Potentiality and Boundaries of Use of Sorafenib in Patients with Hepatocellular Carcinoma: Awaiting the Results of Ongoing Clinical Trials
Abstract
:1. Introduction
2. Boundaries of the Use of Sorafenib
2.1. From Clinical Trials to Clinical Practice: When to Start Sorafenib?
2.2. From Clinical Trials to Clinical Practice: When to Stop Sorafenib? Use of Sorafenib Beyond Progression
2.3. External Validity of the Randomized Trials Testing Sorafenib in Advanced HCC
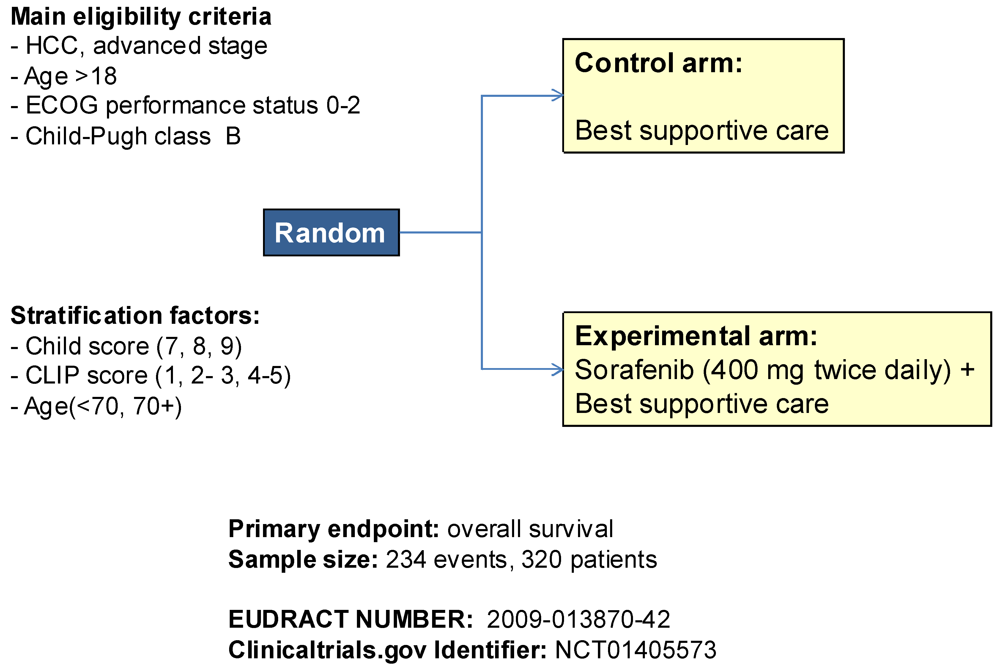
3. Potentialities of the Use of Sorafenib
3.1. Experimental Use of Sorafenib in Patients with Early Stage HCC
3.2. Experimental Use of Sorafenib in Patients with Intermediate Stage HCC
| Trial (ClinicalTrials.gov Identifier) | Type of trial | Treatment arms | Planned number of patients | Expected time for results |
|---|---|---|---|---|
| Eastern Cooperative Oncology Group (NCT01004978) [39] | Phase III | Experimental arm: Patients receive oral sorafenib tosylate twice daily in the absence of disease progression or unacceptable toxicity. Beginning within 2 weeks after a stable dose of sorafenib tosylate is reached, patients undergo TACE comprising doxorubicin hydrochloride, mitomycin C, and cisplatin (closed to accrual in 2010); conventional chemoembolization comprising doxorubicin hydrochloride only; or chemoembolization comprising doxorubicin-eluting beads. Treatment with TACE repeats approximately every 4 weeks for up to 4 courses in the absence of disease progression or unacceptable toxicity. Control arm: Patients receive oral placebo twice daily in the absence of disease progression or unacceptable toxicity. Beginning within 2 weeks after a stable dose of placebo is reached, patients undergo TACE as in experimental arm. | 400 | September 2012 |
| University College of London TACE-2 (NCT01324076) [40] | Phase III | Experimental arm: Patients receive oral sorafenib tosylate twice daily in the absence of disease progression or unacceptable toxicity. Beginning within 2–5 weeks after start of sorafenib tosylate, patients undergo TACE with doxorubicin-eluting beads. Patients may undergo additional sessions of TACE with doxorubicin-eluting beads, in the absence of complete devascularization of the tumor(s). | 412 | November 2014 |
| University College of London TACE-2 (NCT01324076) [40] | Phase III | Control arm: Patients receive oral placebo twice daily in the absence of disease progression or unacceptable toxicity. Beginning within 2–5 weeks after start of placebo, patients undergo TACE with doxorubicin-eluting beads. Patients with disease progression may cross over to the sorafenib tosylate arm at the discretion of the treating clinician. | 412 | November 2014 |
| Japan Liver Oncology Group TACTICS (NCT01217034) [41] | Phase II | Experimental arm: Sorafenib will be administrated at a dose of 400 mg o.d. before the first TACE. After 2 days drug rest, TACE will be conducted. Sorafenib will be resumed at a dose of 400 mg o.d. from 3 days after TACE(the resumption day can be postponed until 21 days after TACE). When tolerability is confirmed at 1 week after resumption, the dose of sorafenib will be increased to 400 mg b.i.d. When tumor increases, TACE will be repeated. Control arm: TACE will be conducted at scheduled day. When tumor increases, TACE will be repeated. | 228 | September 2016 |
3.3. Experimental Use of Sorafenib in Combination with Other Treatments in Patients with Advanced HCC
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Hicklin, D.J.; Wu, Y.; Yanase, K.; Namisaki, T.; Kitade, M.; et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology 2004, 39, 1517–1524. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Yano, H.; Iemura, A.; Ogasawara, S.; Haramaki, M.; Kojiro, M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 1998, 28, 68–77. [Google Scholar]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar]
- Abou-Alfa, G.K.; Schwartz, L.; Ricci, S.; Amadori, D.; Santoro, A.; Figer, A.; de Greve, J.; Douillard, J.Y.; Lathia, C.; Schwartz, B.; et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 4293–4300. [Google Scholar]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar]
- Raoul, J.L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Peck-Radosavljevic, M.; Greten, T.F.; Lammer, J.; Rosmorduc, O.; Sangro, B.; Santoro, A.; Bolondi, L. Consensus on the current use of sorafenib for the treatment of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2010, 22, 391–398. [Google Scholar] [CrossRef]
- Pressiani, T.; Rimassa, L.; Boni, C.; Labianca, R.; Fagiuoli, S.; Ardizzoni, A.; Foa, P.; Cortesi, E.; Porta, C.; Artioli, F.; et al. Phase II randomized trial on dose-escalated sorafenib (S) versus best supportive care (BSC) in patients with advanced hepatocellular carcinoma (HCC) with disease progression on prior S treatment. J. Clin. Oncol. 2011, 29. abstract 4115. [Google Scholar]
- Villanueva, A.; Llovet, J.M. Second-line therapies in HCC: Emergence of resistance to sorafenib. Clin. Cancer Res. 2012, 18, 1824–6. [Google Scholar] [CrossRef]
- BRISK-PS Study with Investigational Compound Brivanib in Hepatocellular Carcinoma Completed. Available online: http://www.reuters.com/article/2011/12/22/idUS205683+22-Dec-2011+BW20111222/ (accessed on 30 May 2012).
- Kane, R.C.; Farrell, A.T.; Madabushi, R.; Booth, B.; Chattopadhyay, S.; Sridhara, R.; Justice, R.; Pazdur, R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009, 14, 95–100. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Amadori, D.; Santoro, A.; Figer, A.; de Greve, J.; Lathia, C.; Voliotis, D.; Anderson, S.; Moscovici, M.; Ricci, S. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest. Cancer Res. 2011, 4, 40–44. [Google Scholar]
- Wörns, M.A.; Weinmann, A.; Pfingst, K.; Schulte-Sasse, C.; Messow, C.M.; Schulze-Bergkamen, H.; Teufel, A.; Schuchmann, M.; Kanzler, S.; Düber, C.; et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J. Clin. Gastroenterol. 2009, 43, 489–495. [Google Scholar] [CrossRef]
- Pinter, M.; Sieghart, W.; Graziadei, I.; Vogel, W.; Maieron, A.; Königsberg, R.; Weissmann, A.; Kornek, G.; Plank, C.; Peck-Radosavljevic, M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist 2009, 14, 70–76. [Google Scholar] [CrossRef]
- Lencioni, R.; Marrero, J.; Venook, A.; Ye, S.L.; Kudo, M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int. J. Clin. Pract. 2010, 64, 1034–1041. [Google Scholar] [CrossRef]
- Marrero, J.A.; Lencioni, R.; Kudo, M.; Ye, S.; Nakajima, K.; Cihon, F.; Venook, A.P. Global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with Sorafenib (GIDEON) second interim analysis in more than 1,500 patients: Clinical findings in patients with liver dysfunction. J. Clin. Oncol. 2011, 29. abstract 4001. [Google Scholar]
- di Maio, M.; Daniele, B.; Perrone, F. Targeted therapies: Role of sorafenib in HCC patients with compromised liver function. Nat. Rev. Clin. Oncol. 2009, 6, 505–506. [Google Scholar] [CrossRef]
- Burak, K.W. Candidacy for sorafenib in HCC patients: Is there a slippery slope beyond a SHARP edge? Oncology (Williston Park NY) 2011, 25, 296–300. [Google Scholar]
- Schwartz, D.; Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Chronic Dis. 1967, 20, 637–648. [Google Scholar] [CrossRef]
- Haynes, B. Can it work? Does it work? Is it worth it? The testing of health care interventions is evolving. BMJ 1999, 319, 652–653. [Google Scholar] [CrossRef]
- Daniele, B.; di Maio, M.; Gallo, C.; Gasbarrini, A.; Carteni, G.; di Costanzo, G.G.; Craxì, A.; Cabibbo, G.; Bolondi, L.; Granito, A.; et al. A randomized phase III trial comparing sorafenib plus best supportive care (BSC) versus BSC alone in Child-Pugh B patients (pts) with advanced hepatocellular carcinoma (HCC): The BOOST study. J. Clin. Oncol. 2012, 30. abstract TPS4151. [Google Scholar]
- Printz, C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM). Cancer 2009, 115, 4646. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Lencioni, R.; de Baere, T.; Burrel, M.; Caridi, J.G.; Lammer, J.; Malagari, K.; Martin, R.C.; O’Grady, E.; Real, M.I.; Vogl, T.J.; et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC bead (DEBDOX): Technical recommendations. Cardiovasc. Intervent. Radiol. 2011. [Google Scholar]
- Lencioni, R.; Chen, X.P.; Dagher, L.; Venook, A.P. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: How can outcomes be improved? Oncologist 2010, 15, S42–S52. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Virmani, S.; Rhee, T.K.; Ryu, R.K.; Sato, K.T.; Lewandowski, R.J.; Mulcahy, M.F.; Kulik, L.M.; Szolc-Kowalska, B.; Woloschak, G.E.; Yang, G.Y.; et al. Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors. J. Vasc. Interv. Radiol. 2008, 19, 1483–1489. [Google Scholar]
- Jiang, H.; Meng, Q.; Tan, H.; Pan, S.; Sun, B.; Xu, R.; Sun, X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int. J. Cancer 2007, 121, 416–424. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef]
- Strebel, B.M.; Dufour, J.F. Combined approach to hepatocellular carcinoma: A new treatment concept for nonresectable disease. Expert. Rev. Anticancer Ther. 2008, 8, 1743–1749. [Google Scholar] [CrossRef]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.Y.; Takayama, T.; Yoon, J.H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar]
- Dufour, J.F.; Hoppe, H.; Heim, M.H.; Helbling, B.; Maurhofer, O.; Szucs-Farkas, Z.; Kickuth, R.; Borner, M.; Candinas, D.; Saar, B. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: Results of a phase I study. Oncologist 2010, 15, 1198–1204. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Reyes, D.K.; Cosgrove, D.; Kamel, I.R.; Bhagat, N.; Geschwind, J.F. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J. Clin. Oncol. 2011, 29, 3960–3967, Erratum: J. Clin. Oncol. 2011, 29, 4596–4598. [Google Scholar]
- Lencioni, R.; Zou, J.; Leberre, M.; Meinhardt, G.; Voliotis, D.; Bruix, J.; Llovet, J.M. Sorafenib or placebo in combination with transarterial chemoembolization for intermediate-stage hepatocellular carcinoma (SPACE). J. Clin. Oncol. 2010, 28. no. 15_suppl TPS178. [Google Scholar]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Leberre, M.A.; Niu, W.; Nicholson, K.; Meinhardt, G.; Bruix, J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. J. Clin. Oncol. 2012, 30. abstract LBA154. [Google Scholar]
- Chemoembolization With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed By Surgery. Available online: http://clinicaltrials.gov/ct2/show/NCT01004978/ (accessed on 30 May 2012).
- Transarterial Chemoembolization Using Doxorubicin Beads With or Without Sorafenib Tosylate in Treating Patients With Liver Cancer That Cannot Be Removed By Surgery. Available online: http://clinicaltrials.gov/ct2/show/NCT01324076/ (accessed on 30 May 2012).
- Transcatheter Arterial Chemoembolization Therapy In Combination With Sorafenib (TACTICS). Available online: http://clinicaltrials.gov/ct2/show/NCT01217034/ (accessed on 30 May 2012).
- Chao, Y.; Yoon, J.; Li, C.; Kim, B.; Lee, R.; Chang, C.; Lee, T.; Hwang, J.; Chung, Y. START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. In 2010 Gastrointestinal Cancers Symposium, Orlando, FL, USA, 22-24 January 2010. abstract 211.
- di Maio, M.; de Maio, E.; Perrone, F.; Pignata, S.; Daniele, B. Hepatocellular carcinoma: Systemic treatments. J. Clin. Gastroenterol. 2002, 35, S109–S114. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Johnson, P.; Knox, J.J.; Capanu, M.; Davidenko, I.; Lacava, J.; Leung, T.; Gansukh, B.; Saltz, L.B. Doxorubicin plus sorafenib versus doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA 2010, 304, 2154–2160. [Google Scholar]
- Girard, N.; Mornex, F. Sorafenib and radiotherapy association for hepatocellular carcinoma. Cancer Radiother. 2011, 15, 77–80. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Di Maio, M.; Daniele, G.; Piccirillo, M.C.; Giordano, P.; Signoriello, G.; Daniele, B.; Perrone, F. Potentiality and Boundaries of Use of Sorafenib in Patients with Hepatocellular Carcinoma: Awaiting the Results of Ongoing Clinical Trials. Cancers 2012, 4, 549-565. https://doi.org/10.3390/cancers4020549
Di Maio M, Daniele G, Piccirillo MC, Giordano P, Signoriello G, Daniele B, Perrone F. Potentiality and Boundaries of Use of Sorafenib in Patients with Hepatocellular Carcinoma: Awaiting the Results of Ongoing Clinical Trials. Cancers. 2012; 4(2):549-565. https://doi.org/10.3390/cancers4020549
Chicago/Turabian StyleDi Maio, Massimo, Gennaro Daniele, Maria Carmela Piccirillo, Pasqualina Giordano, Giuseppe Signoriello, Bruno Daniele, and Francesco Perrone. 2012. "Potentiality and Boundaries of Use of Sorafenib in Patients with Hepatocellular Carcinoma: Awaiting the Results of Ongoing Clinical Trials" Cancers 4, no. 2: 549-565. https://doi.org/10.3390/cancers4020549
APA StyleDi Maio, M., Daniele, G., Piccirillo, M. C., Giordano, P., Signoriello, G., Daniele, B., & Perrone, F. (2012). Potentiality and Boundaries of Use of Sorafenib in Patients with Hepatocellular Carcinoma: Awaiting the Results of Ongoing Clinical Trials. Cancers, 4(2), 549-565. https://doi.org/10.3390/cancers4020549





