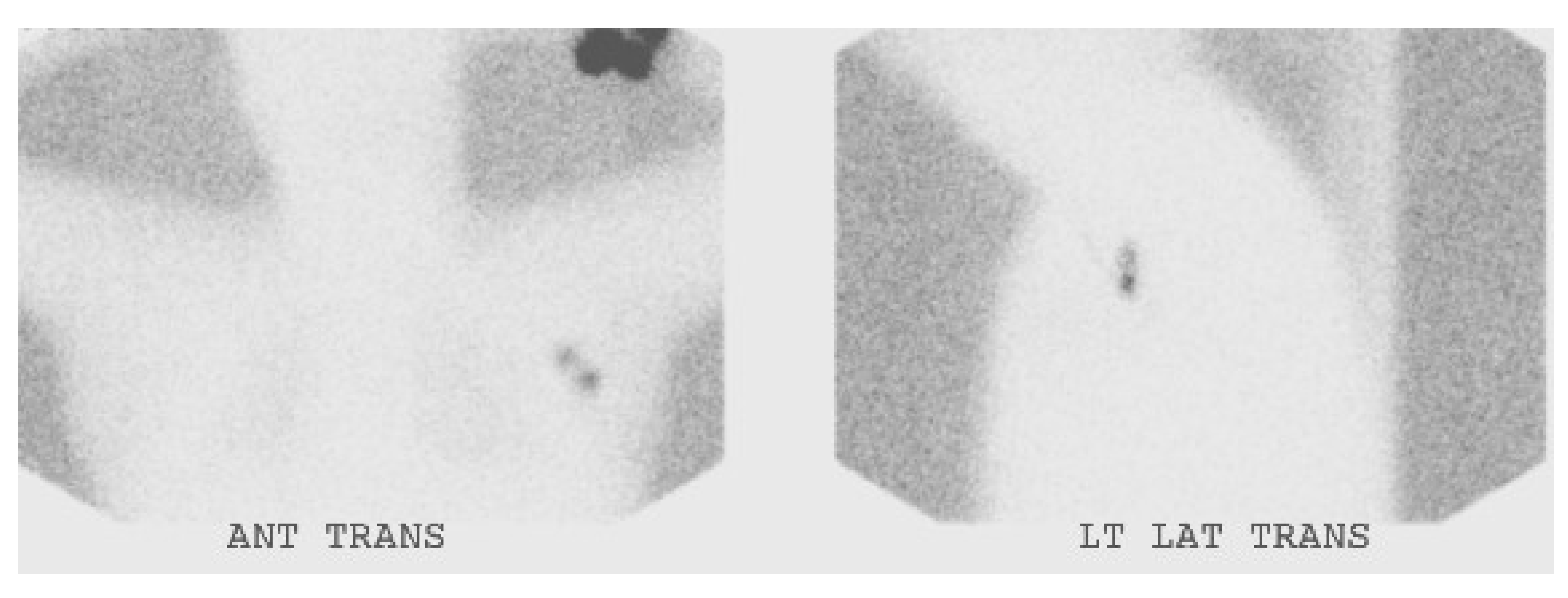
4. Conclusions
Conflicts of Interest
References
- Toker, C. Trabecular carcinoma of skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Five hundred patients with merkel cell carcinoma evaluated at a single institution. Ann. Surg. 2011, 254, 465–475. [Google Scholar] [CrossRef]
- Tadmor, T.; Aviv, A.; Polliack, A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: An old bond with possible new viral ties. Ann. Oncol. 2011, 22, 250–256. [Google Scholar] [CrossRef]
- Medina-Franco, H.; Urist, M.M.; Fiveash, J.; Heslin, M.J.; Bland, K.I.; Beenken, S.W. Multimodality treatment of Merkel cell carcinoma: Case series and literature review of 1024 cases. Ann. Surg. Oncol. 2001, 8, 204–208. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Bichakjian, C.K.; Lowe, L.; Griffith, K.A.; Frohm, M.L.; Fullen, D.R.; Hayman, J.A.; Lao, C.D.; Shah, K.S.; McLean, S.A.; et al. Clinicopathologic features of primary Merkel cell carcinoma: A detailed descriptive analysis of a large contemporary cohort. Dermatol. Surg. 2013, 39, 1009–1016. [Google Scholar] [CrossRef]
- Hruby, G.; Scolyer, R.A.; Thompson, J.F. The important role of radiation treatment in the management of Merkel cell carcinoma. Br. J. Dermatol. 2013, 169, 975–982. [Google Scholar] [CrossRef]
- Allen, P.J.; Bowne, W.B.; Jaques, D.P.; Brennan, M.F.; Busam, K.; Coit, D.G. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef]
- Maza, S.; Trefzer, U.; Hofmann, M.; Schneider, S.; Voit, C.; Krossin, T.; Zander, A.; Audring, H.; Sterry, W.; Munz, D.L. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: Results of a prospective study and review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 433–440. [Google Scholar] [CrossRef]
- Gollub, M.J.; Gruen, D.R.; Dershaw, D.D. Merkel cell carcinoma: CT findings in 12 patients. Am. J. Roentgenol. 1996, 167, 617–620. [Google Scholar] [CrossRef]
- Gupta, S.G.; Wang, L.C.; Penas, P.F.; Gellenthin, M.; Lee, S.J.; Nghiem, P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma—The dana-farber experience and meta-analysis of the literature. Arch. Dermatol. 2006, 142, 685–690. [Google Scholar]
- Carrasquillo, J.A.; Chen, C.C. Molecular imaging of neuroendocrine tumors. Semin. Oncol. 2010, 37, 662–679. [Google Scholar] [CrossRef]
- Von Moll, L.; McEwan, A.J.; Shapiro, B.; Sisson, J.C.; Gross, M.D.; Lloyd, R.; Beals, E.; Beierwaltes, W.H.; Thompson, N.W. Iodine-131 MIBG scintigraphy of neuroendocrine tumors other than pheochromocytoma and neuroblastoma. J. Nucl. Med. 1987, 28, 979–988. [Google Scholar]
- Castagnoli, A.; Biti, G.; de Cristofaro, M.T.; Ferri, P.; Magrini, S.M.; Papi, M.G.; Bianchi, S. Merkel cell carcinoma and iodine-131 metaiodobenzylguanidine scan. Eur. J. Nucl. Med. 1992, 19, 913–916. [Google Scholar] [CrossRef]
- Watanabe, N.; Shimizu, M.; Kageyama, M.; Kitagawa, K.; Hayasaka, S.; Seto, H. I-123-mibg spect of Merkel cell carcinoma. Br. J. Radiol. 1998, 71, 886–887. [Google Scholar]
- Papotti, M.; Macri, L.; Pagani, A.; Aloi, F.; Bussolati, G. Quantitation of somatostatin receptor type 2 in neuroendocrine (Merkel cell) carcinoma of the skin by competitive RT-PCR. Endocr. Pathol. 1999, 10, 37–46. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Hoff, A.M.; Lamberts, S.W.J.; Oei, H.Y.; Krenning, E.P. Somatostatin analog scintigraphy—A simple and sensitive method for the in vivo visualization of Merkel cell tumors and their metastases. Arch. Dermatol. 1992, 128, 818–821. [Google Scholar] [CrossRef] [Green Version]
- Durani, B.K.; Klein, A.; Henze, M.; Haberkorn, U.; Hartschuh, W. Somatostatin analogue scintigraphy in Merkel cell tumours. Br. J. Dermatol. 2003, 148, 1135–1140. [Google Scholar] [CrossRef]
- Guitera-Rovel, P.; Lumbroso, J.; Gautier-Gougis, M.S.; Spatz, A.; Mercier, S.; Margulis, A.; Mamelle, G.; Kolb, F.; Lartigau, E.; Avril, M.F. Indium-111 octreotide scintigraphy of Merkel cell carcinomas and their metastases. Ann. Oncol. 2001, 12, 807–811. [Google Scholar] [CrossRef]
- Peloschek, P.; Novotny, C.; Mueller-Mang, C.; Weber, M.; Sailer, J.; Dawid, M.; Czerny, C.; Dudczak, R.; Kletter, K.; Becherer, A. Diagnostic imaging in Merkel cell carcinoma: Lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur. J. Radiol. 2010, 73, 317–323. [Google Scholar] [CrossRef]
- Abgral, R.; Leboulleux, S.; Deandreis, D.; Auperin, A.; Lumbroso, J.; Dromain, C.; Duvillard, P.; Elias, D.; de Baere, T.; Guigay, J.; et al. Performance of (18)fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (≥10%) well-differentiated endocrine carcinoma staging. J. Clin. Endocrinol. Metab. 2011, 96, 665–671. [Google Scholar] [CrossRef]
- Lu, Y.; Fleming, S.E.; Fields, R.C.; Coit, D.G.; Carrasquillo, J.A. Comparison of 18F-FDG PET/CT and 111In-pentetreotide scan for detection of Merkel cell carcinoma. Clin. Nucl. Med. 2012, 37, 759–762. [Google Scholar] [CrossRef]
- Meier, G.; Waldherr, C.; Herrmann, R.; Maecke, H.; Mueller-Brand, J.; Pless, M. Successful targeted radiotherapy with 90Y-DOTATOC in a patient with Merkel cell carcinoma—A case report. Oncology 2004, 66, 160–163. [Google Scholar] [CrossRef]
- Salavati, A.; Prasad, V.; Schneider, C.P.; Herbst, R.; Baum, R.P. Peptide receptor radionuclide therapy of Merkel cell carcinoma using (177)lutetium-labeled somatostatin analogs in combination with radiosensitizing chemotherapy: A potential novel treatment based on molecular pathology. Ann. Nucl. Med. 2012, 26, 365–369. [Google Scholar] [CrossRef]
- Epstude, M.; Tornquist, K.; Riklin, C.; di Lenardo, F.; Winterhalder, R.; Hug, U.; Strobel, K. Comparison of 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT imaging in metastasized Merkel cell carcinoma. Clin. Nucl. Med. 2013, 38, 283–284. [Google Scholar] [CrossRef]
- Schmidt, M.C.; Uhrhan, K.; Markiefka, B.; Hasselbring, L.; Schlaak, M.; Cremer, B.; Kunze, S.; Baum, R.P.; Dietlein, M. 68Ga-DOTATATE PET-CT followed by peptide receptor radiotherapy in combination with capecitabine in two patients with Merkel cell carcinoma. Int. J. Clin. Exp. Med. 2012, 5, 363–366. [Google Scholar]
- Talbot, J.N.; Kerrou, K.; Missoum, F.; Grahek, D.; Aide, N.; Lumbroso, J.; Montravers, F. 6-[F-18]Fluoro-l-DOPA positron emission tomography in the imaging of Merkel cell carcinoma: Preliminary report of three cases with 2-Deoxy-2-[F-18]fluoro-d-glucose positron emission tomography or pentetreotide-(111In) SPECT data. Mol. Imaging Biol. 2005, 7, 257–261. [Google Scholar] [CrossRef]
- Belhocine, T.; Pierard, G.E.; Fruhling, J.; Letesson, G.; Bolle, S.; Hustinx, R.; Dargent, J.L.; Flamen, P.; Rigo, P. Clinical added-value of 18FDG PET in neuroendocrine-Merkel cell carcinoma. Oncol. Rep. 2006, 16, 347–352. [Google Scholar]
- Concannon, R.; Larcos, G.S.; Veness, M. The impact of 18F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: Results from Westmead Hospital, Sydney, Australia. J. Am. Acad. Dermatol. 2010, 62, 76–84. [Google Scholar] [CrossRef]
- Iagaru, A.; Quon, A.; McDougall, I.R.; Gambhir, S.S. Merkel cell carcinoma: Is there a role for 2-Deoxy-2-[f-18]fluoro-d-glucose-positron emission tomography/computed tomography? Mol. Imaging Biol. 2006, 8, 212–217. [Google Scholar] [CrossRef]
- Hawryluk, E.B.; O’Regan, K.N.; Sheehy, N.; Guo, Y.; Dorosario, A.; Sakellis, C.G.; Jacene, H.A.; Wang, L.D.C. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J. Am. Acad. Dermatol. 2013, 68, 592–599. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Ahronowitz, I.; McCalmont, T.H.; Hernandez Pampaloni, M.; Ryan, J.L.; Yu, S.S. 18F-Fluorodeoxyglucose positron emission tomography-computed tomography imaging in the management of Merkel cell carcinoma: A single-institution retrospective study. Dermatol. Surg. 2013, 39, 1323–1333. [Google Scholar] [CrossRef]
- Siva, S.; Byrne, K.; Seel, M.; Bressel, M.; Jacobs, D.; Callahan, J.; Laing, J.; MacManus, M.P.; Hicks, R.J. 18F-FDG PET provides high-impact and powerful prognostic stratification in the staging of Merkel cell carcinoma: A 15-year institutional experience. J. Nucl. Med. 2013, 54, 1223–1229. [Google Scholar] [CrossRef]
- Treglia, G.; Kakhki, V.R.; Giovanella, L.; Sadeghi, R. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: A systematic review and meta-analysis. Am. J. Clin. Dermatol. 2013, 14, 437–447. [Google Scholar] [CrossRef]
- Colgan, M.B.; Tarantola, T.I.; Weaver, A.L.; Wiseman, G.A.; Roenigk, R.K.; Brewer, J.D.; Otley, C.C. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J. Am. Acad. Dermatol. 2012, 67, 1250–1256. [Google Scholar] [CrossRef]
- Golan, H.; Volkov, O.; Linchinsky, O.; Melloul, M. FDG-PET imaging in Merkel cell carcinoma—Value of head-to-toe scan. Nucl. Med. Rev. 2005, 8, 135–136. [Google Scholar]
- Alex, J.C.; Krag, D.N. Gamma-probe guided localization of lymph-nodes. Surg. Oncol. 1993, 2, 137–143. [Google Scholar] [CrossRef]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: Analysis of 153 patients from a single institution. Ann. Surg. Oncol. 2011, 18, 2529–2537. [Google Scholar]
- Schwartz, J.L.; Griffith, K.A.; Lowe, L.; Wong, S.L.; McLean, S.A.; Fullen, D.R.; Lao, C.D.; Hayman, J.A.; Bradford, C.R.; Rees, R.S.; et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J. Clin. Oncol. 2011, 29, 1036–1041. [Google Scholar] [CrossRef]
- Stokes, J.B.; Graw, K.S.; Dengel, L.T.; Swenson, B.R.; Bauer, T.W.; Slingluff, C.L.; Ledesma, E.J. Patients with Merkel cell carcinoma tumors ≤1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J. Clin. Oncol. 2009, 27, 3772–3777. [Google Scholar] [CrossRef]
- Kachare, S.D.; Wong, J.H.; Vohra, N.A.; Zervos, E.E.; Fitzgerald, T.L. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann. Surg. Oncol. 2014, 21, 1624–1630. [Google Scholar] [CrossRef]
- Fritsch, V.A.; Camp, E.R.; Lentsch, E.J. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: Not a predictor of survival. Head Neck 2014, 36, 571–579. [Google Scholar] [CrossRef]
- Shibayama, Y.; Imafuku, S.; Takahashi, A.; Nakayama, J. Role of sentinel lymph node biopsy in patients with Merkel cell carcinoma: Statistical analysis of 403 reported cases. Int. J. Clin. Oncol. 2014. [Google Scholar] [CrossRef]
- Li, L.X.; Scolyer, R.A.; Ka, V.S.; McKinnon, J.G.; Shaw, H.M.; McCarthy, S.W.; Thompson, J.F. Pathologic review of negative sentinel lymph nodes in melanoma patients with regional recurrence: A clinicopathologic study of 1152 patients undergoing sentinel lymph node biopsy. Am. J. Surg. Pathol. 2003, 27, 1197–1202. [Google Scholar] [CrossRef]
- Nccn Clinical Practice Guidelines In Oncology. Available online: http://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf (accessed on 3 December 2013).
- Heuveling, D.A.; van Schie, A.; Vugts, D.J.; Hendrikse, N.H.; Yaqub, M.; Hoekstra, O.S.; Karagozoglu, K.H.; Leemans, C.R.; van Dongen, G.; de Bree, R. Pilot study on the feasibility of PET/CT lymphoscintigraphy with 89Zr-nanocolloidal albumin for sentinel node identification in oral cancer patients. J. Nucl. Med. 2013, 54, 585–589. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beylergil, V.; Carrasquillo, J.A. Molecular Imaging and Therapy of Merkel Cell Carcinoma. Cancers 2014, 6, 1020-1030. https://doi.org/10.3390/cancers6021020
Beylergil V, Carrasquillo JA. Molecular Imaging and Therapy of Merkel Cell Carcinoma. Cancers. 2014; 6(2):1020-1030. https://doi.org/10.3390/cancers6021020
Chicago/Turabian StyleBeylergil, Volkan, and Jorge A. Carrasquillo. 2014. "Molecular Imaging and Therapy of Merkel Cell Carcinoma" Cancers 6, no. 2: 1020-1030. https://doi.org/10.3390/cancers6021020




