Three-Times Daily Ultrafractionated Radiation Therapy, A Novel and Promising Regimen for Glioblastoma Patients
Abstract
:1. Introduction
2. Experimental Studies
2.1. In Vitro Studies
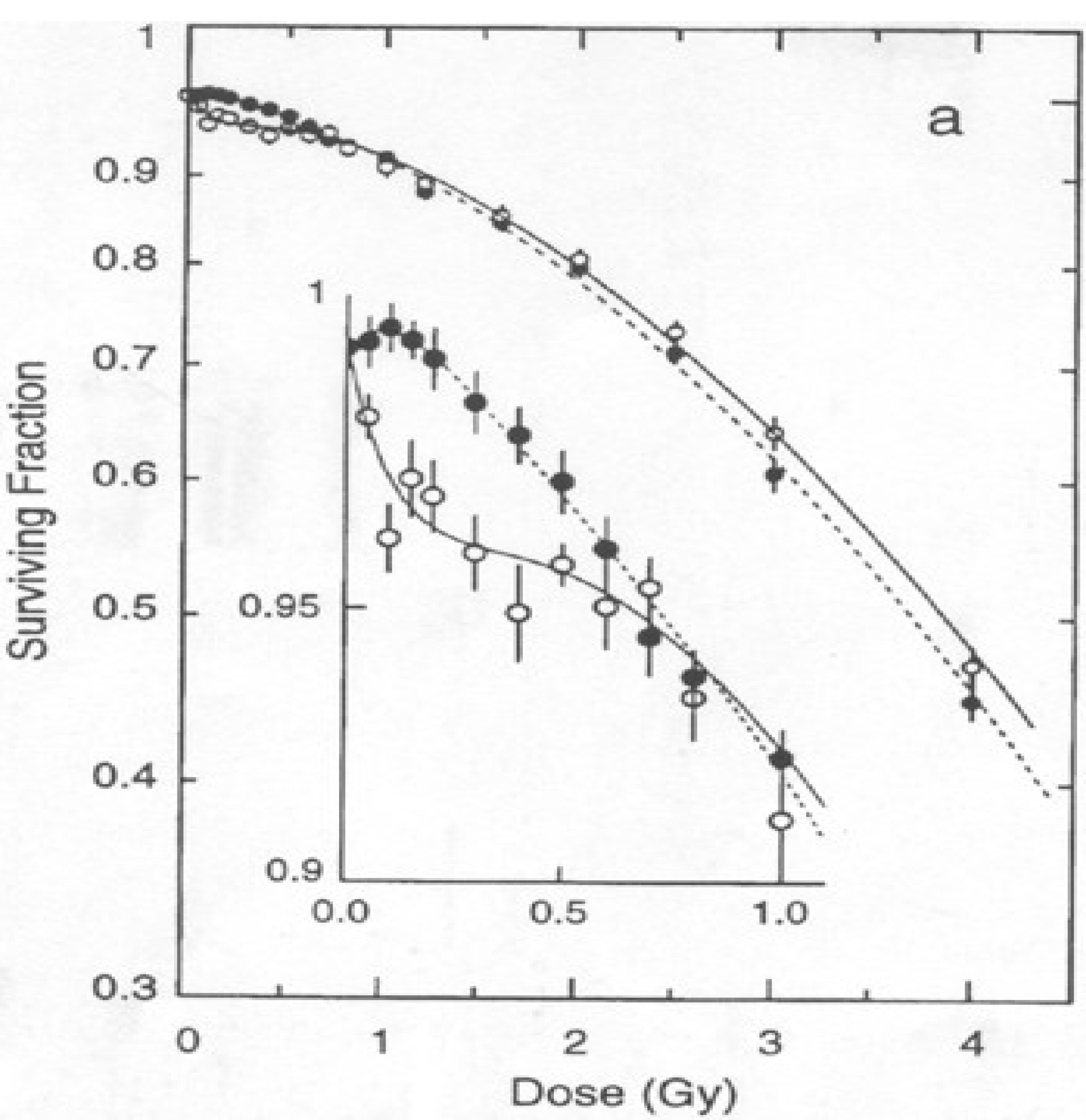

2.2. In Vivo Studies

2.3. Mechanisms of HRS
2.4. Anti-Neoplastic Agents
3. Clinical Studies
3.1. De Novo Tumors

3.2. Recurrent Tumors
3.3. Pulse Reduced Dose Rate Radiotherapy
4. Conclusions
Conflicts of Interest
References
- Behin, A.; Hoang-Xuan, K.; Carpentier, A.F.; Delattre, J.Y. Primary brain tumours in adults. Lancet 2003, 36, 323–331. [Google Scholar]
- De Angelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef]
- Stewart, L.A. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002, 359, 1011–1018. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Joiner, M.C.; Denekamp, J. The effect of small radiation doses on mouse skin. Br. J. Cancer 1986, 7, 63–66. [Google Scholar]
- Joiner, M.C.; Denekamp, J.; Maughan, R.L. The use of “top-up” experiments to investigate the effect of very small doses per fraction in mouse skin. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 49, 565–580. [Google Scholar]
- Joiner, M.C.; Johns, H. Renal damage in the mouse: The response to very small doses per fraction. Radiat. Res. 1988, 114, 385–398. [Google Scholar] [CrossRef]
- Marples, B.; Joiner, M.C. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 1993, 133, 41–51. [Google Scholar] [CrossRef]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turessson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar]
- Marples, B.; Collis, S.J. Low-dose hyper-radiosensitivity: Past, present, and future. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1310–1318. [Google Scholar] [CrossRef]
- Martin, L.M.; Marples, B.; Lynch, T.H.; Hollywood, D.; Marignol, L. Exposure to low dose ionising radiation: Molecular and clinical consequences. Cancer Lett. 2013, 338, 209–218. [Google Scholar] [CrossRef]
- Lambin, P.; Marples, B.; Fertil, B.; Joiner, M.C. Hypersensitivity of a human tumour cell line to very low radiation doses. Int. J. Radiat. Biol. 1993, 63, 639–650. [Google Scholar] [CrossRef]
- Lambin, P.; Malaise, E.P.; Joiner, M.C. Megafractionnement: Une methode pour agir sur les tumeurs intrinsequement radioresistantes? Bull. Cancer Radiother. 1993, 80, 417–423. [Google Scholar]
- Lambin, P.; Malaise, E.P.; Joiner, M.C. Might intrinsic radioresistance of human tumour cells be induced by radiation? Int. J. Radiat. Biol. 1996, 69, 279–290. [Google Scholar] [CrossRef]
- Short, S.C.; Mayes, C.R.; Woodcock, M.; Joiner, M.C. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int. J. Radiat. Biol. 1999, 75, 847–855. [Google Scholar] [CrossRef]
- Short, S.C.; Mitchell, S.A.; Boulton, P.; Joiner, M.C. The response of human glioma cell lines to low-dose radiation exposure. Int. J. Radiat. Biol. 1999, 75, 1341–1348. [Google Scholar] [CrossRef]
- Beauchesne, P.; Bertrand, S.; Branche, R.; Linke, S.P.; Revel, R.; Dore, J.F.; Pedeux, R.M. Human malignant glioma cell lines are sensitive to low radiation doses. Int. J. Cancer 2003, 105, 33–40. [Google Scholar] [CrossRef]
- Joiner, M.C.; Marples, B.; Johns, H. The response of tissues to very low doses per fraction: A reflection of induced repair? Cancer Res. 1993, 130, 27–40. [Google Scholar]
- Joiner, M.C.; Lambin, P.; Malaise, E.P.; Robson, T.; Arrand, J.E.; Skov, K.A.; Marples, B. Hypersensitivity to very-low single radiation doses: Its relationship to the adaptive response and induced radioresistance. Mutat. Res. 1996, 358, 171–183. [Google Scholar] [CrossRef]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, M.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Lambin, P.; Skov, K.A.; Joiner, M.C. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int. J. Radiat. Biol. 1997, 71, 721–735. [Google Scholar] [CrossRef]
- Singh, B.; Arrand, J.E.; Joiner, M.C. Hypersensitive response of normal human lung epithelial cells at low radiation doses. Int. J. Radiat. Biol. 1994, 65, 457–464. [Google Scholar] [CrossRef]
- Turesson, I.; Joiner, M.C. Clinical evidence of hypersensitivity to low doses in radiotherapy. Radiother. Oncol. 1996, 40, 1–3. [Google Scholar] [CrossRef]
- Wouters, B.G.; Sky, A.M.; Skarsgard, L.D. Low dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat. Res. 1996, 146, 399–413. [Google Scholar] [CrossRef]
- Short, S.C.; Kelly, J.; Mayes, C.R.; Woodcock, M.; Joiner, M.C. Low-dose hypersensitivity after fractionated low- dose irradiation in vitro. Int. J. Radiat. Biol. 2001, 77, 655–664. [Google Scholar] [CrossRef]
- Pedeux, R.; Boniol, M.; Dore, J.F.; Beauchesne, P. Ultrafractionation radiation therapy of human gliomas; a pre-clinical model. Int. J. Cancer 2003, 107, 334. [Google Scholar] [CrossRef]
- Beck-Bornholdt, H.P.; Maurer, T.; Becker, S.; Omniczynski, M.; Vogler, H.; Würschmidt, F. Radiotherapy of the rhabdomyosarcoma R1H of the rat: Hyperfractionation–126 fractions applied within 6 weeks. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 701–705. [Google Scholar] [CrossRef]
- Krause, M.; Hessel, F.; Wohlfarth, J.; Zips, D.; Hoinkis, C.; Foest, H.; Petersen, C.; Short, S.C.; Joiner, M.C.; Baumann, M. Ultrafractionation in A7 human malignant glioma in nude mice. Int. J. Cancer 2003, 79, 377–383. [Google Scholar]
- Harney, J.; Short, S.C.; Shah, N.; Joiner, M.; Saunders, M.I. Low dose hyper-radiosensitivity in metastatic tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1190–1195. [Google Scholar] [CrossRef]
- Harney, J.; Shah, N.; Short, S.C.; Daley, F.; Groom, N.; Wilson, G.D.; Joiner, M.C.; Saunders, M.I. The evaluation of low dose hyper-radiosensitivity in normal human skin. Radiother. Oncol. 2004, 70, 319–329. [Google Scholar] [CrossRef]
- Shajahan, S.; Brown, B.; Dey, S.; Lele, S.M.; Valentino, J.; Jones, R.; Mohiuddin, M.; Ahmed, M.M.; Spring, P.M.; Arnold, S.M. Low dose fractionated radiation potentiates the effects of taxotere in nude mice xenografts of squamous cell carcinoma of head and neck. Cell Cycle 2004, 3, 479–485. [Google Scholar]
- Dey, S.; Spring, P.M.; Arnold, S.; Valentino, J.; Chendil, D.; Regine, W.F.; Mohiuddin, M.; Ahmed, M.M. Low-dose fractionated radiation potentiates the effects of Paclitaxel in wild-type and mutant p53 head and neck tumor cell lines. Clin. Cancer Res. 2003, 9, 1557–1565. [Google Scholar]
- Beauchesne, P.; Bernier, V.; Carnin, C.; Taillandier, L.; Djabri, M.; Martin, L.; Michel, X.; Maire, J.P.; Khalil, T.; Kerr, C.; et al. Prolonged survival for patients with newly diagnosed, inoperable glioblastoma with 3-times daily ultrafractionated radiation therapy. Neuro-oncology 2010, 12, 595–602. [Google Scholar] [CrossRef]
- Athanassiou, H.; Synodinou, M.; Maragoudakis, E.; Paraskevaidis, M.; Verigos, C.; Misailidou, D.; Antonadou, D.; Saris, G.; Beroukas, K.; Karageorgis, P. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2005, 23, 2372–2377. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology 2003, 5, 79–88. [Google Scholar]
- Beauchesne, P.; Faure, G.; Noel, G.; Schmitt, T.; Martin, L.; Jadaud, E.; Carnin, C. TEMOFRAC—A phase II trial. concurrent 3-times daily ultrafractionated radiation therapy and temozolomide for newly inoperable glioblastomas. J. Clin. Oncol. 2011, 29, Abstract 2073. [Google Scholar]
- Siker, M.L.; Firat, S.Y.; Mueller, W.; Krouwer, H.; Schultz, C.J. Semicontinuous low-dose-rate teletherapy for the treatment of recurrent glial brain tumors: Final report of a phase I/II study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 765–772. [Google Scholar] [CrossRef]
- Jahraus, C.D.; Friedman, A.H. Chemopotentiation by unltrafractionated radiotherapy in glioblastoma resistant to conventional therapy. Tumori 2010, 96, 771–775. [Google Scholar]
- Park, S.S.; Chunta, J.L.; Robertson, J.M.; Martinez, A.A.; Wong, C.Y.O.; Amin, M.; Wilson, G.D.; Marples, B. MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsed low-dose irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 885–892. [Google Scholar] [CrossRef]
- Dilworth, J.T.; Ktueger, S.A.; Dabian, M.; Grills, I.S.; Torma, J.; Wilson, G.D.; Marples, B. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: Effects on vascularization and tumor control. Radiother. Oncol. 2013, 108, 149–154. [Google Scholar] [CrossRef]
- Cannon, G.M.; Tomé, W.A.; Robins, H.I.; Howard, S.P. Pulsed reduced dose-rate radiotherapy: Case report: A novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J. Neurooncol. 2007, 83, 307–311. [Google Scholar] [CrossRef]
- Adkison, J.B.; Tomé, W.; Seo, S.; Richards, G.M.; Robins, H.I.; Rassmussen, K.; Welsh, J.S.; Mahler, P.A.; Howard, S.P. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 835–841. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beauchesne, P. Three-Times Daily Ultrafractionated Radiation Therapy, A Novel and Promising Regimen for Glioblastoma Patients. Cancers 2013, 5, 1199-1211. https://doi.org/10.3390/cancers5041199
Beauchesne P. Three-Times Daily Ultrafractionated Radiation Therapy, A Novel and Promising Regimen for Glioblastoma Patients. Cancers. 2013; 5(4):1199-1211. https://doi.org/10.3390/cancers5041199
Chicago/Turabian StyleBeauchesne, Patrick. 2013. "Three-Times Daily Ultrafractionated Radiation Therapy, A Novel and Promising Regimen for Glioblastoma Patients" Cancers 5, no. 4: 1199-1211. https://doi.org/10.3390/cancers5041199



