Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Molecular Strategies for Viral Gene Therapy of the GBM
2.1. Viral Vector Types Proposed for Gene Therapy of GBM
2.2. Adenovirus-Based Vectors
2.3. Herpes Simplex Virus-Based Vectors
2.4. Vectors Based on other Viral Backgrounds
2.5. Evaluating Vector Efficacy
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, Y.; Uhrbom, L. On the origin of glioma. Upsala J. Med. Sci. 2012, 117, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, G.R.C.B.; Weller, H.-G.W.M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Bent, M.J.V.D.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Schaich, M.; Kestel, L.; Pfirrmann, M.; Robel, K.; Illmer, T.; Kramer, M.; Dill, C.; Ehninger, G.; Schackert, G.; Krex, D. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann. Oncol. 2009, 20, 175–181. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neuro Oncol. 2015, 122, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, A.; Sofi, R.; Tong, L.; Teschemacher, A.G.; Kasparov, S. In Search of a Breakthrough Therapy for Glioblastoma Multiforme. Neuroglia 2018, 1, 292–310. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-C.; Galanis, E.; Kirn, D.H. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; O’Leary, M.P.; Fong, Y.; Chen, N.G. From Benchtop to Bedside: A Review of Oncolytic Virotherapy. Biomedicines 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide Gene Therapy for Cancer—Current Strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). Available online: http://www.ictvonline.org/ (accessed on 15 October 2020).
- Harrach, B. Adenoviruses: General Features. In Encyclopedia of Virology; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 1–9. [Google Scholar]
- Knipe, D.M.; Howley, P.M. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Pelka, P.; Miller, M.S.; Cecchini, M.; Yousef, A.F.; Bowdish, D.M.E.; Dick, F.; Whyte, P.; Mymryk, J.S. Adenovirus E1A Directly Targets the E2F/DP-1 Complex. J. Virol. 2011, 85, 8841–8851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wold, W.S.M.; Toth, K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr. Gene Ther. 2014, 13, 421–433. [Google Scholar] [CrossRef]
- Ganly, I.; Kirn, D.; Eckhardt, G.; I Rodriguez, G.; Soutar, D.S.; Otto, R.; Robertson, A.G.; Park, O.; Gulley, M.L.; Heise, C.; et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000, 6, 798–806. [Google Scholar]
- Rothmann, T.; Hengstermann, A.; Whitaker, N.J.; Scheffner, M.; Hausen, H.Z. Replication of ONYX-015, a Potential Anticancer Adenovirus, Is Independent of p53 Status in Tumor Cells. J. Virol. 1998, 72, 9470–9478. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.; Dix, B.R.; Myers, C.J.; Dobson-Le, D.; Huschtscha, L.; Hibma, M.; Royds, J.; Braithwaite, A.W. Evidence that Replication of the Antitumor Adenovirus ONYX-015 Is Not Controlled by the p53 and p14ARF Tumor Suppressor Genes. J. Virol. 2002, 76, 12483–12490. [Google Scholar] [CrossRef] [Green Version]
- Marina, N.; Christie, I.N.; Korsak, A.; Doronin, M.; Brazhe, A.; Hosford, P.S.; Wells, J.A.; Sheikhbahaei, S.; Humoud, I.; Paton, J.F.R.; et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.; Polson, J.W.; Murphy, D.; Paton, J.F.R.; Kasparov, S. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J. 2002, 16, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science 2010, 329, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fueyo-margareto, J.; Manzano-gomez, C.; Conrad, C.; Lang, F.; Yung, W.A.; Tufaro, F. Treatment of Brain Cancer with Oncolytic Adenovirus. WIPO Patent No. WO 2014/204814 A1, 24 December 2014. [Google Scholar]
- Philbrick, B.; Adamson, C. DNX-2401: An investigational drug for the treatment of recurrent glioblastoma. Expert Opin. Investig. Drugs 2019, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shin, D.H.; Nguyen, T.T.; Fueyo, J.; Fan, X.; Henry, V.; Carrillo, C.C.; Yi, Y.; Alonso, M.M.; Collier, T.L.; et al. Localized Treatment with Oncolytic Adenovirus Delta-24-RGDOX Induces Systemic Immunity against Disseminated Subcutaneous and Intracranial Melanomas. Clin. Cancer Res. 2019, 25, 6801–6814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufaro, F.; Fueyo-Margareto, J.; Gomez-Manzano, C.; Conrad, C.; Yung, A.W.; Jiang, H. Adenovirus Expressing Immune Cell Stimulatory Receptor Agonist(s). WIPO Patent No. WO 2015/077624 Al, 28 May 2015. [Google Scholar]
- Smitt, P.S.; Driesse, M.; Wolbers, J.; Kros, M.; Avezaat, C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol. Ther. 2003, 7, 851–858. [Google Scholar] [CrossRef]
- Kasparov, S. Suitability of hCMV for viral gene expression in the brain. Nat. Methods 2007, 4, 379. [Google Scholar] [CrossRef]
- Germano, I.M.; Fable, J.; Gultekin, S.H.; Silvers, A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: Preliminary results of a phase I trial in patients with recurrent malignant gliomas. J. Neuro-Oncol. 2003, 65, 279–289. [Google Scholar] [CrossRef]
- Smith, R.L.; Traul, D.L.; Schaack, J.; Clayton, G.H.; Staley, K.J.; Wilcox, C.L. Characterization of Promoter Function and Cell-Type-Specific Expression from Viral Vectors in the Nervous System. J. Virol. 2000, 74, 11254–11261. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, P.; Castro, M.G. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of malignant brain tumors: From mice to clinical trials for glioma patients. Clin. Immunol. 2018, 189, 43–51. [Google Scholar] [CrossRef]
- Vilaboa, N.; Boellmann, F.; Voellmy, R.W. Gene Switches for Deliberate Regulation of Transgene Expression: Recent Advances in System Development and Uses. J. Genet. Syndr. Gene Ther. 2011, 2. [Google Scholar] [CrossRef]
- Barrett, J.A.; Cai, H.; Miao, J.; Khare, P.D.; Gonzalez, P.; Dalsing-Hernandez, J.; Sharma, G.; Chan, T.; Cooper, L.J.; Lebel, F. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018, 25, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Johnston, S.C.; Tang, J.; Stahl, M.; Tobin, J.F.; Somers, W.S. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000, 19, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Smith, K.M.; McKinney, B.; Palmer, C.; Rosenfeld, S.; Lillehei, K.; Hamilton, A.; DeMasters, B.K.; Judy, K.; Kirn, D. A Phase I Trial of Ad.hIFN-β Gene Therapy for Glioma. Mol. Ther. 2008, 16, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.K.; Prados, M.; Levin, V.A.; Fetell, M.R.; Bennett, J.; Mahaley, M.S.; Salcman, M.; Etcubanas, E. Intravenous Recombinant Interferon beta in Patients With Recurrent Malignant Gliomas: A Phase I/II Study. J. Clin. Oncol. 1991, 9, 1945–1949. [Google Scholar] [CrossRef]
- Breitbart, E.; Leubitz, A.; Feige, E.; Penson, R. Treatment Methods Using Adenovirus. WIPO Patent No. WO 2014/060848 A2, 24 April 2014. [Google Scholar]
- Artusi, S.; Miyagawa, Y.; Goins, W.F.; Cohen, J.B.; Glorioso, J.C. Herpes Simplex Virus Vectors for Gene Transfer to the Central Nervous System. Diseases 2018, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Gale, M.; Katze, M.G. Molecular Mechanisms of Interferon Resistance Mediated by Viral-Directed Inhibition of PKR, the Interferon-Induced Protein Kinase. Pharmacol. Ther. 1998, 78, 29–46. [Google Scholar] [CrossRef]
- Cheng, G.; Gross, M.; Brett, M.-E.; He, B. AlaArg Motif in the Carboxyl Terminus of the γ134.5 Protein of Herpes Simplex Virus Type 1 Is Required for the Formation of a High-Molecular-Weight Complex That Dephosphorylates eIF-2α. J. Virol. 2001, 75, 3666–3674. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, H.H.; Thompson, T.W.; Konen, A.J.; Haenchen, S.D.; Hilliard, J.G.; Macdonald, S.J.; Morrison, L.A.; Davido, D.J. Herpes Simplex Virus 1 Mutant with Point Mutations inUL39Is Impaired for Acute Viral Replication in Mice, Establishment of Latency, and Explant-Induced Reactivation. J. Virol. 2018, 92, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef] [Green Version]
- Cassady, K.A.; Bauer, D.F.; Roth, J.; Chambers, M.R.; Shoeb, T.; Coleman, J.; Prichard, M.; Gillespie, G.Y.; Markert, J.M. Pre-clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-human Primates. Mol. Ther. Oncolytics 2017, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cinatl, J.; Michaelis, M.; Driever, P.H.; Činátl, J.; Hraběta, J.; Suhan, T.; Doerr, H.W.; Vogel, J.-U. Multimutated Herpes Simplex Virus G207 Is a Potent Inhibitor of Angiogenesis1. Neoplasia 2004, 6, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiocca, E.A.; Nakashima, H.; Kasai, K.; Fernandez, S.A.; Oglesbee, M. Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol. Ther. Methods Clin. Dev. 2020, 17, 871–893. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Yoshimura, H.; Suzuki, T.; Ishiwata, T. Nestin: Neural Stem/Progenitor Cell Marker in Brain Tumors. In Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications; IntechOpen: London, UK, 2013. [Google Scholar]
- Peters, C.; Rabkin, S.D. Designing herpes viruses as oncolytics. Mol. Ther. Oncolytics 2015, 2, 15010. [Google Scholar] [CrossRef]
- Strong, J.E.; Coffey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998, 17, 3351–3362. [Google Scholar] [CrossRef] [Green Version]
- Werner, K. Use of a Virus Regimen for the Treatment of Diseases. WIPO Patent No. PCT/EP2009/003074, 12 November 2009. [Google Scholar]
- Biederer, C.; Ries, S.; Brandts, C.H.; McCormick, F. Replication-selective viruses for cancer therapy. J. Mol. Med. 2001, 80, 163–175. [Google Scholar] [CrossRef]
- Lazar, I.; Yaacov, B.; Shiloach, T.; Eliahoo, E.; Kadouri, L.; Lotem, M.; Perlman, R.; Zakay-Rones, Z.; Panet, A.; Ben-Yehuda, D. The Oncolytic Activity of Newcastle Disease Virus NDV-HUJ on Chemoresistant Primary Melanoma Cells Is Dependent on the Proapoptotic Activity of the Inhibitor of Apoptosis Protein Livin. J. Virol. 2009, 84, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Marchini, A.; Bonifati, S.; Scott, E.M.; Angelova, A.L.; Rommelaere, J. Oncolytic parvoviruses: From basic virology to clinical applications. Virol. J. 2015, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Merrill, M.K.; Bernhardt, G.; Sampson, J.H.; Wikstrand, C.J.; Bigner, D.D.; Gromeier, M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004, 6, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Perez, O.D.; Logg, C.R.; Hiraoka, K.; Diago, O.; Burnett, R.; Inagaki, A.; Jolson, D.; Amundson, K.; Buckley, T.; Lohse, D.; et al. Design and Selection of Toca 511 for Clinical Use: Modified Retroviral Replicating Vector With Improved Stability and Gene Expression. Mol. Ther. 2012, 20, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Hogan, D.J.; Zhu, J.-J.; Diago, O.R.; Gammon, D.K.; Haghighi, A.; Lu, G.; Das, A.; Gruber, H.E.; Jolly, D.J.; Ostertag, D. Molecular Analyses Support the Safety and Activity of Retroviral Replicating Vector Toca 511 in Patients. Clin. Cancer Res. 2018, 24, 4680–4693. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.T.; Hlavaty, J.; Ostertag, D.; Espinoza, F.L.; Martin, B.; Petznek, H.; Rodriguez-Aguirre, M.; E Ibanez, C.; Kasahara, N.; Gunzburg, W.; et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013, 20, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Hruby, D. Vaccinia virus vectors: New strategies for producing recombinant vaccines. Clin. Microbiol. Rev. 1990, 3, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Splinter, P.L.; Greiner, S.; Myers, R.; Peng, K.-W.; Federspiel, M.J.; Russell, S.J.; LaRusso, N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006, 44, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- A Database of Privately and Publicly Funded Clinical Studies Conducted Around the World (ClinicalTrials.com Database). Available online: https://clinicaltrials.gov/ (accessed on 5 November 2020).
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Alonso, M.M.; García-Moure, M.; Gonzalez-Huarriz, M.; Marigil, M.; Hernandez-Alcoceba, R.; Buñales, M.; Hervás, S.; Gallego, J.; Gomez-Manzano, C.; Fueyo, J.; et al. Abstract CT027: Oncolytic virus DNX-2401 with a short course of temozolomide for glioblastoma at first recurrence: Clinical data and prognostic biomarkers. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Regeneron. Phase 1b open-label randomized study of the oncolytic adenovirus DNX- 2401 administered with or without interferon gamma for recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 3008. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A Phase I Open-Label, Dose-Escalation, Multi-Institutional Trial of Injection with an E1B-Attenuated Adenovirus, ONYX-015, into the Peritumoral Region of Recurrent Malignant Gliomas, in the Adjuvant Setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Manzanera, A.G.; Bell, S.D.; Cavaliere, R.; McGregor, J.M.; Grecula, J.C.; Newton, H.B.; Lo, S.S.; Badie, B.; Portnow, J.; et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro-oncology 2016, 18, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Ji, N.; Weng, D.; Liu, C.; Gu, Z.; Chen, S.; Guo, Y.; Fan, Z.; Wang, X.; Chen, J.; Zhao, Y.; et al. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 2015, 7, 4369–4378. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, P.R.; A Orringer, D.; Sagher, O.; Heth, J.; Hervey-Jumper, S.L.; Mammoser, A.G.; Junck, L.; Leung, D.; Umemura, Y.; Lawrence, T.S.; et al. First-in-human phase I trial of the combination of two adenoviral vectors expressing HSV1-TK and FLT3L for the treatment of newly diagnosed resectable malignant glioma: Initial results from the therapeutic reprogramming of the brain immune system. J. Clin. Oncol. 2019, 37, 2019. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Yu, J.S.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M.; et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11, eaaw5680. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.J.; Cohen, Y.; Vredenburgh, J.J.; Peters, K.B.; Breitbart, E.; Bangio, L.; Sher, N.; Harats, D.; Wen, P.Y. Phase I/II dose-escalation study of VB-111, an antiangiogenic gene therapy, in patients with recurrent glioblastoma multiforme. J. Clin. Oncol. 2013. [Google Scholar] [CrossRef]
- Cloughesy, T.; Brenner, A.; De Groot, J.F.; A Butowski, N.; Zach, L.; Campian, J.L.; Ellingson, B.M.; Freedman, L.S.; Cohen, Y.C.; Lowenton-Spier, N.; et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2019, 22, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Rampling, R.; Cruickshank, G.; Papanastassiou, V.; Nicoll, J.A.R.; Hadley, D.M.; Brennan, D.C.; Petty, R.; MacLean, A.; Harland, J.; A McKie, E.; et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000, 7, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5−) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Harrow, S.; Papanastassiou, V.; Harland, J.; Mabbs, R.; Petty, R.D.; Fraser, M.J.; Hadley, D.M.; Patterson, J.; Brown, S.M.; Rampling, R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004, 11, 1648–1658. [Google Scholar] [CrossRef] [Green Version]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; A Palmer, C.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; A Palmer, C.; et al. Phase Ib Trial of Mutant Herpes Simplex Virus G207 Inoculated Pre-and Post-tumor Resection for Recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.-C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A Phase 1 Trial of Oncolytic HSV-1, G207, Given in Combination With Radiation for Recurrent GBM Demonstrates Safety and Radiographic Responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef] [Green Version]
- A Forsyth, P.; Roldán, G.; George, D.J.; Wallace, C.; Palmer, C.A.; Morris, D.; Cairncross, G.; Matthews, M.V.; Markert, J.M.; Gillespie, Y.; et al. A Phase I Trial of Intratumoral Administration of Reovirus in Patients with Histologically Confirmed Recurrent Malignant Gliomas. Mol. Ther. 2008, 16, 627–632. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geletneky, K.; Hüsing, J.; Rommelaere, J.; Schlehofer, J.; Leuchs, B.; Dahm, M.; Krebs, O.; Doeberitz, M.V.K.; Huber, B.; Hajda, J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II Trial of Intravenous NDV-HUJ Oncolytic Virus in Recurrent Glioblastoma Multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Ii, J.E.H.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Petrecca, K.; Walbert, T.; Butowski, N.; Salacz, M.; Perry, J.; Damek, D.; Bota, D.; Bettegowda, C.; Zhu, J.-J.; et al. Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial. JAMA Oncol. 2020, 33612. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra75. [Google Scholar] [CrossRef] [Green Version]
- Jolly, D.J.; Robbins, J.M.; Ostertag, D.; Ibañez, C.; Kasahara, N.; Gruber, H.; Kalkanis, S.N.; Vogelbaum, M.; Aghi, M.K.; Cloughesy, T.; et al. 61. Ascending Dose Trials of a Retroviral Replicating Vector (Toca 511) in Patients with Recurrent High-Grade Glioma: Clinical Update, Molecular Analyses, and Proposed Mechanism of Action. Mol. Ther. 2016, 24, S27. [Google Scholar] [CrossRef]
- Cloughesy, T.; Landolfi, J.; A Vogelbaum, M.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Kalkanis, S.N.; Aghi, M.K.; Cloughsy, T.F.; Kaptain, G.; Portnow, J.; Vogelbaum, M.A.; Kesari, S.; Mikkelsen, T.; Elder, J.B.; Chen, C.C.; et al. DDEL-06 Preliminary Safety of Toca 511, a Retroviral Replicating Vector, in Patients with Recurrent High Grade Glioma across Three Separate Phase 1 Studies. Neuro Oncol. 2015, 17, v74. [Google Scholar] [CrossRef] [Green Version]
- Lyle, C.; McCormick, F. Integrin αvβ5 is a primary receptor for adenovirus in CAR-negative cells. Virol. J. 2010, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.R.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duale, H.; Kasparov, S.; Paton, J.F.R.; Teschemacher, A.G. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp. Physiol. 2005, 90, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Paton, J.F.R.; Kasparov, S. Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.-C.; Li, H.-P.; Hung, Y.-H.; Leu, Y.-W.; Wu, W.-H.; Wang, F.-S.; Lee, K.-D.; Chang, P.-J.; Wu, C.-S.; Lu, Y.-J.; et al. Targeted methylation of CMV and E1A viral promoters. Biochem. Biophys. Res. Commun. 2010, 402, 228–234. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Tian, Z.; Zhang, X.; Xu, D.; Li, Q.; Zhang, J.; Wang, T. The EF-1α promoter maintains high-level transgene expression from episomal vectors in transfected CHO-K1 cells. J. Cell. Mol. Med. 2017, 21, 3044–3054. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D. Glioblastoma Heterogeneity and Cancer Cell Plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef]
- Sharma, P.; Khuc, K. Summary Basis for Regulatory Action. 2018. Available online: https://www.fda.gov/media/125157/download (accessed on 10 October 2020).
- Al-Zaidy, S.A.; Mendell, J.R. From Clinical Trials to Clinical Practice: Practical Considerations for Gene Replacement Therapy in SMA Type 1. Pediatr. Neurol. 2019, 100, 3–11. [Google Scholar] [CrossRef]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.-L.; Sánchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; E Deverman, B.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef]

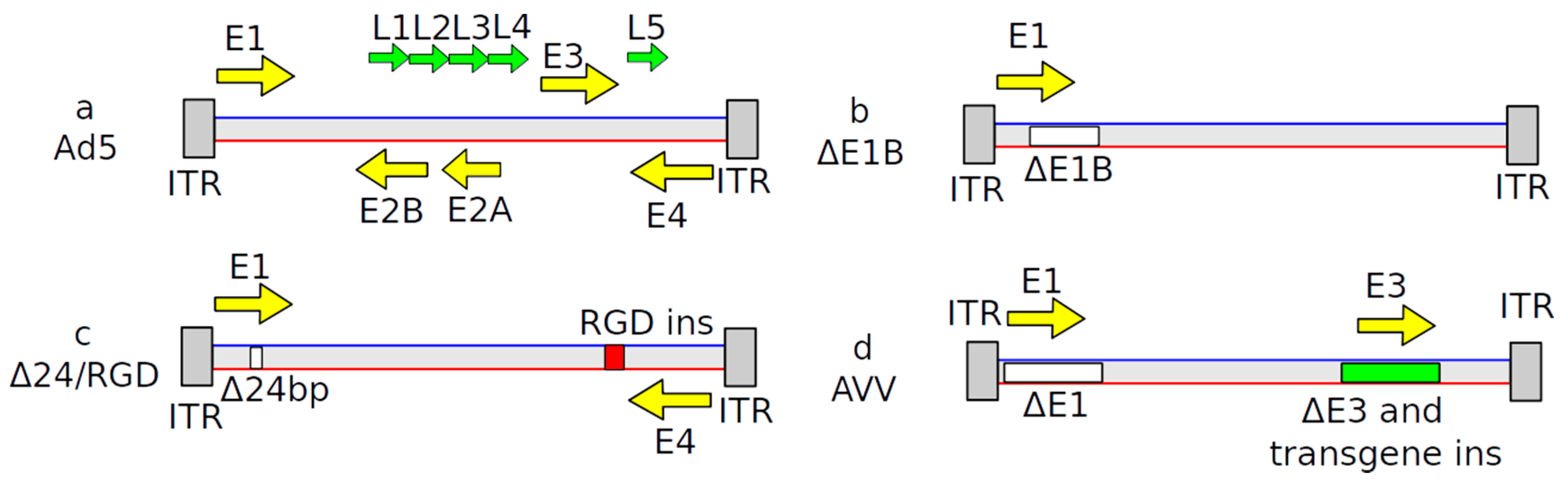
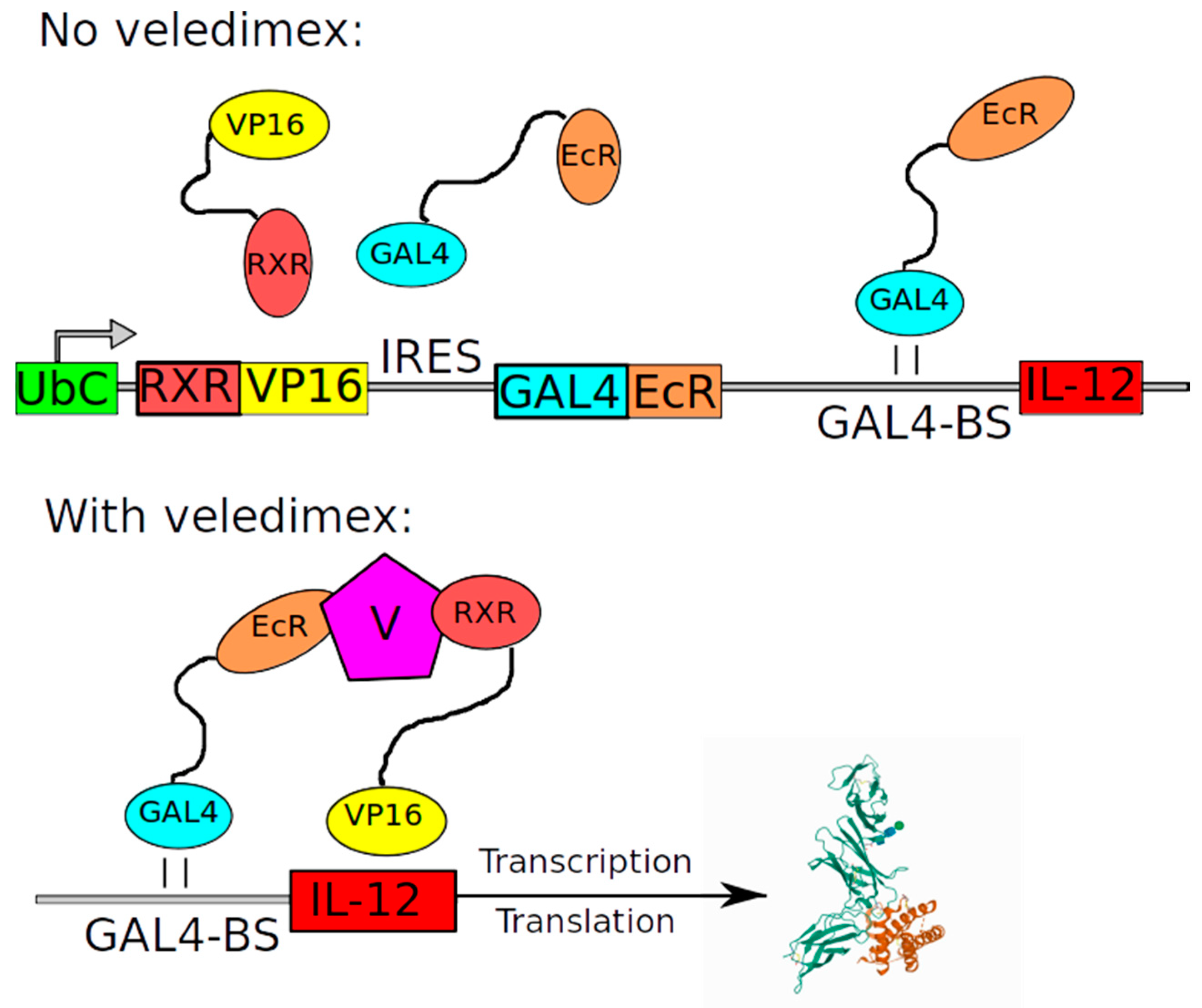
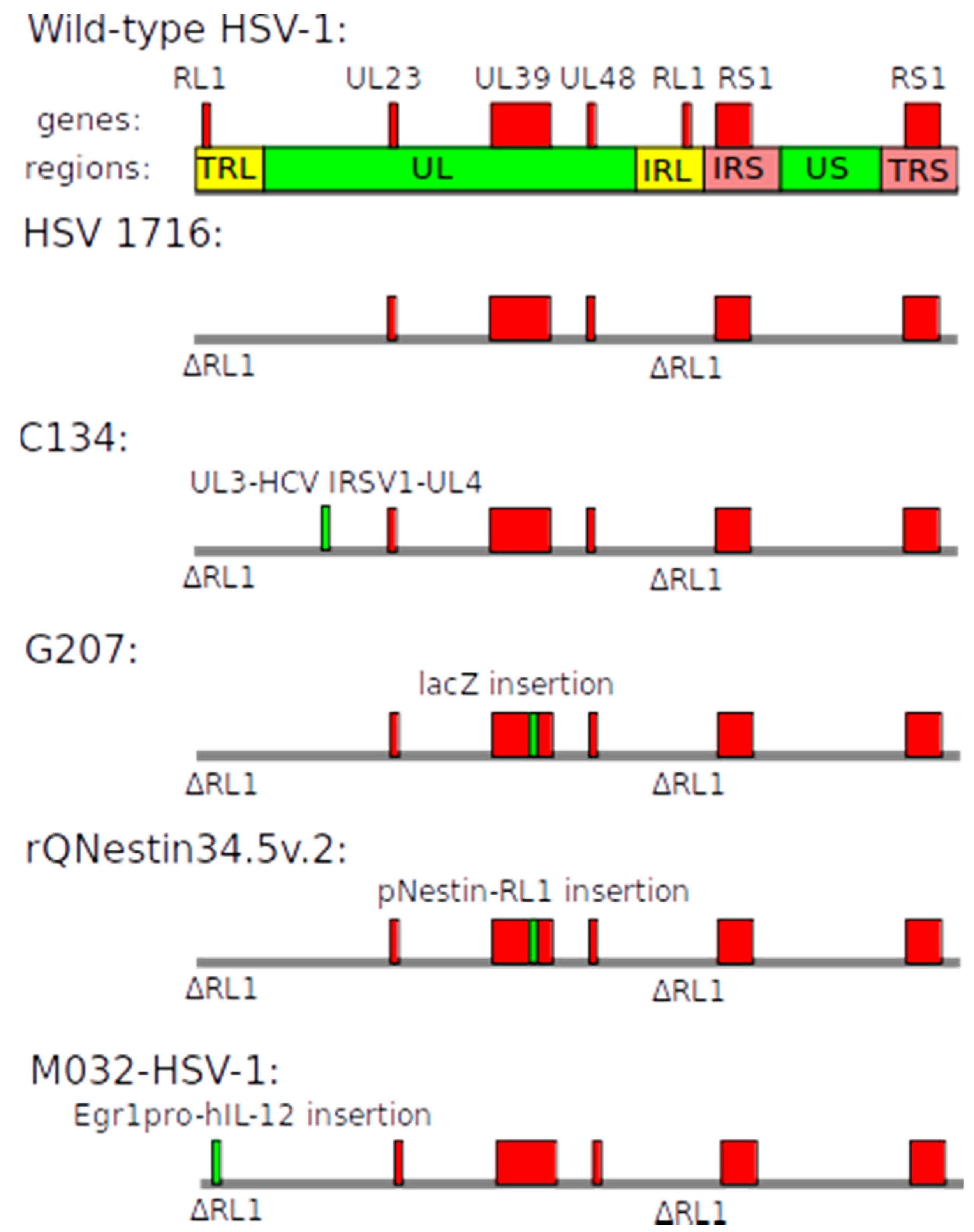
| Name | Structure of Vector | Mechanism of Action | Specificity | Replication Competent |
|---|---|---|---|---|
| DNX2401 | Ad5 | Lytic viral cycle in targeted cells | Replicate in cells defective in the Rb/p16 tumor suppressor pathway and expressing integrins αvβ3 and αvβ5 | ± |
| DNX2440 | Ad5 | Lytic viral cycle in targeted cells and immunomodulatory effect | Replicate in cells defective in the Rb/p16 tumor suppressor pathway and expressing integrins αvβ3 and αvβ5 | ± |
| ONYX-015 | chimeric Ad2 and Ad5 | Lytic viral cycle in targeted cells | Replicate in tumor cells with altered p53 pathway | ± |
| Ad-hCMV-TK | Ad5 | Converts harmless ganciclovir to toxic product in transduced cells | Transduce CAR-expressing cells. CMV-dependent expression mechanism | − |
| ADV/HSV-tk | Ad5 | Converts harmless ganciclovir to toxic product in transduced cells | Transduce CAR-expressing cells. RSV-dependent expression mechanism | − |
| Ad-hCMV-Flt3L | Ad5 | Immunomodulatory effect by stimulating both the proliferation of dendritic cells (DCs) and their migration to the tumor site | Transduce CAR expressing cells | − |
| Ad-RTS-hIL12 | Ad5 | Immunomodulatory effect by activation of immune system via IL-12 release | Transduce CAR-expressing cells | − |
| Ad.hIFN-β | Ad5 | Immunomodulatory effect by activation of immune system via human Interferon-β release | Transduce CAR-expressing cells | − |
| VB-111 | Ad5 | Decrease excessive angiogenesis via inhibition of endothelial cells | Transduce CAR-expressing cells, promotor initializes transcription only in endothelial cells undergoing angiogenesis | − |
| HSV 1716 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient cells | ± |
| G207 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient and fast dividing cells | ± |
| C134 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient and fast dividing cells | ± |
| rQNestin34.5v.2 | HSV-1 | Lytic viral cycle in targeted cells and indirect T-cell mediated cell death | Replication in PKR-deficient, Nestin-positive and fast dividing cells | ± |
| M032-HSV-1 | HSV-1 | Lytic viral cycle in targeted cells, indirect T-cell mediated cell death and immune system stimulation via IL12 release | Replication in PKR-defective and fast dividing cells | ± |
| Pelareorep (Reolysin) | Wild-type reovirus | Lytic viral cycle in targeted cells | Replication in ras-positive cells | + |
| ParvOryx | Wild-type parvovirus | Lytic viral cycle in targeted cells | Replication in fast dividing cells | + |
| NDV-HUJ | Wild-type HUJ strain of Newcastle disease virus | Livin-mediated apoptosis | Replication in fast dividing cells, apoptosis of livin-positive cells | + |
| PVSRIPO | Recombinant poliovirus type 1 | Lytic viral cycle in targeted cells | Replication restricted to CD155-expressing non-neuronal cells | + |
| Toca 511 | Recombinant Gammaretrovirus | CD-mediated prodrug conversion to cytotoxic drug in transduced cells | Replication in fast dividing cells | + |
| TG6002 | Recombinant vaccinia virus | Lytic viral cycle in targeted cells, CD-mediated prodrug conversion | Replication in cells expressing ribonucleotide reductase | + |
| MV-CEA | Recombinant measles virus | Lytic viral cycle in targeted cells | Transduce CD46-expressing cells | + |
| Vector | A Unique Identification Code Given to Clinical Study Registered on ClinicalTrials.gov | Study Date | Study Type (Safety/Trials in Recurrent GBM/Trials in Newly Diagnosed GBM) | Results/Comments |
|---|---|---|---|---|
| DNX2401 | NCT00805376 | 2008 | Dose-escalation study in recurrent GBM | Reported in 2018: DNX-2401 is safe, improves clinical outcome. Post-treatment histology examination of biopsy revealed sites of necrosis in GBM [64]. |
| - | NCT01582516 | 2012 | Dose-escalation study in recurrent GBM | No posted results. |
| - | NCT01956734 | 2013 | Safety and efficacy study in recurrent GBM DNX2401 + TMZ vs. TMZ alone | Reported in 2017: The safety objective of the trial was achieved with no severe toxicities related to DNX-2401 [65]. |
| - | NCT02197169 | 2014 | Safety and efficacy study in recurrent GBM, DNX2401 + IFN vs. DMX2401 alone | Reported in 2017: DNX-2401 was well tolerated as monotherapy. The addition of interferon did not improve survival [66]. |
| - | NCT02798406 | 2016 | Safety and efficacy study in recurrent GBM, DNX2401 + pembrolizumab | No posted results. |
| DNX2440 | NCT03714334 | 2018 | Safety and efficacy study in recurrent GBM, DNX2440 alone | No posted results. |
| ONYX-015 | Was not registered at ClinicalTrials.gov | - | Dose-escalation study | Reported in 2004: None of the 24 patients experienced serious adverse events related to ONYX-015 [67]. |
| ADV/HSV-tk | NCT00589875 | 2008 | Study of AdV-tk + valacyclovir Gene therapy in combination with standard radiation therapy for malignant glioma | Reported in 2016: Addition of ADV/HSV-tk to SoC improves outcome [68]. |
| - | NCT00870181 | 2009 | Safety and efficacy of intravenous-administered ADV/HSV-tk in recurrent GBM vs. surgery or systemic chemotherapy or palliative care | Reported in 2016: ADV/HSV-tk is safe and can provide benefits [69]. |
| - | NCT03603405 | 2018 | Safety and efficacy study of standard treatment + ADV/HSV-tk in newly diagnosed GBM | No results posted. |
| - | NCT03596086 | 2018 | Safety and efficacy of ADV/HSV-tk in recurrent GBM | No results posted. |
| Ad-hCMV-Flt3L + 4. Ad-hCMV-TK (combination) | NCT01811992 | 2013 | Dose-escalation study in newly diagnosed GBM + standard treatment | Reported in 2019: Examination of tumor samples reveals increase in the infiltration of inflammatory cells. Preliminary data suggest that virotherapy can improve outcomes [70]. |
| Ad-RTS-hIL12 | NCT02026271 | 2014 | Safety and tolerability of a single tumor injection of Ad-RTS-hIL-12 given with oral veledimex (the activator of RTS promoter) in patients with recurrent or progressive GBM | Reported in 2019: The clinical trial demonstrated tolerability of veledimex-induced hIL-12 expression [71]. |
| - | NCT04006119 | 2019 | Safety and efficacy of intratumoral Ad-RTS-hIL-12 and oral veledimex in combination with cemiplimab-rwlc in patients with recurrent or progressive GBM | No results posted. |
| Ad.hIFN-β | Was not registered | - | Dose-escalation study | Reported in 2008: The most common adverse events were considered by the investigator as being unrelated to treatment [38]. |
| VB-111 | NCT01260506 | 2010 | Dose-escalation study of VB-111 in combination with bevacizumab in recurrent GBM. | Reported in 2013: VB-111 was safe and well tolerated in patients with recurrent GBM with repeat doses of up to 1 × 1013 VPs. Tumor responses were seen [72]. |
| - | NCT02511405 | 2015 | Comparison of VB-111 plus bevacizumab to bevacizumab in patients with recurrent GBM | Reported in 2020: Upfront concomitant administration of VB-111 and bevacizumab failed to improve outcomes [73]. |
| HSV 1716 | Was not registered | - | Safety and feasibility of intratumoral administration of HSV1716 | Reported in 2000: HSV1716 is safe when injected into sites around the post-resection tumor cavity [74]. |
| - | Was not registered | - | Efficacy of HSV1716 | Reported in 2002: HSV1716 replicates in HGG without causing toxicity [75]. |
| - | Was not registered | - | Efficacy of HSV1716 | Reported in 2004:Study demonstrates that HSV1716 injections can provide benefits [76]. |
| G207 | Was not registered | - | Dose-escalation study | Reported in 2000: No viral-related toxicity; evidence of antitumor activity. While adverse events were noted in some patients, no toxicity or serious adverse events could unequivocally be ascribed to G207 [77]. |
| - | NCT00028158 | 2001 | Dose-escalation study. Doses 1E9, 3E9 and 1E10 pfu were tested | Reported in 2009:No encephalitis; evidence of antitumor activity and viral replication [78]. |
| - | NCT00157703 | 2005 | De-escalation study. First patients received the highest dose (1E10 pfu). and if excessive toxicity had occurred, the dose would be reduced for the following patients | As reported in 2014: Treatment was well tolerated with signs of improving outcomes [79]. |
| C134 | NCT03657576 | 2018 | Dose-escalation study in recurrent/progressive GBM, anaplastic astrocytoma, or gliosarcoma | No results posted. |
| rQNestin34.5v.2 | NCT03152318 | 2017 | Dose-escalation study of in patients with recurrent GBM | No results posted. |
| M032-HSV-1 | NCT02062827 | 2014 | Dose escalation in recurrent/progressive GBM, anaplastic astrocytoma or gliosarcoma | No results posted. |
| Pelareorep (Reolysin) | NCT02444546 | 2015 | Dose-escalation study of Pelareorep in combination with sargramostim in recurrent/progressive GBM | No results posted. |
| - | NCT00528684 | 2007 | Dose-escalation study of Pelareorep in recurrent GBM | Reported in 2008: The intratumoral administration of the genetically unmodified reovirus was well tolerated using these doses and schedule in patients with recurrent GBM [80]. |
| ParvOryx | NCT01301430 | 2011 | Dose-escalation study of ParvOryx in patients with progressive or recurrent GBM | Reported in 2012 and 2017: No dose-limiting toxicity was reported but clinical response did not depend on the dose or mode of ParvOryx administration. No statistical confirmation of efficacy [81,82]. |
| NDV-HUJ | Was not registered | - | Dose-escalation study of NDV-HUJ | Reported in 2006: Toxicity was minimal with Grade I/II constitutional fever being seen in five patients. Maximum tolerated dose was not achieved [83]. |
| - | NCT01174537 | 2010 | Safety and efficacy of single dose intravenously administered | No results posted. |
| PVSRIPO | NCT02986178 | 2016 | Safety and efficacy of single dose PVSRIPO administered intratumorally in patients with recurrent GBM | No results posted. |
| - | NCT03973879 | 2019 | Safety and efficacy of single dose PVSRIPO administered intratumorally with atezolizumab treatment in patients with recurrent GBM | Withdrawn. |
| - | NCT01491893 | 2011 | Dose-escalation study of PVSRIPO administered intratumorally in patients with recurrent GBM | Reported in 2018: Intratumoral infusion of PVSRIPO in patients with recurrent WHO grade IV malignant glioma confirmed the absence of neurovirulent potential [84]. |
| Toca 511 | NCT04105374 | 2019 | Toca 511, Toca FC and standard of care vs. standard of care in newly diagnosed GBM | Withdrawn. |
| - | NCT02414165 | 2015 | Toca 511/Toca FC vs. Lomustine, Temozolomide, or Bevacizumab in recurrent GBM | Reported in 2020: administration of Toca 511 and Toca FC, compared with SoC, did not improve overall survival (11.10 months vs. 12.22 months, respectively) or other end points [85]. |
| - | NCT01470794 | 2011 | Dose-escalation study of Toca 511/Toca FC administered by injections into resection cavity wall in patients with recurrent GBM | Reported in 2016, 2016, 2018: Toca 511/Toca FC is safe and can provide durable complete response in some patients [86,87,88]. |
| - | NCT01156584 | 2010 | Dose-escalation study of Toca 511/Toca FC administered by intratumoral injections in patients with recurrent GBM | Reported in 2015, 2016:Safe and well tolerated [87,88,89]. |
| - | NCT01985256 | 2013 | Dose-escalation study of Toca 511/Toca FC administered by intravenously in patients with recurrent GBM | Reported in 2016: Injections were well tolerated [87]. |
| TG6002 | NCT03294486 | 2017 | Dose-escalation study of TG6002 in patients with recurrent GBM | No results posted. |
| MV-CEA | NCT00390299 | 2006 | Dose-escalation study of MV-CEA in patients with recurrent GBM | No results posted. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozhei, O.; G. Teschemacher, A.; Kasparov, S. Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers 2020, 12, 3724. https://doi.org/10.3390/cancers12123724
Mozhei O, G. Teschemacher A, Kasparov S. Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers. 2020; 12(12):3724. https://doi.org/10.3390/cancers12123724
Chicago/Turabian StyleMozhei, Oleg, Anja G. Teschemacher, and Sergey Kasparov. 2020. "Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma" Cancers 12, no. 12: 3724. https://doi.org/10.3390/cancers12123724






