The Protective Role of Vitamin D Signaling in Non-Melanoma Skin Cancer
Abstract
:1. Introduction
2. Vitamin D Regulation of Proliferation and Differentiation: Role of the CTNNB and HH Pathways
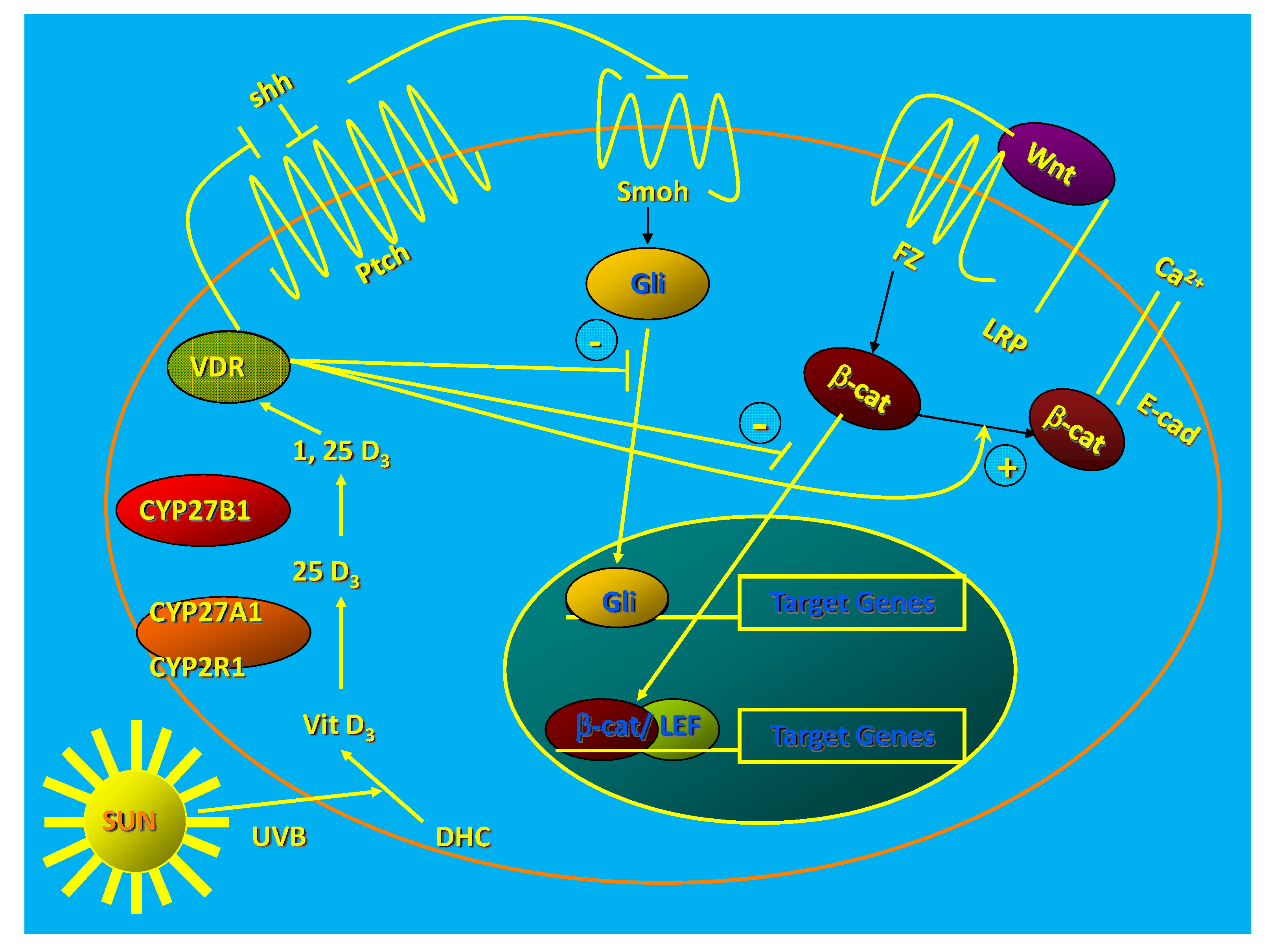
2.1. Role of CTNNB Pathway
2.2. Role of HH Pathway
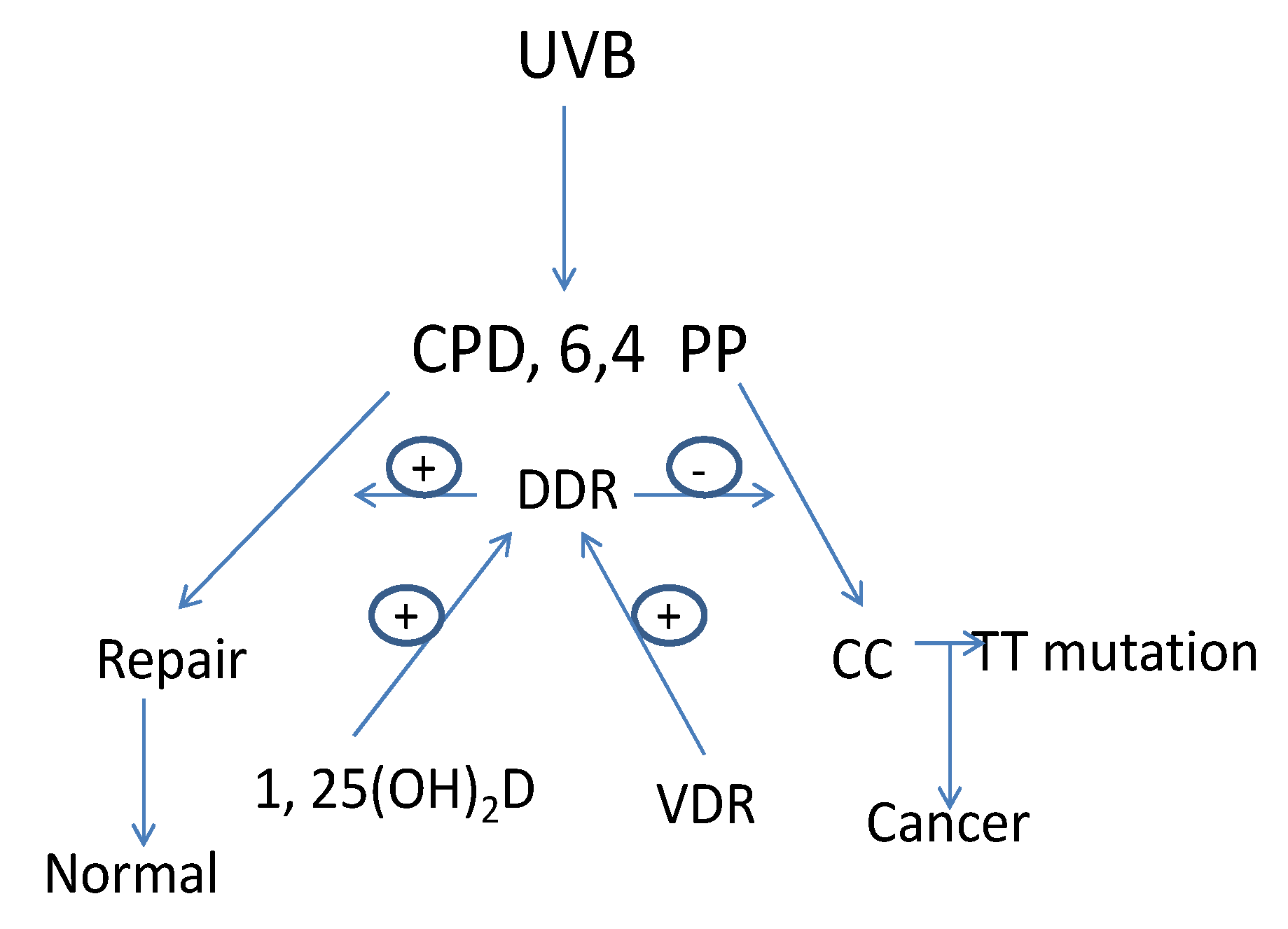
3. Vitamin D Regulation of the DNA Damage Response (DDR)
4. Vitamin D Regulation of Long Non-Coding RNA (lncRNA) Expression
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer statistics 2001. CA Cancer J. Clin. 2001, 51, 15–36. [Google Scholar] [CrossRef]
- Garland, C.; Barrett-Connor, E.; Rossof, A.H.; Shekelle, R.B.; Criqui, M.H.; Paul, O. Dietary vitamin D and calcium and risk of colorectal cancer: A 19-year prospective study in men. Lancet 1985, 1, 307–309. [Google Scholar]
- Bostick, R.M.; Potter, J.D.; Sellers, T.A.; McKenzie, D.R.; Kushi, L.H.; Folsom, A.R. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am. J. Epidemiol. 1993, 137, 1302–1317. [Google Scholar]
- Kearney, J.; Giovannucci, E.; Rimm, E.B.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Wing, A.; Kampman, E.; Willett, W.C. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am. J. Epidemiol. 1996, 143, 907–917. [Google Scholar] [CrossRef]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Young, J.F. Geographic variation in breast cancer mortality in the United States: A hypothesis involving exposure to solar radiation. Prev. Med. 1990, 19, 614–622. [Google Scholar] [CrossRef]
- Hanchette, C.L.; Schwartz, G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer 1992, 70, 2861–2869. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Huang, Z.; Giovannucci, E.; Rimm, E.B.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am. J. Clin. Nutr. 2000, 71, 135–141. [Google Scholar]
- Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann. Epidemiol. 1992, 2, 231–239. [Google Scholar] [CrossRef]
- Weinstock, M.A.; Stampfer, M.J.; Lew, R.A.; Willett, W.C.; Sober, A.J. Case-control study of melanoma and dietary vitamin D: Implications for advocacy of sun protection and sunscreen use. J. Invest. Dermatol. 1992, 98, 809–811. [Google Scholar]
- Asgari, M.M.; Tang, J.; Warton, M.E.; Chren, M.M.; Quesenberry, C.P., Jr.; Bikle, D.; Horst, R.L.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J. Invest. Dermatol. 2010, 130, 1438–1443. [Google Scholar] [CrossRef]
- Lucas, R.M.; McMichael, A.J.; Armstrong, B.K.; Smith, W.T. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 2008, 37, 654–667. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- English, D.R.; Armstrong, B.K.; Kricker, A.; Winter, M.G.; Heenan, P.J.; Randell, P.L. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int. J. Cancer 1998, 77, 347–353. [Google Scholar]
- Rosso, S.; Tang, J.; Warton, M.E.; Chren, M.M.; Quesenberry, C.P., Jr.; Bikle, D.; Horst, R.L.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. The multicentre south European study “Helios”. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br. J. Cancer 1996, 73, 1447–1454. [Google Scholar] [CrossRef]
- Zinser, G.M.; Sundberg, J.P.; Welsh, J. Vitamin D3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 2002, 23, 2103–2109. [Google Scholar] [CrossRef]
- Indra, A.K.; Castaneda, E.; Antal, M.C.; Jiang, M.; Messaddeq, N.; Meng, X.J.; Loehr, C.V.; Gariglio, P.; Kato, S.; Wahli, W.; et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J. Invest. Dermatol. 2007, 127, 1250–1260. [Google Scholar] [CrossRef]
- Ellison, T.I.; Smith, M.K.; Gilliam, A.C.; MacDonald, P.N. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J. Invest. Dermatol. 2008, 128, 2508–2517. [Google Scholar] [CrossRef]
- Teichert, A.E.; Elalieh, H.; Elias, P.M.; Welsh, J.; Bikle, D.D. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Invest. Dermatol. 2011, 131, 2289–2297. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism and function in the skin. Mol. Cell Endocrinol. 2011, 347, 80–89. [Google Scholar] [CrossRef]
- Chan, E.F.; Gat, U.; McNiff, J.M.; Fuchs, E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat. Genet. 1999, 21, 410–413. [Google Scholar] [CrossRef]
- Hahn, H.; Wicking, C.; Zaphiropoulos, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef]
- Daya-Grosjean, L.; Sarasin, A. The role of UV induced lesions in skin carcinogenesis: An overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat. Res. 2005, 571, 43–56. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Bikle, D.D. LncRNA profiling reveals a new mechanism for VDR protection against skin cancer formation. J. Steroid Biochem. Mol. Biol. 2013, in press. [Google Scholar]
- Bikle, D.D.; Oda, Y.; Teichert, A. The vitamin D receptor: A tumor suppressor in skin. Discovery Med. 2011, 11, 7–17. [Google Scholar]
- Bikle, D.D.; Elalieh, H.; Chang, S.; Xie, Z.; Sundberg, J.P. Development and progression of alopecia in the vitamin D receptor null mouse. J. Cell Physiol. 2006, 207, 340–353. [Google Scholar] [CrossRef]
- Oda, Y.; Ishikawa, M.H.; Hawker, N.P.; Yun, Q.C.; Bikle, D.D. Vitamin D receptor and coactivators SRC 2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J. Invest. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef]
- Oda, Y.; Ishikawa, M.H.; Hawker, N.P.; Yun, Q.C.; Bikle, D.D. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J. Steroid Biochem. Mol. Biol. 2007, 103, 776–780. [Google Scholar] [CrossRef]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-Myc as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Xie, Z.; Bikle, D.D. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007, 282, 8695–8703. [Google Scholar] [CrossRef]
- Bikle, D.D. The vitamin D receptor: A tumor suppressor in skin. Discov. Med. 2011, 11, 7–17. [Google Scholar]
- Bienz, M. beta-Catenin: A pivot between cell adhesion and Wnt signaling. Curr. Biol. 2005, 15, R64–R67. [Google Scholar] [CrossRef]
- Tu, C.; Chang, W.; Xie, Z.; Bikle, D.D. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J. Biol.Chem. 2008, 283, 3519–3528. [Google Scholar]
- Bikle, D.D. University of California San Francisco: San Francisco, CA, USA; Unpublished work. 2013.
- Jiang, Y.J.; Teichert, A.E.; Fong, F.; Oda, Y.; Bikle, D.D. 1,25(OH)2-Dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the B-catenin pathway. J. Steroid Biochem. Mol. Biol. 2013, 136, 229–232. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Niemann, C.; Owens, D.M.; Hulsken, J.; Birchmeier, W.; Watt, F.M. Repression of B-catenin signaling in mouse epidermis results in transdifferentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 2002, 129, 95–109. [Google Scholar]
- Gat, U.; DasGupta, R.; Degenstein, L.; Fuchs, E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998, 95, 605–614. [Google Scholar] [CrossRef]
- Palmer, H.G.; Anjos-Afonso, F.; Carmeliet, G.; Takeda, H.; Watt, F.M. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One 2008, 3, e1483. [Google Scholar]
- Aszterbaum, M.; Rothman, A.; Johnson, R.L.; Fisher, M.; Xie, J.; Bonifas, J.M.; Zhang, X.; Scott, M.P.; Epstein, E.H., Jr. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J. Invest. Dermatol. 1998, 110, 885–888. [Google Scholar] [CrossRef]
- Barnfield, P.C.; Zhang, X.; Thanabalasingham, V.; Yoshida, M.; Hui, C.C. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation 2005, 73, 397–405. [Google Scholar] [CrossRef]
- Svard, J.; Heby-Henricson, K.; Persson-Lek, M.; Rozell, B.; Lauth, M.; Bergstrom, A.; Ericson, J.; Toftgard, R.; Teglund, S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell 2006, 10, 187–197. [Google Scholar] [CrossRef]
- Regl, G.; Kasper, M.; Schnidar, H.; Eichberger, T.; Neill, G.W.; Ikram, M.S.; Quinn, A.G.; Philpott, M.P.; Frischauf, A.M.; Aberger, F. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene 2004, 23, 1263–1274. [Google Scholar] [CrossRef]
- Regl, G.; Kasper, M.; Schnidar, H.; Eichberger, T.; Neill, G.W.; Philpott, M.P.; Esterbauer, H.; Hauser-Kronberger, C.; Frischauf, A.M.; Aberger, F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004, 64, 7724–7731. [Google Scholar] [CrossRef]
- Regl, G.; Neill, G.W.; Eichberger, T.; Kasper, M.; Ikram, M.S.; Koller, J.; Hintner, H.; Quinn, A.G.; Frischauf, A.M.; Aberger, F. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene 2002, 21, 5529–5539. [Google Scholar] [CrossRef]
- Grachtchouk, M.; Mo, R.; Yu, S.; Zhang, X.; Sasaki, H.; Hui, C.C.; Dlugosz, A.A. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat. Genet. 2000, 24, 216–217. [Google Scholar] [CrossRef]
- Nilsson, M.; Unden, A.B.; Krause, D.; Malmqwist, U.; Raza, K.; Zaphiropoulos, P.G.; Toftgard, R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl. Acad. Sci. USA 2000, 97, 3438–3443. [Google Scholar] [CrossRef]
- Bijlsma, M.F.; Spek, C.A.; Zivkovic, D.; van de Water, S.; Rezaee, F.; Peppelenbosch, M.P. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006, 4, e232. [Google Scholar] [CrossRef]
- Tang, J.Y.; Xiao, T.Z.; Oda, Y.; Chang, K.S.; Shpall, E.; Wu, A.; So, P.L.; Hebert, J.; Bikle, D.; Epstein, E.H., Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev. Res. 2011, 4, 744–751. [Google Scholar] [CrossRef]
- Youssef, K.K.; Lapouge, G.; Bouvrée, K.; Rorive, S.; Brohéem, S.; Appelstein, O.; Larsimont, J.-C.; Sukumaran, V.; van de Sande, B.; Pucci, D.; et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat. Cell Biol. 2012, 14, 1282–1294. [Google Scholar] [CrossRef]
- Yang, S.H.; Andl, T.; Grachtchouk, V.; Wang, A.; Liu, J.; Syu, L.J.; Ferris, J.; Wang, T.S.; Glick, A.B.; Millar, S.E.; et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat. Genet. 2008, 40, 1130–1135. [Google Scholar] [CrossRef]
- Schneider, F.T.; Schanzer, A.; Czupalla, C.J.; Thom, S.; Engels, K.; Schmidt, M.H.; Plate, K.H.; Liebner, S. Sonic hedgehog acts as a negative regulator of {beta}-catenin signaling in the adult tongue epithelium. Am. J. Pathol. 2010, 177, 404–414. [Google Scholar] [CrossRef]
- Freeman, S.E.; Hacham, H.; Gange, R.W.; Maytum, D.J.; Sutherland, J.C.; Sutherland, B.M. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc. Natl. Acad. Sci. USA 1989, 86, 5605–5609. [Google Scholar] [CrossRef]
- Besaratinia, A.; Synold, T.W.; Chen, H.H.; Chang, C.; Xi, B.; Riggs, A.D.; Pfeifer, G.P. DNA lesions induced by UV A1 and B radiation in human cells: Comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc. Natl. Acad. Sci. USA 2005, 102, 10058–10063. [Google Scholar]
- Hussein, M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef]
- Ziegler, A.; Leffell, D.J.; Kunala, S.; Sharma, H.W.; Gailani, M.; Simon, J.A.; Halperin, A.J.; Baden, H.P.; Shapiro, P.E.; Bale, A.E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc. Natl. Acad. Sci. USA 1993, 90, 4216–4220. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the onset of skin cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef]
- Bito, T.; Ueda, M.; Ahmed, N.U.; Nagano, T.; Ichihashi, M. Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J. Cutan. Pathol. 1995, 22, 427–434. [Google Scholar] [CrossRef]
- Chen, R.H.; Maher, V.M.; McCormick, J.J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/−)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc. Natl. Acad. Sci. USA 1990, 87, 8680–8684. [Google Scholar] [CrossRef]
- Wood, R.D. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie 1999, 81, 39–44. [Google Scholar] [CrossRef]
- Mellon, I.; Bohr, V.A.; Smith, C.A.; Hanawalt, P.C. Preferential DNA repair of an active gene in human cells. Proc. Natl. Acad. Sci. USA 1986, 83, 8878–8882. [Google Scholar] [CrossRef]
- Hanawalt, P.C. Transcription-coupled repair and human disease. Science 1994, 266, 1957–1958. [Google Scholar]
- Wood, R.D.; Mitchell, M.; Sgouros, J.; Lindahl, T. Human DNA repair genes. Science 2001, 291, 1284–1289. [Google Scholar] [CrossRef]
- Demetriou, S.K.; Ona-Vu, K.; Teichert, A.E.; Cleaver, J.E.; Bikle, D.D.; Oh, D.H. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J. Invest. Dermatol. 2012, 132, 2097–2100. [Google Scholar] [CrossRef]
- Dixon, K.M.; Deo, S.S.; Wong, G.; Slater, M.; Norman, A.W.; Bishop, J.E.; Posner, G.H.; Ishizuka, S.; Halliday, G.M.; Reeve, V.E.; et al. Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. J. Steroid Biochem. Mol. Biol. 2005, 97, 137–143. [Google Scholar] [CrossRef]
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Invest. Dermatol. 2007, 127, 707–715. [Google Scholar] [CrossRef]
- Sequeira, V.B.; Rybchyn, M.S.; Tongkao-On, W.; Gordon-Thomson, C.; Malloy, P.J.; Nemere, I.; Norman, A.W.; Reeve, V.E.; Halliday, G.M.; Feldman, D.; et al. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol. Endocrinol. 2012, 26, 574–582. [Google Scholar] [CrossRef]
- Moll, P.R.; Sander, V.; Frischauf, A.M.; Richter, K. Expression profiling of vitamin D treated primary human keratinocytes. J. Cell Biochem. 2007, 100, 574–592. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Mattick, J.S. Long noncoding RNAs in cell and developmental biology. Semin. Cell Dev. Biol. 2011, 22. [Google Scholar] [CrossRef]
- Spitale, R.C.; Crisalli, P.; Flynn, R.A.; Torre, E.A.; Kool, E.T.; Chang, H.Y. RNA SHAPE analysis in living cells. Nat. Chem. Biol. 2013, 9, 18–20. [Google Scholar]
- Gibb, E.A.; Vucic, E.A.; Enfield, K.S.S.; Stewart, G.L.; Lonergan, K.M.; Kennett, J.Y.; Becker-Santos, D.D.; MacAulay, C.E.; Lam, S.; Brown, C.J.; et al. Human cancer long non-coding RNA transcriptomes. PLoS One 2011, 6, e25915. [Google Scholar] [CrossRef]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Fu, X.; Han, W. Long Noncoding RNAs: Insights from biological features and functions to diseases. Med. Res. Rev. 2013, 33, 517–553. [Google Scholar] [CrossRef]
- Barsyte-Lovejoy, D.; Lau, S.K.; Boutros, P.C.; Khosravi, F.; Jurisica, I.; Andrulis, I.L.; Tsao, M.S.; Penn, L.Z. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006, 66, 5330–5337. [Google Scholar] [CrossRef]
- Matouk, I.J.; DeGroot, N.; Mezan, S.; Ayesh, S.; Abu-lail, R.; Hochberg, A.; Galun, E. The H19 non-coding RNA is essential for human tumor growth. PLoS One 2007, 2, e845. [Google Scholar] [CrossRef]
- Berteaux, N.; Lottin, S.; Monté, D.; Pinte, S.; Quatannens, B.; Coll, J.; Hondermarck, H.; Curgy, J.-J.; Dugimont, T.; Adriaenssens, E. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J. Biol. Chem. 2005, 280, 29625–29636. [Google Scholar] [CrossRef]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A.; et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef]
- Pandey, R.R.; Mondal, T.; Mohammad, F.; Enroth, S.; Redrup, L.; Komorowski, J.; Nagano, T.; Mancini-Dinardo, D.; Kanduri, C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 2008, 32, 232–246. [Google Scholar] [CrossRef]
- Huarte, M.; Guttman, M.; Feldser, D.; Garber, M.; Koziol, M.J.; Kenzelmann-Broz, D.; Khalil, A.M.; Zuk, O.; Amit, I.; Rabani, M.; et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010, 142, 409–419. [Google Scholar] [CrossRef]
- Moumen, A.; Magill, C.; Dry, K.L.; Jackson, S.P. ATM-dependent phosphorylation of heterogeneous nuclear ribonucleoprotein K promotes p53 transcriptional activation in response to DNA damage. Cell Cycle 2013, 12, 698–704. [Google Scholar] [CrossRef]
- Redrup, L.; Branco, M.R.; Perdeaux, E.R.; Krueger, C.; Lewis, A.; Santos, F.; Nagano, T.; Cobb, B.S.; Fraser, P.; Reik, W. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development 2009, 136, 525–530. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bikle, D.D.; Jiang, Y. The Protective Role of Vitamin D Signaling in Non-Melanoma Skin Cancer. Cancers 2013, 5, 1426-1438. https://doi.org/10.3390/cancers5041426
Bikle DD, Jiang Y. The Protective Role of Vitamin D Signaling in Non-Melanoma Skin Cancer. Cancers. 2013; 5(4):1426-1438. https://doi.org/10.3390/cancers5041426
Chicago/Turabian StyleBikle, Daniel D., and Yan Jiang. 2013. "The Protective Role of Vitamin D Signaling in Non-Melanoma Skin Cancer" Cancers 5, no. 4: 1426-1438. https://doi.org/10.3390/cancers5041426



