Spatiotemporal pH Heterogeneity as a Promoter of Cancer Progression and Therapeutic Resistance
Abstract
1. Introduction
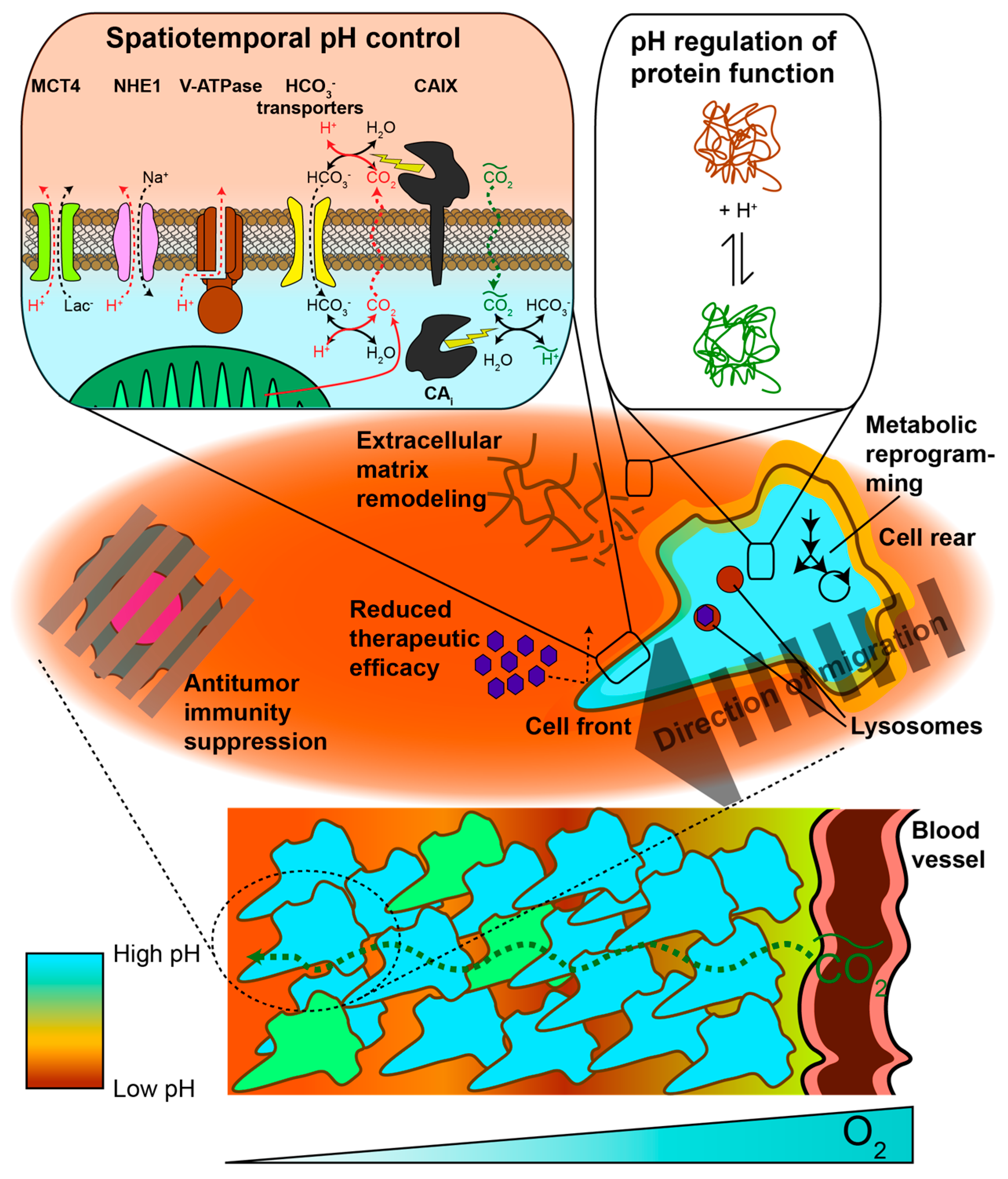
2. pH Heterogeneity in Space
2.1. Protonation as a Post-Translational Modification
2.2. Intracellular pH
2.2.1. Spatial Regulation of Protein Activity via Subcellular pH Heterogeneity
2.2.2. Intercellular pHi Heterogeneity within Tumors
2.3. Extracellular pH
2.3.1. Metabolic and Physiological Contributors to Spatial Gradients and Acidic pHe
2.3.2. Acidic pHe Can Alter Tumor Metabolism
2.3.3. Spatial pHe Gradients Promote Healthy Cell Death, Tumor Aggressiveness, and Therapeutic Resistance
3. pH Heterogeneity in Time
3.1. Carbonic Anhydrase Kinetics
3.2. Effects of pHi Transients on Tumor Cells
3.3. Tumor pHe Decreases Over Time During Tumorigenesis and Disease Progression
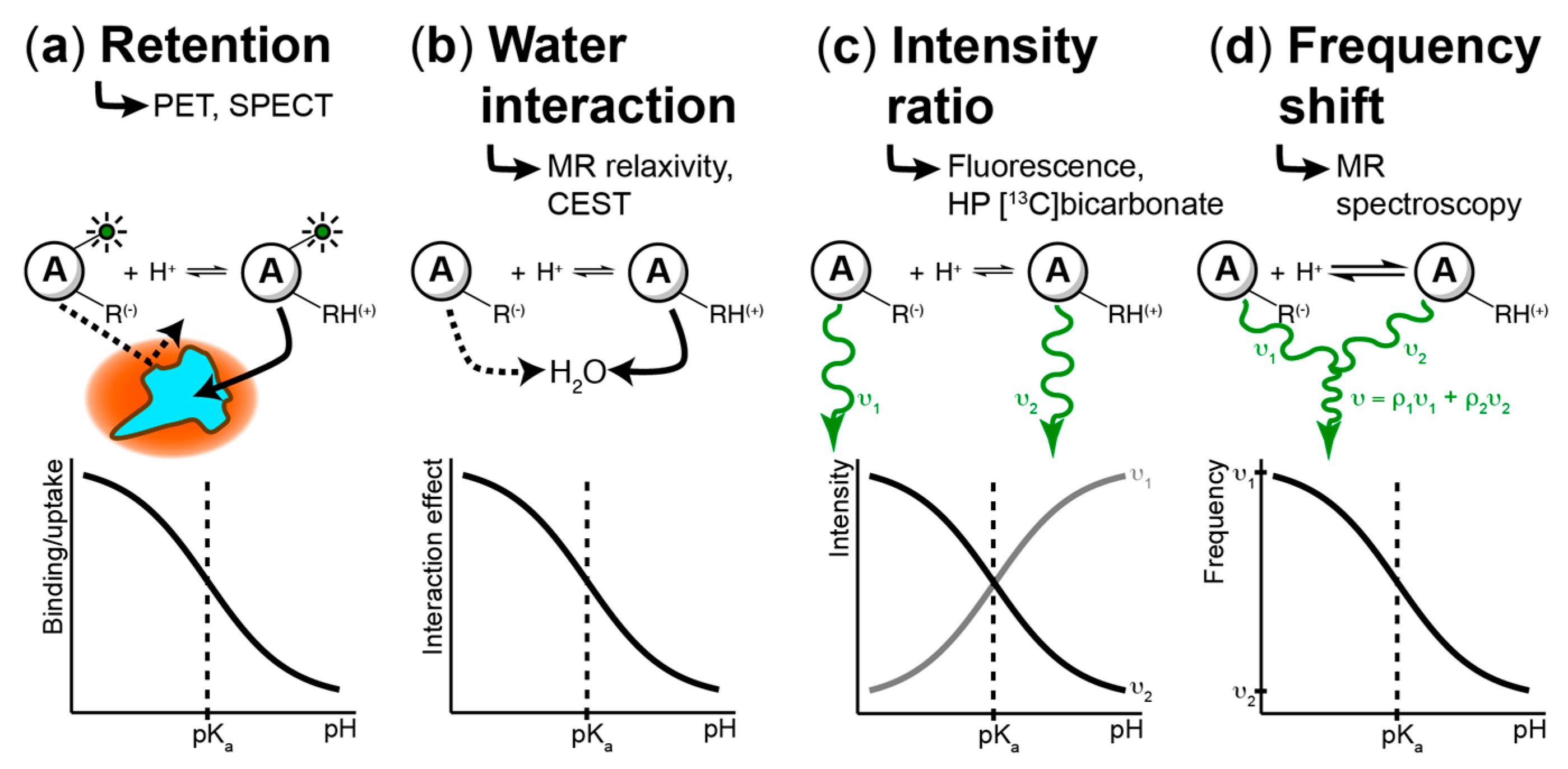
4. Techniques to Measure pH Spatiotemporal Heterogeneity
4.1. Fluorescence-Based Measurements
4.2. PET/SPECT-Based Imaging Methods
4.3. MR-Based Techniques
4.3.1. Chemical Exchange Saturation Transfer (CEST)
4.3.2. MR Relaxometry
4.3.3. MR Spectroscopic Approaches
4.3.4. Hyperpolarized (HP) 13C MR Imaging
5. Conclusions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 1–12. [Google Scholar] [CrossRef]
- Wike-Hooley, J.L.; Haveman, J.; Reinhold, H.S. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver's seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Comms. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Schönichen, A.; Webb, B.A.; Jacobson, M.P.; Barber, D.L. Considering Protonation as a Posttranslational Modification Regulating Protein Structure and Function. Annu. Rev. Biophys. 2013, 42, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cheung, W.Y. H+ is involved in the activation of calcineurin by calmodulin. J. Biol. Chem. 1994, 269, 22067–22074. [Google Scholar]
- Webb, B.A.; White, K.A.; Grillo-Hill, B.K.; Schönichen, A.; Choi, C.; Barber, D.L. A Histidine Cluster in the Cytoplasmic Domain of the Na-H Exchanger NHE1 Confers pH-sensitive Phospholipid Binding and Regulates Transporter Activity. J. Biol. Chem. 2016, 291, 24096–24104. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Barreiro, G.; Dominguez, L.; Chen, X.; Eddy, R.; Condeelis, J.; Kelly, M.J.S.; Jacobson, M.P.; Barber, D.L. Cofilin is a pH sensor for actin free barbed end formation: Role of phosphoinositide binding. J. Cell Biol. 2008, 183, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Barreiro, G.; Groscurth, S.; Gingras, A.R.; Goult, B.T.; Critchley, D.R.; Kelly, M.J.S.; Jacobson, M.P.; Barber, D.L. Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc. Natl. Acad. Sci. USA 2008, 105, 14436–14441. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Ruiz, D.G.; Szpiech, Z.A.; Strauli, N.B.; Hernandez, R.D.; Jacobson, M.P.; Barber, D.L. Cancer-associated arginine-to-histidine mutations confer a gain in pH sensing to mutant proteins. Sci. Signal. 2017, 10, eaam9931. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Schwab, A. Protons make tumor cells move like clockwork. Pflugers Arch.—Eur. J. Physiol. 2009, 458, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Szpiech, Z.A.; Strauli, N.B.; White, K.A.; Ruiz, D.G.; Jacobson, M.P.; Barber, D.L.; Hernandez, R.D. Prominent features of the amino acid mutation landscape in cancer. PLoS ONE 2017, 12, e0183273-12. [Google Scholar] [CrossRef] [PubMed]
- Kallunki, T.; Olsen, O.D.; Jäättelä, M. Cancer-associated lysosomal changes: Friends or foes? Oncogene 2013, 32, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Stransky, L.; Cotter, K.; Forgac, M. The Function of V-ATPases in Cancer. Physiol. Rev. 2016, 96, 1071–1091. [Google Scholar] [CrossRef] [PubMed]
- Grillo-Hill, B.K.; Choi, C.; Jimenez-Vidal, M.; Barber, D.L. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife 2015, 2015, 1–31. [Google Scholar] [CrossRef]
- Humez, S.; Monet, M.; van Coppenolle, F.; Delcourt, P.; Prevarskaya, N. The role of intracellular pH in cell growth arrest induced by ATP. Am. J. Physiol., Cell Physiol. 2004, 287, C1733–C1746. [Google Scholar] [CrossRef][Green Version]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 768–777. [Google Scholar] [CrossRef]
- Schornack, P.A.; Gillies, R.J. Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors. Neoplasia 2003, 5, 135–145. [Google Scholar] [CrossRef]
- Latour, L.L.; Svoboda, K.; Mitra, P.P.; Sotak, C.H. Time-dependent diffusion of water in a biological model system. Proc. Natl. Acad. Sci. USA 1994, 91, 1229–1233. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Montcourrier, P.; Silver, I.; Farnoud, R.; Bird, I.; Rochefort, H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin. Exp. Metastasis 1997, 15, 382–392. [Google Scholar] [CrossRef]
- Newell, K.; Franchi, A.; Pouyssegur, J.; Tannock, I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc. Natl. Acad. Sci. USA 1993, 90, 1127–1131. [Google Scholar] [CrossRef]
- Yamagata, M.; Hasuda, K.; Stamato, T.; Tannock, I.F. The contribution of lactic acid to acidification of tumours: Studies of variant cells lacking lactate dehydrogenase. Br. J. Cancer 1998, 77, 1726–1731. [Google Scholar] [CrossRef]
- Hulikova, A.; Black, N.; Hsia, L.-T.; Wilding, J.; Bodmer, W.F.; Swietach, P. Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proc. Natl. Acad. Sci. USA 2016, 113, E5344–E5353. [Google Scholar] [CrossRef]
- Swietach, P.; Wigfield, S.; Cobden, P.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008, 283, 20473–20483. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef]
- Lee, S.-H.; McIntyre, D.; Honess, D.; Hulikova, A.; Pacheco-Torres, J.; Cerdán, S.; Swietach, P.; Harris, A.L.; Griffiths, J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer 2018, 119, 622–630. [Google Scholar] [CrossRef]
- McIntyre, A.; Hulikova, A.; Ledaki, I.; Snell, C.; Singleton, D.; Steers, G.; Seden, P.; Jones, D.; Bridges, E.; Wigfield, S.; et al. Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer Res. 2016, 76, 3744–3755. [Google Scholar] [CrossRef]
- Swietach, P.; Hulikova, A.; Vaughan-Jones, R.D.; Harris, A.L. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 2010, 29, 6509–6521. [Google Scholar] [CrossRef]
- Ippolito, J.E.; Piwnica-Worms, D. A Fluorescence-Coupled Assay for Gamma Aminobutyric Acid (GABA) Reveals Metabolic Stress-Induced Modulation of GABA Content in Neuroendocrine Cancer. PLoS ONE 2014, 9, e88667-15. [Google Scholar] [CrossRef]
- Ippolito, J.E.; Brandenburg, M.W.; Ge, X.; Crowley, J.R.; Kirmess, K.M.; Som, A.; D'Avignon, D.A.; Arbeit, J.M.; Achilefu, S.; Yarasheski, K.E.; et al. Extracellular pH Modulates Neuroendocrine Prostate Cancer Cell Metabolism and Susceptibility to the Mitochondrial Inhibitor Niclosamide. PLoS ONE 2016, 11, e0159675. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006, 66, 5216–5223. [Google Scholar] [CrossRef]
- Park, H.J.; Lyons, J.C.; Ohtsubo, T.; Song, C.W. Acidic environment causes apoptosis by increasing caspase activity. Br. J. Cancer 1999, 80, 1892–1897. [Google Scholar] [CrossRef]
- Williams, A.C.; Collard, T.J.; Paraskeva, C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: Implications for clonal selection during colorectal carcinogenesis. Oncogene 1999, 18, 3199–3204. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Raghunand, N.; He, X.; Van Sluis, R.; Mahoney, B.; Baggett, B.; Taylor, C.W.; Paine-Murrieta, G.; Roe, D.; Bhujwalla, Z.M.; Gillies, R.J. Enhancement of chemotherapy by manipulation of tumour pH. Br. J. Cancer 1999, 80, 1005–1011. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; De Milito, A.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of Microenvironment Acidity Reverses Anergy in Human and Murine Tumor-Infiltrating T Lymphocytes. Cancer Res. 2012, 72, 2746–2756. [Google Scholar] [CrossRef]
- Vermeulen, M.; Giordano, M.; Trevani, A.S.; Sedlik, C.; Gamberale, R.; Fernandez-Calotti, P.; Salamone, G.; Raiden, S.; Sanjurjo, J.; Geffner, J.R. Acidosis Improves Uptake of Antigens and MHC Class I-Restricted Presentation by Dendritic Cells. J. Immunol. 2004, 172, 3196–3204. [Google Scholar] [CrossRef]
- Gottfried, E. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Zhou, C.F.; Yuan, J.; Liu, L.; Guo, X.R.; Wang, X.L.; Ding, Y.; Wang, X.N.; Li, D.S.; Tu, H.J. NaHCO3 enhances the antitumor activities of cytokine-induced killer cells against hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2016, 12, 3167–3174. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef]
- Suzuki, A.; Maeda, T.; Baba, Y.; Shimamura, K.; Kato, Y. Acidic extracellular pH promotes epithelial mesenchymal transition in Lewis lung carcinoma model. Cancer Cell Int. 2014, 14, 129. [Google Scholar] [CrossRef]
- Singh, S.; Lomelino, C.L.; Mboge, M.Y.; Frost, S.C.; McKenna, R. Cancer Drug Development of Carbonic Anhydrase Inhibitors beyond the Active Site. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 2013, 13, 611–623. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent Development of pH-Responsive Polymers for Cancer Nanomedicine. Molecules 2018, 24. [Google Scholar] [CrossRef]
- Taresco, V.; Alexander, C.; Singh, N.; Pearce, A.K. Stimuli-Responsive Prodrug Chemistries for Drug Delivery. Adv. Ther. 2018, 1. [Google Scholar] [CrossRef]
- Sedlakova, O.; Svastova, E.; Takacova, M.; Kopacek, J.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front. Physiol. 2014, 4, 400. [Google Scholar] [CrossRef]
- Geers, C.; Gros, G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 2000, 80, 681–715. [Google Scholar] [CrossRef]
- Hallerdei, J.; Scheibe, R.J.; Parkkila, S.; Waheed, A.; Sly, W.S.; Gros, G.; Wetzel, P.; Endeward, V. T Tubules and Surface Membranes Provide Equally Effective Pathways of Carbonic Anhydrase-Facilitated Lactic Acid Transport in Skeletal Muscle. PLoS ONE 2010, 5, e15137-11. [Google Scholar] [CrossRef]
- Svastova, E.; Hulı́ková, A.; Rafajová, M.; Zat'ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef]
- Gallagher, F.A.; Sladen, H.; Kettunen, M.I.; Serrao, E.M.; Rodrigues, T.B.; Wright, A.; Gill, A.B.; McGuire, S.; Booth, T.C.; Boren, J.; et al. Carbonic Anhydrase Activity Monitored In Vivo by Hyperpolarized 13C-Magnetic Resonance Spectroscopy Demonstrates Its Importance for pH Regulation in Tumors. Cancer Res. 2015, 75, 4109–4118. [Google Scholar] [CrossRef]
- Hulikova, A.; Aveyard, N.; Harris, A.L.; Vaughan-Jones, R.D.; Swietach, P. Intracellular carbonic anhydrase activity sensitizes cancer cell pH signaling to dynamic changes in CO2 partial pressure. J. Biol. Chem. 2014, 289, 25418–25430. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Novak, I.; Alves, F.; Schwab, A.; Pardo, L.A. Alternating pH landscapes shape epithelial cancer initiation and progression: Focus on pancreatic cancer. BioEssays 2017, 39, 1600253-10. [Google Scholar] [CrossRef]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef]
- Greenberg, N.M.; Demayo, F.; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate Cancer in a Transgenic Mouse. Proc. Natl. Acad. Sci. USA 1995, 92, 3439–3443. [Google Scholar] [CrossRef]
- Albers, M.J.; Bok, R.; Chen, A.P.; Cunningham, C.H.; Zierhut, M.L.; Zhang, V.Y.; Kohler, S.J.; Tropp, J.; Hurd, R.E.; Yen, Y.F.; et al. Hyperpolarized 13C Lactate, Pyruvate, and Alanine: Noninvasive Biomarkers for Prostate Cancer Detection and Grading. Cancer Res. 2008, 68, 8607–8615. [Google Scholar] [CrossRef]
- Ibrahim-Hashim, A.; Cornnell, H.H.; Abrahams, D.; Lloyd, M.; Bui, M.; Gillies, R.J.; Gatenby, R.A. Systemic Buffers Inhibit Carcinogenesis in TRAMP Mice. JURO 2012, 188, 624–631. [Google Scholar] [CrossRef]
- Korenchan, D.E.; Bok, R.; Sriram, R.; Delos Santos, R.; Qin, H.; Vigneron, D.B.; Wilson, D.M.; Kurhanewicz, J.; Flavell, R.R. Hyperpolarized in vivo pH imaging reveals grade-dependent interstitial acidification. In Proceedings of the International Society for Magnetic Resonance in Medicine, Paris, France, 16–21 June 2018. Abstract #3711. [Google Scholar]
- Rottenberg, D.A.; Ginos, J.Z.; Kearfott, K.G. In vivo measurement of brain tumor pH using [11C] DMO and positron emission tomography. Ann. Neurol. 1985, 17, 70–79. [Google Scholar] [CrossRef]
- Jones, K.M.; Randtke, E.A.; Yoshimaru, E.S.; Howison, C.M.; Chalasani, P.; Klein, R.R.; Chambers, S.K.; Kuo, P.H.; Pagel, M.D. Clinical Translation of Tumor Acidosis Measurements with AcidoCEST MRI. Mol. Imaging Biol. 2017, 1–9. [Google Scholar] [CrossRef]
- Anemone, A.; Consolino, L.; Arena, F.; Capozza, M.; Longo, D.L. Imaging tumor acidosis: A survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev. 2019, 8, 705–725. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]
- Grillo-Hill, B.K.; Webb, B.A.; Barber, D.L. Ratiometric Imaging of pH Probes. In Quantitative Imaging in Cell Biology, 1st ed.; Waters, J.C., Wittman, T., Eds.; Elsevier: San Diego, CA, USA, 2014; Volume 123, pp. 429–448. [Google Scholar]
- Ni, Y.; Wu, J. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Buxton, R.B.; Alpert, N.M.; Babikian, V.; Weise, S. Evaluation of the 11C02 Positron Emission Tomographic Method for Measuring Brain pH. I. pH Changes Measured in States of Altered Pco2. J. Cereb. Blood Flow Metab. 1987, 7, 709–719. [Google Scholar] [CrossRef]
- Bauwens, M.; De Saint-Hubert, M.; Cleynhens, J.; Brams, L.; Devos, E.; Mottaghy, F.M.; Verbruggen, A. Radioiodinated Phenylalkyl Malonic Acid Derivatives as pH-Sensitive SPECT Tracers. PLoS ONE 2012, 7, e38428. [Google Scholar] [CrossRef]
- Flavell, R.R.; Truillet, C.; Regan, M.K.; Ganguly, T.; Blecha, J.E.; Kurhanewicz, J.; VanBrocklin, H.F.; Keshari, K.R.; Chang, C.J.; Evans, M.J.; et al. Caged [18F]FDG Glycosylamines for Imaging Acidic Tumor Microenvironments Using Positron Emission Tomography. Bioconjug. Chem. 2016, 27, 170–178. [Google Scholar] [CrossRef]
- Chen, L.Q.; Pagel, M.D. Evaluating pH in the Extracellular Tumor Microenvironment Using CEST MRI and Other Imaging Methods. Adv. Radiol. 2015, 2015, 1–25. [Google Scholar] [CrossRef]
- Lindeman, L.R.; Randtke, E.A.; High, R.A.; Jones, K.M.; Howison, C.M.; Pagel, M.D. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn. Reson. Med. 2018, 79, 2766–2772. [Google Scholar] [CrossRef]
- Longo, D.L.; Bartoli, A.; Consolino, L.; Bardini, P.; Arena, F.; Schwaiger, M.; Aime, S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016, 76, 6463–6470. [Google Scholar] [CrossRef]
- Martin, M.G.; Martinez, G.V. High resolution pHe imaging of rat glioma using pH-dependent relaxivity. Magn. Reson. Med. 2006, 309–315. [Google Scholar] [CrossRef]
- Kálmán, F.K.; Woods, M.; Caravan, P.; Jurek, P.; Spiller, M.; Tircsó, G.; Király, R.; Brücher, E.; Sherry, A.D. Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH. Inorg. Chem. 2007, 46, 5260–5270. [Google Scholar] [CrossRef]
- Raghunand, N.; Howison, C.; Sherry, A.D.; Zhang, S.; Gillies, R.J. Renal and systemic pH imaging by contrast-enhanced MRI. Magn. Reson. Med. 2003, 49, 249–257. [Google Scholar] [CrossRef]
- Gillies, R.J.; Liu, Z.; Bhujwalla, Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol., Cell Physiol. 1994, 267, C195–C203. [Google Scholar] [CrossRef]
- McCoy, C.L.; Parkins, C.S.; Chaplin, D.J.; Griffiths, J.R.; Rodrigues, L.M.; Stubbs, M. The Effect of Blood-Flow Modification on Intra- and Extracellular Ph Measured by P-31 Magnetic-Resonance Spectroscopy in Murine Tumors. Br. J. Cancer 1995, 72, 905–911. [Google Scholar] [CrossRef][Green Version]
- Bhujwalla, Z.M.; McCoy, C.L.; Glickson, J.D.; Gillies, R.J.; Stubbs, M. Estimations of intra- and extracellular volume and pH by P-31 magnetic resonance spectroscopy: Effect of therapy on RIF-1 tumours. Br. J. Cancer 1998, 78, 606–611. [Google Scholar] [CrossRef]
- Garcia-Martin, M.L.; Herigault, G.; Remy, C.; Farion, R.; Ballesteros, P.; Coles, J.A.; Cerdan, S.; Ziegler, A. Mapping extracellular pH in rat brain gliomas in vivo by H-1 magnetic resonance spectroscopic imaging: Comparison with maps of metabolites. Cancer Res. 2001, 61, 6524–6531. [Google Scholar]
- Provent, P.; Benito, M.; Hiba, B.; Farion, R.; Lopez-Larrubia, P.; Ballesteros, P.; Remy, C.; Segebarth, C.; Cerdan, S.; Coles, J.A.; et al. Serial In vivo Spectroscopic Nuclear Magnetic Resonance Imaging of Lactate and Extracellular pH in Rat Gliomas Shows Redistribution of Protons Away from Sites of Glycolysis. Cancer Res. 2007, 67, 7638–7645. [Google Scholar] [CrossRef]
- Pacheco-Torres, J.; Mukherjee, N.; Walko, M.; López-Larrubia, P.; Ballesteros, P.; Cerdán, S.; Kocer, A. Image guided drug release from pH-sensitive Ion channel-functionalized stealth liposomes into an in vivo glioblastoma model. Nanomedicine 2015, 11, 1345–1354. [Google Scholar] [CrossRef]
- Ojugo, A.S.E.; McSheehy, P.M.J.; McIntyre, D.J.O.; McCoy, C.; Stubbs, M.; Leach, M.O.; Judson, I.R.; Griffiths, J.R. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: A comparison of exogenous19F and31P probes. NMR Biomed. 1999, 12, 495–504. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Wilson, D.M.; Keshari, K.R.; Larson, P.E.Z.; Chen, A.P.; Hu, S.; Criekinge, M.V.; Bok, R.; Nelson, S.J.; Macdonald, J.M.; Vigneron, D.B.; et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J. Magn. Reson. 2010, 205, 141–147. [Google Scholar] [CrossRef]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.-E.; Ardenkjaer-Larsen, J.H.; Zandt, R.I.T.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Korenchan, D.E.; Flavell, R.R.; Baligand, C.; Sriram, R.; Neumann, K.; Sukumar, S.; VanBrocklin, H.; Vigneron, D.B.; Wilson, D.M.; Kurhanewicz, J. Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging. Chem. Commun. 2016, 52, 3030–3033. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Kadlecek, S.J.; Pourfathi, M.; Rizi, R.R. Efficient production of hyperpolarized bicarbonate by chemical reaction on a DNP precursor to measure pH. Magn. Reson. Med. 2014, 74, 1406–1413. [Google Scholar] [CrossRef]
- Drachman, N.; Kadlecek, S.; Pourfathi, M.; Xin, Y.; Profka, H.; Rizi, R. In vivo pH mapping of injured lungs using hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 2016, 78, 1121–1130. [Google Scholar] [CrossRef]
- Scholz, D.J.; Janich, M.A.; Köllisch, U.; Schulte, R.F.; Ardenkjaer-Larsen, J.H.; Frank, A.; Haase, A.; Schwaiger, M.; Menzel, M.I. Quantified pH imaging with hyperpolarized 13C-bicarbonate. Magn. Reson. Med. 2014, 73, 2274–2282. [Google Scholar] [CrossRef]
- Scholz, D.J.; Otto, A.M.; Hintermair, J.; Schilling, F.; Frank, A.; Köllisch, U.; Janich, M.A.; Schulte, R.F.; Schwaiger, M.; Haase, A.; et al. Parameterization of hyperpolarized 13C-bicarbonate-dissolution dynamic nuclear polarization. Magn. Reson. Mater. Phys. 2015, 28, 591–598. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Swietach, P.; Atherton, H.J.; Gallagher, F.A.; Lee, P.; Radda, G.K.; Clarke, K.; Tyler, D.J. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: A 13C and 31P magnetic resonance spectroscopy study. Cardiovasc. Res. 2010, 86, 82–91. [Google Scholar] [CrossRef]
- Lau, A.Z.; Miller, J.J.; Tyler, D.J. Mapping of intracellular pH in the in vivo rodent heart using hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 2017, 77, 1810–1817. [Google Scholar] [CrossRef]
- Martínez-Santiesteban, F.M.; Dang, T.P.; Lim, H.; Chen, A.P.; Scholl, T.J. T1nuclear magnetic relaxation dispersion of hyperpolarized sodium and cesium hydrogencarbonate-13C. NMR Biomed. 2017, 30, e3749-8. [Google Scholar] [CrossRef]
- Maptue, N.; Jiang, W.; Harrison, C.; Funk, A.M.; Sharma, G.; Malloy, C.R.; Sherry, D.; Khemtong, C. Esterase-Catalyzed Production of Hyperpolarized 13C-Enriched Carbon Dioxide in Tissues for Measuring pH. ACS Sensors 2018, 3, 2232–2236. [Google Scholar] [CrossRef]
- Lau, A.Z.; Chen, A.P.; Cunningham, C.H. Integrated Bloch-Siegert B₁ mapping and multislice imaging of hyperpolarized ¹³C pyruvate and bicarbonate in the heart. Magn. Reson. Med. 2012, 67, 62–71. [Google Scholar] [CrossRef]
- Korenchan, D.E.; Gordon, J.W.; Subramaniam, S.; Sriram, R.; Baligand, C.; VanCriekinge, M.; Bok, R.; Vigneron, D.B.; Wilson, D.M.; Larson, P.E.Z.; et al. Using bidirectional chemical exchange for improved hyperpolarized [13 C]bicarbonate pH imaging. Magn. Reson. Med. 2019, 82, 959–972. [Google Scholar] [CrossRef]
- Flavell, R.R.; von Morze, C.; Blecha, J.E.; Korenchan, D.E.; Van Criekinge, M.; Sriram, R.; Gordon, J.W.; Chen, H.-Y.; Subramaniam, S.; Bok, R.; et al. Application of Good's buffers to pH imaging using hyperpolarized 13C MRI. Chem. Commun. 2015, 51, 14119–14122. [Google Scholar] [CrossRef]
- Korenchan, D.E.; Taglang, C.; von Morze, C.; Blecha, J.E.; Gordon, J.W.; Sriram, R.; Larson, P.E.Z.; Vigneron, D.B.; VanBrocklin, H.F.; Kurhanewicz, J.; et al. Dicarboxylic acids as pH sensors for hyperpolarized (13)C magnetic resonance spectroscopic imaging. Analyst 2017, 142, 1429–1433. [Google Scholar] [CrossRef]
- Düwel, S.; Hundshammer, C.; Gersch, M.; Feuerecker, B.; Steiger, K.; Buck, A.; Walch, A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; et al. Imaging of pH in vivo using hyperpolarized. Nature 2017, 8, 1–9. [Google Scholar]
- Hundshammer, C.; Düwel, S.; Köcher, S.S.; Maltee, G.; Feuerecker, B.; Scheurer, C.; Haase, A.; Glaser, S.J.; Schwaiger, M.; Schilling, F. Deuteration of Hyperpolarized 13C-Labeled Zymonic Acid Enables Sensitivity-Enhanced Dynamic MRI of pH. ChemPhysChem 2017, 18, 2422–2425. [Google Scholar] [CrossRef]
- Hundshammer, C.; Düwel, S.; Ruseckas, D.; Topping, G.; Dzien, P.; Müller, C.; Feuerecker, B.; Hövener, J.B.; Haase, A.; Schwaiger, M.; et al. Hyperpolarized Amino Acid Derivatives as Multivalent Magnetic Resonance pH Sensor Molecules. Sensors 2018, 18, 600. [Google Scholar] [CrossRef]
- Jensen, P.R.; Meier, S. Hyperpolarised organic phosphates as NMR reporters of compartmental pH. Chem. Commun. 2016, 52, 2288–2291. [Google Scholar] [CrossRef]
- Hurd, R.E.; Yen, Y.-F.; Mayer, D.; Chen, A.; Wilson, D.; Kohler, S.; Bok, R.; Vigneron, D.; Kurhanewicz, J.; Tropp, J.; et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn. Reson. Med. 2010, 63, 1137–1143. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korenchan, D.E.; Flavell, R.R. Spatiotemporal pH Heterogeneity as a Promoter of Cancer Progression and Therapeutic Resistance. Cancers 2019, 11, 1026. https://doi.org/10.3390/cancers11071026
Korenchan DE, Flavell RR. Spatiotemporal pH Heterogeneity as a Promoter of Cancer Progression and Therapeutic Resistance. Cancers. 2019; 11(7):1026. https://doi.org/10.3390/cancers11071026
Chicago/Turabian StyleKorenchan, David E., and Robert R. Flavell. 2019. "Spatiotemporal pH Heterogeneity as a Promoter of Cancer Progression and Therapeutic Resistance" Cancers 11, no. 7: 1026. https://doi.org/10.3390/cancers11071026
APA StyleKorenchan, D. E., & Flavell, R. R. (2019). Spatiotemporal pH Heterogeneity as a Promoter of Cancer Progression and Therapeutic Resistance. Cancers, 11(7), 1026. https://doi.org/10.3390/cancers11071026




