Circulating miRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study
Abstract
:1. Introduction
2. Results
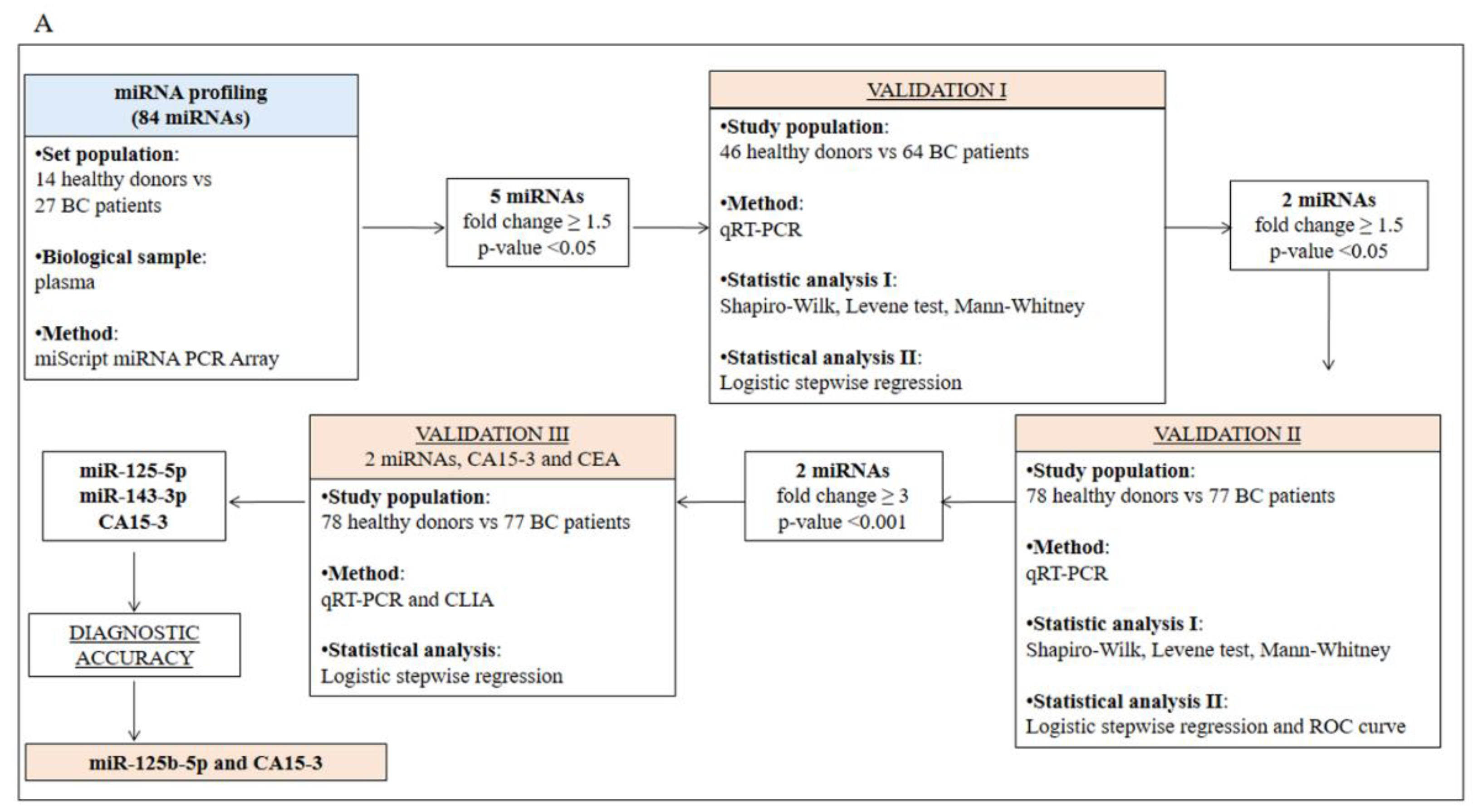
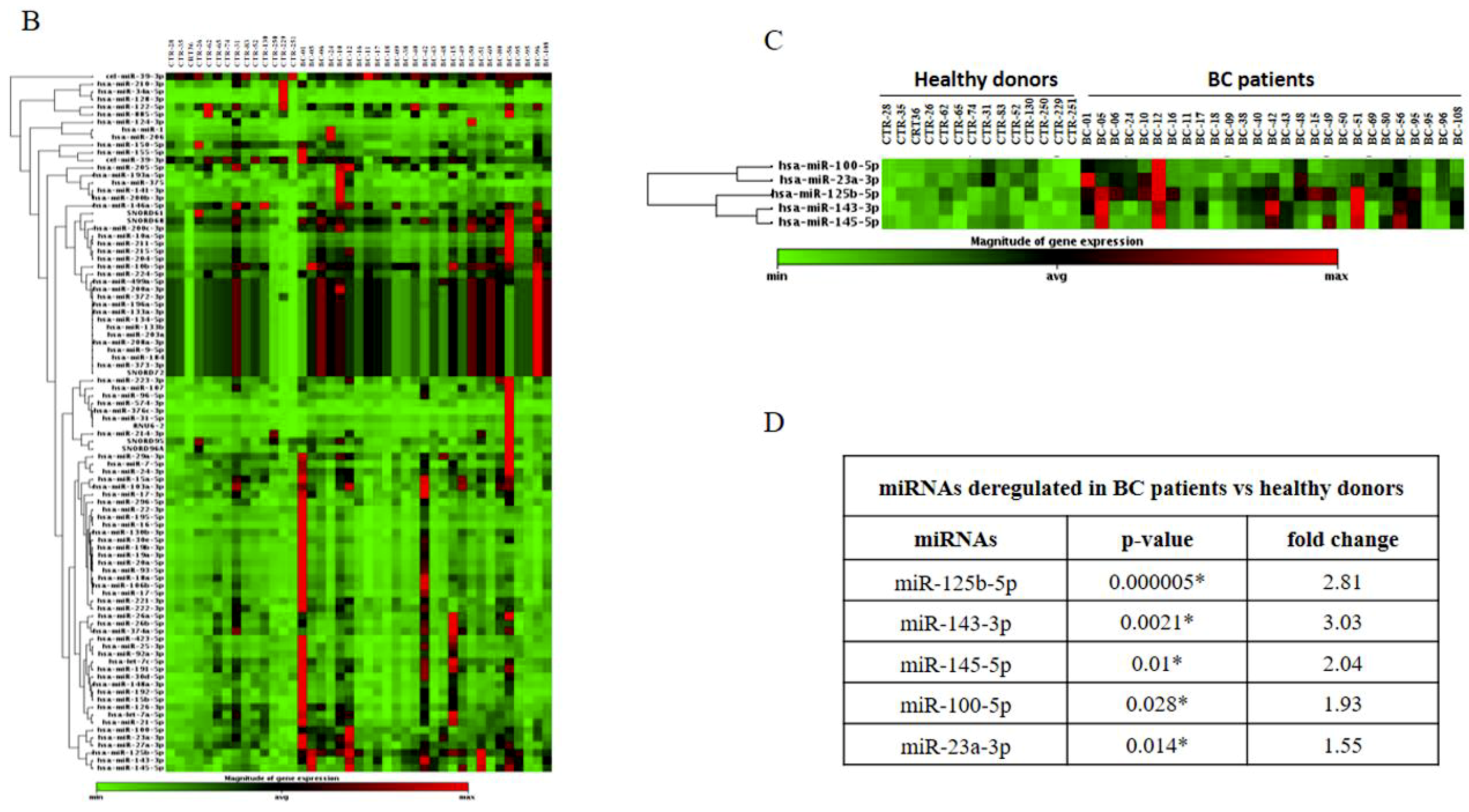
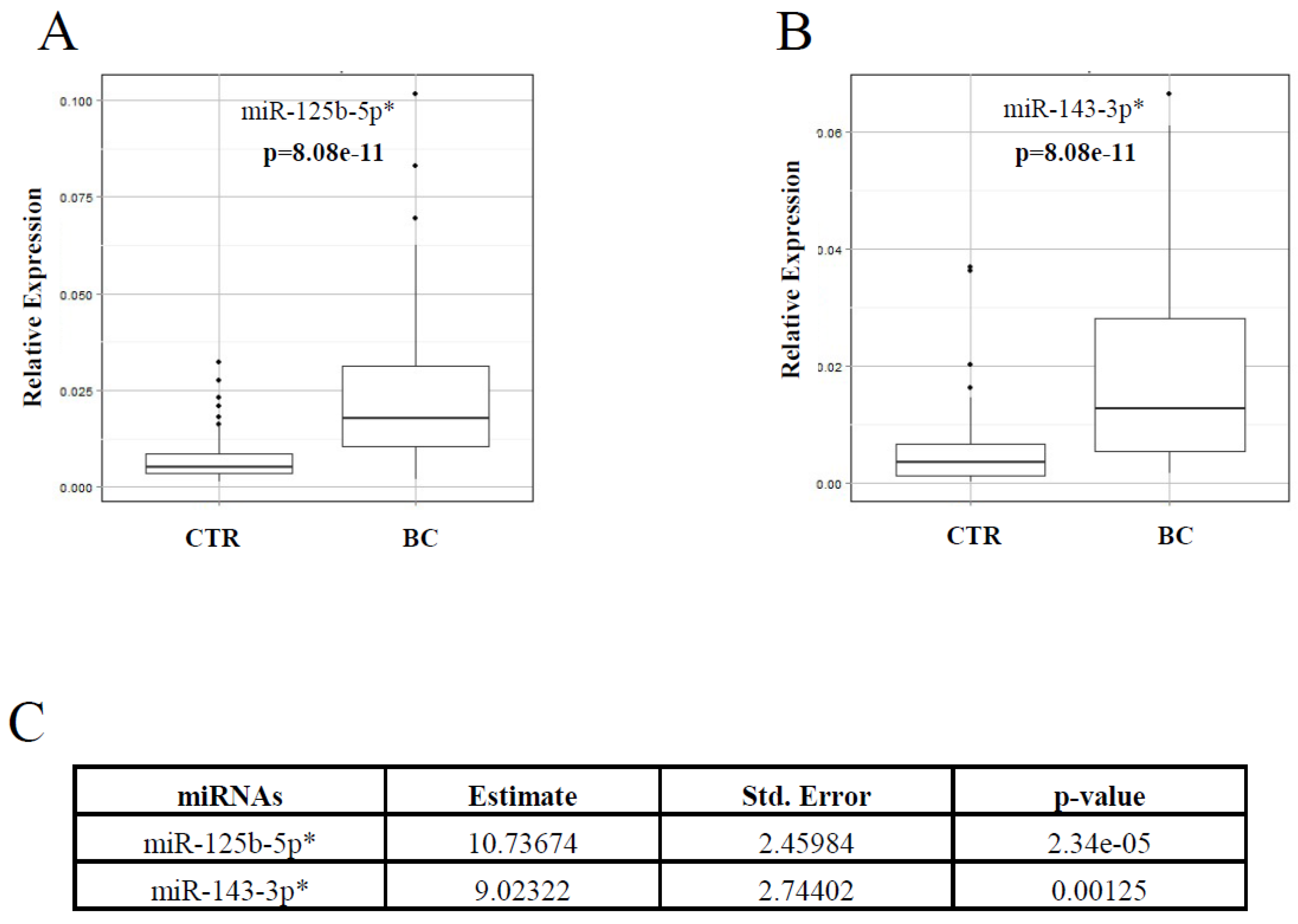
2.1. Identification and Validation of a Plasma miRNA Signature for Breast Cancer Detection
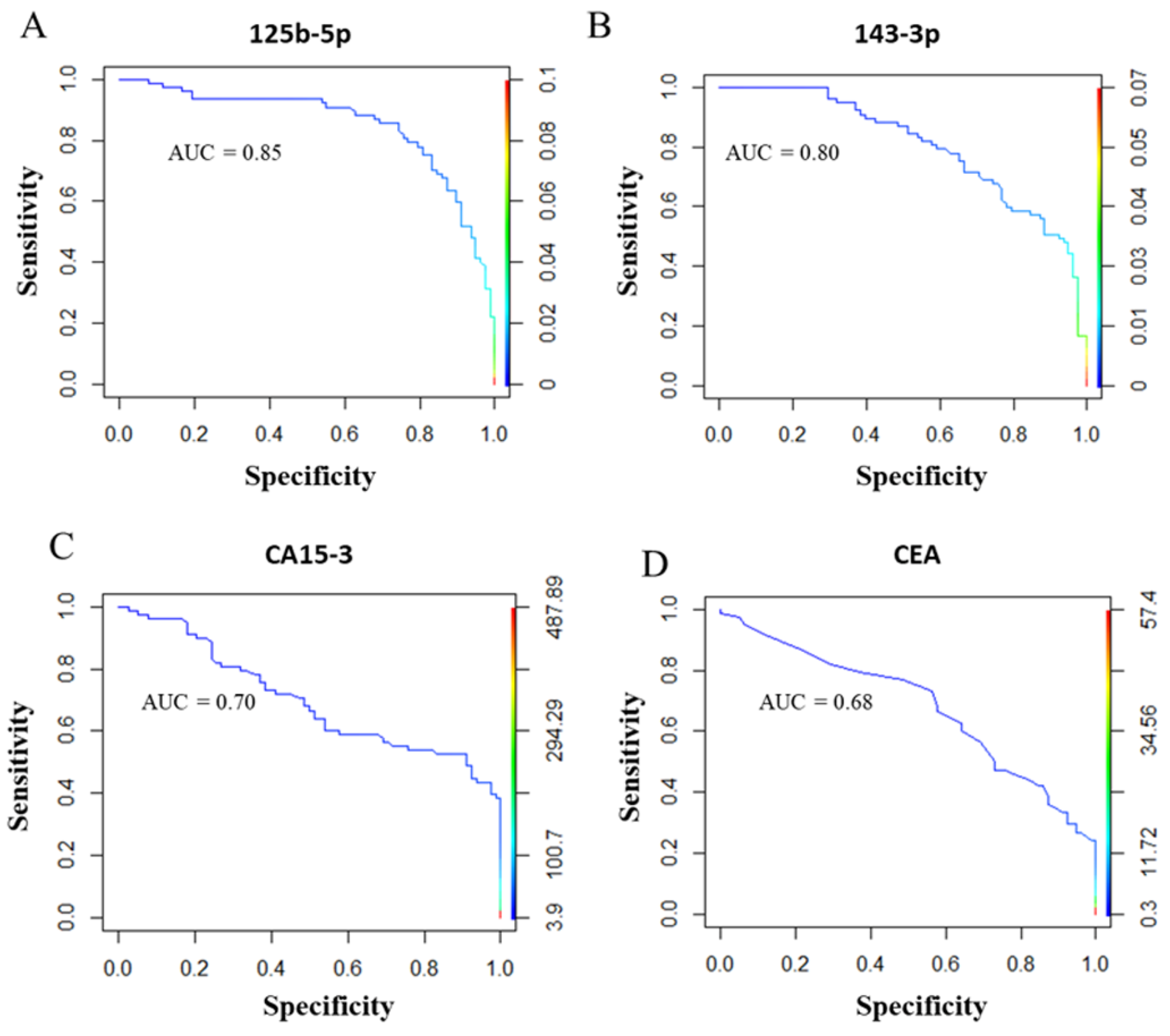
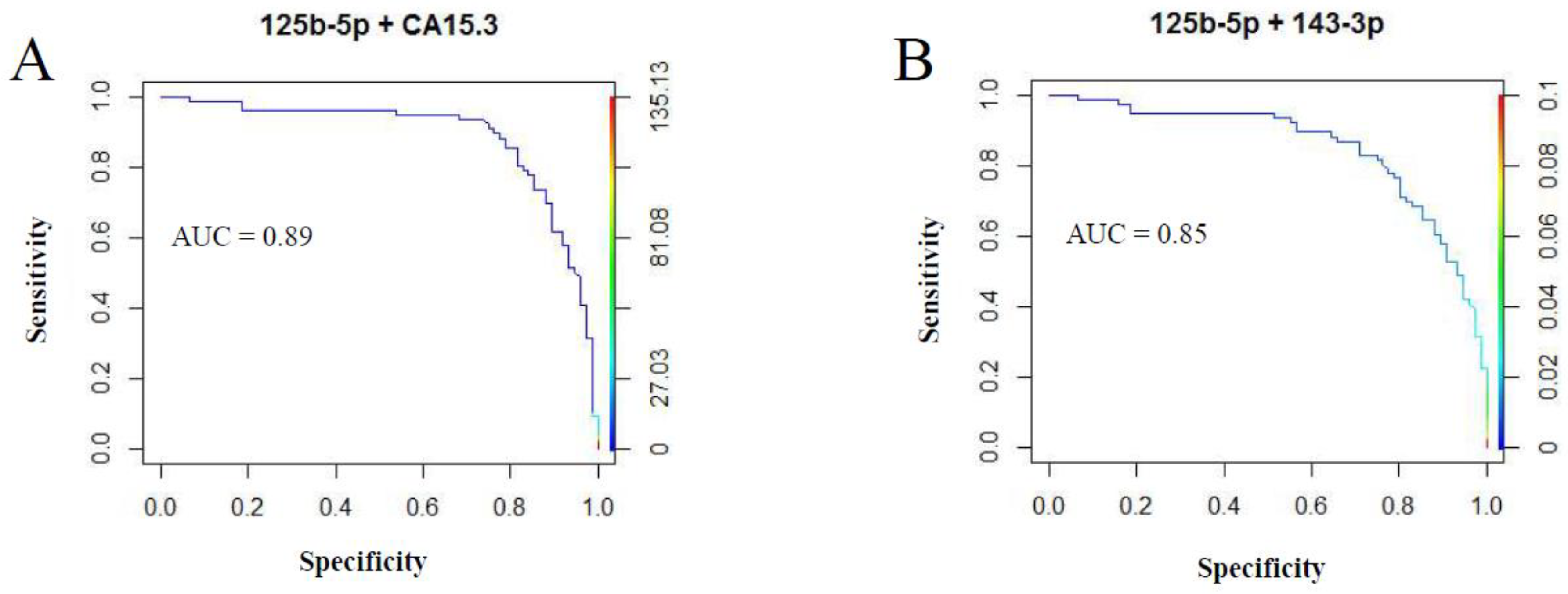
2.2. Diagnostic miRNA Signature-Based Model Compared with Established BC Markers
2.3. Co-Expression Analysis of Circulating miR-125b-5p, miR-143-3p and miR-145-5p and Their Association with Immunohistochemical Markers of BC
2.4. Correlation Analysis of Circulating miR-125b-5p, miR-143-3p and miR-145-5p with Quantitative Imaging Parameters of Tumour Lesions
2.5. In Silico Studies
2.6. Cellular Origin of miR-125b-5p, miR-143-3p and miR-145-5p in Normal and BC Tissues
2.7. Combining Diagnostic test Results to Increase Accuracy
3. Discussion
4. Materials and Methods
4.1. Participants and Study Design
4.2. PET/MR Data Acquisition
4.3. Data Processing and Multiparametric Analysis
4.4. Plasma Sampling, RNA Extraction and Reverse Transcription
4.5. miRNA Microarray and Validation by Real-Time PCR (qRT-PCR)
4.6. TCGA Data Processing
4.7. In Situ Hybridization
4.8. Measurement of CA 15-3
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, P.A.; Sickles, E.A.; Monsees, B.S.; Bassett, L.W.; Brenner, R.J.; Feig, S.A.; Smith, R.A.; Rosenberg, R.D.; Bogart, T.A.; Browning, S.; et al. Identifying Minimally Acceptable Interpretive Performance Criteria for Screening Mammography. Radiology 2010, 255, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Bohm-Velez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA J. Am. Med Assoc. 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Maric, P.; Ozretic, P.; Levanat, S.; Oreskovic, S.; Antunac, K.; Beketic-Oreskovic, L. Tumor Markers in Breast Cancer—Evaluation of their Clinical Usefulness. Coll. Antropol. 2011, 35, 241–247. [Google Scholar] [PubMed]
- Duffy, M.J. Serum tumor markers in breast cancer: Are they of clinical value? Clin. Chem. 2006, 52, 345–351. [Google Scholar] [CrossRef]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Thomssen, C.; Cardoso, F.; Cameron, D.; Cufer, T.; Fallowfield, L.; Francis, P.A.; Kyriakides, S.; Pagani, O.; Senkus, E.; et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: Surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast 2013, 22, 203–210. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—MicroRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Kent, O.A.; Mendell, J.T. A small piece in the cancer puzzle: MicroRNAs as tumor suppressors and oncogenes. Oncogene 2006, 25, 6188–6196. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.; Hoefig, K.; Merz, H.; Feller, A.C.; Kausch, I.; Jocham, D.; Warnecke, J.M.; Sczakiel, G. A robust methodology to study urine microRNA as tumor marker: MicroRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Todd, N.W.; Liu, Z.Q.; Zhan, M.; Fang, H.B.; Peng, H.; Alattar, M.; Deepak, J.; Stass, S.A.; Jiang, F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.T.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Salta, S.; Henrique, R.; Jeronimo, C. Decoding the usefulness of non-coding RNAs as breast cancer markers. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, D.; Cuk, K.; Burwinkel, B.; Yang, R. Cancer diagnosis and prognosis decoded by blood-based circulating microRNA signatures. Front. Genet. 2013, 4, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidner, R.S.; Li, L.; Thompson, C.L. Dampening Enthusiasm for Circulating MicroRNA in Breast Cancer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Mema, E.; Himoto, Y.; Veeraraghavan, H.; Brenton, J.D.; Snyder, A.; Weigelt, B.; Vargas, H.A. Unravelling tumour heterogeneity using next-generation imaging: Radiomics, radiogenomics, and habitat imaging. Clin. Radiol. 2017, 72, 3–10. [Google Scholar] [CrossRef]
- Incoronato, M.; Grimaldi, A.M.; Cavaliere, C.; Inglese, M.; Mirabelli, P.; Monti, S.; Ferbo, U.; Nicolai, E.; Soricelli, A.; Catalano, O.A.; et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: A PET/MRI study. Eur. J. Nucl. Med. Mol. Imaging 2018. [Google Scholar] [CrossRef]
- Catalano, O.A.; Daye, D.; Signore, A.; Iannace, C.; Vangel, M.; Luongo, A.; Catalano, M.; Filomena, M.; Mansi, L.; Soricelli, A.; et al. Staging performance of whole-body DWI, PET/CT and PET/MRI in invasive ductal carcinoma of the breast. Int. J. Oncol. 2017, 51, 281–288. [Google Scholar] [CrossRef]
- Catalano, O.A.; Nicolai, E.; Rosen, B.R.; Luongo, A.; Catalano, M.; Iannace, C.; Guimaraes, A.; Vangel, M.G.; Mahmood, U.; Soricelli, A.; et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br. J. Cancer 2015, 112, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botsikas, D.; Bagetakos, I.; Picarra, M.; Barisits, A.C.D.C.A.; Boudabbous, S.; Montet, X.; Giang Thanh, L.; Mainta, I.; Kalovidouri, A.; Becker, M. What is the diagnostic performance of 18-FDG-PET/MR compared to PET/CT for the N- and M- staging of breast cancer? Eur. Radiol. 2019, 29, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.S.; Wang, D.W.; Sun, X.B.; Shi, H.; Pang, T.; Dong, G.Q.; Zhang, C.Q. Quantitative analysis of 3-Tesla magnetic resonance imaging in the differential diagnosis of breast lesions. Exp. Ther. Med. 2015, 9, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, K.; Sun, X.L.; Wang, K.Z.; Sun, Y.Y.; Zhang, G.F.; Shen, B.Z. Parameters of Dynamic Contrast-Enhanced MRI as Imaging Markers for Angiogenesis and Proliferation in Human Breast Cancer. Med. Sci. Monit. 2015, 21, 376–382. [Google Scholar] [PubMed] [Green Version]
- Ma, C.X.; Ellis, M.J. The Cancer Genome Atlas: Clinical Applications for Breast Cancer. Oncology (New York) 2013, 27, 1263–1269. [Google Scholar]
- Matamala, N.; Vargas, M.T.; Gonzalez-Campora, R.; Minambres, R.; Arias, J.I.; Menendez, P.; Andres-Leon, E.; Gomez-Lopez, G.; Yanowsky, K.; Calvete-Candenas, J.; et al. Tumor MicroRNA Expression Profiling Identifies Circulating MicroRNAs for Early Breast Cancer Detection. Clin. Chem. 2015, 61, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, E.K.O.; Li, R.F.N.; Shin, V.Y.; Jin, H.C.; Leung, C.P.H.; Ma, E.S.K.; Pang, R.; Chua, D.; Chu, K.M.; Law, W.L.; et al. Circulating microRNAs as Specific Biomarkers for Breast Cancer Detection. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Thakur, S.; Grover, R.K.; Gupta, S.; Yadav, A.K.; Das, B.C. Identification of Specific miRNA Signature in Paired Sera and Tissue Samples of Indian Women with Triple Negative Breast Cancer. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Kodahl, A.R.; Lyng, M.B.; Binder, H.; Cold, S.; Gravgaard, K.; Knoop, A.S.; Ditzel, H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: A case control study. Mol. Oncol. 2014, 8, 874–883. [Google Scholar] [CrossRef]
- Chan, M.; Liaw, C.S.; Ji, S.M.; Tan, H.H.; Wong, C.Y.; Thike, A.A.; Tan, P.H.; Ho, G.H.; Lee, A.S.G. Identification of Circulating MicroRNA Signatures for Breast Cancer Detection. Clin. Cancer Res. 2013, 19, 4477–4487. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Tan, G.; Dong, L.; Cheng, L.; Li, K.J.; Wang, Z.Y.; Luo, H.F. Circulating MiR-125b as a Marker Predicting Chemoresistance in Breast Cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Mar-Aguilar, F.; Mendoza-Ramirez, J.A.; Malagon-Santiago, I.; Espino-Silva, P.K.; Santuario-Facio, S.K.; Ruiz-Flores, P.; Rodriguez-Padilla, C.; Resendez-Perez, D. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis. Mark. 2013, 34, 163–169. [Google Scholar] [CrossRef]
- Liu, B.Q.; Su, F.; Chen, M.W.; Li, Y.; Qi, X.Y.; Xiao, J.B.; Li, X.M.; Liu, X.C.; Liang, W.T.; Zhang, Y.F.; et al. Serum miR-21 and miR-125b as markers predicting neoadjuvant chemotherapy response and prognosis in stage II/III breast cancer. Hum. Pathol. 2017, 64, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.A.; Horn, G.L.; Signore, A.; Iannace, C.; Lepore, M.; Vangel, M.; Luongo, A.; Catalano, M.; Lehman, C.; Salvatore, M.; et al. PET/MR in invasive ductal breast cancer: Correlation between imaging markers and histological phenotype. Br. J. Cancer 2017, 116, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.; Calin, G.A. The miR-143/miR-145 cluster and the tumor microenvironment: Unexpected roles. Genome Med. 2016, 8. [Google Scholar] [CrossRef]
- Berindan-Neagoe, I.; Calin, G.A. Molecular Pathways: MicroRNAs, Cancer Cells, and Microenvironment. Clin. Cancer Res. 2014, 20, 6247–6253. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, H.; Zhang, Z.; Li, N. miR-125b-5p inhibits breast cancer cell proliferation, migration and invasion by targeting KIAA1522. Biochem. Biophys. Res. Commun. 2018, 504, 277–282. [Google Scholar] [CrossRef]
- Feliciano, A.; Castellvi, J.; Artero-Castro, A.; Leal, J.A.; Romagosa, C.; Hernandez-Losa, J.; Peg, V.; Fabra, A.; Vidal, F.; Kondoh, H.; et al. miR-125b Acts as a Tumor Suppressor in Breast Tumorigenesis via Its Novel Direct Targets ENPEP, CK2-alpha, CCNJ, and MEGF9. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Barak, V.; van Dalen, A.; Duffy, M.J.; Einarsson, R.; Gion, M.; Goike, H.; Lamerz, R.; Nap, M.; Soletormos, G.; et al. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumor Biol. 2005, 26, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Incoronato, M. Usefulness of Traditional Serum Biomarkers for Management of Breast Cancer Patients. Biomed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, B.; Xu, B.; Meng, X.; Li, L.; Cong, Y.; Liu, J.; Wang, Q.; Xuan, L.; Song, Q.; et al. A panel containing PD-1, IL-2R alpha, IL-10, and CA15-3 as a biomarker to discriminate breast cancer from benign breast disease. Cancer Manag. Res. 2018, 10, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Zaleski, M.; Kobilay, M.; Schroeder, L.; Debald, M.; Semaan, A.; Hettwer, K.; Uhlig, S.; Kuhn, W.; Hartmann, G.; Holdenrieder, S. Improved sensitivity for detection of breast cancer by combination of miR-34a and tumor markers CA 15-3 or CEA. Oncotarget 2018, 9, 22523–22536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajkowska, M.; Glazewska, E.K.; Bedkowska, G.E.; Chorazy, P.; Szmitkowski, M.; Lawicki, S. Diagnostic Power of Vascular Endothelial Growth Factor and Macrophage Colony-Stimulating Factor in Breast Cancer Patients Based on ROC Analysis. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef]
- Kirschner, M.B.; Edelman, J.J.B.; Kao, S.C.H.; Vallely, M.P.; van Zandwijk, N.; Reid, G. The Impact of Hemolysis on Cell-Free microRNA Biomarkers. Front. Genet. 2013, 4, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, O.A.; Rosen, B.R.; Sahani, D.V.; Hahn, P.F.; Guimaraes, A.R.; Vangel, M.G.; Nicolai, E.; Soricelli, A.; Salvatore, M. Clinical Impact of PET/MR Imaging in Patients with Cancer Undergoing Same-Day PET/CT: Initial Experience in 134 Patients-A Hypothesis-generating Exploratory Study. Radiology 2013, 269, 857–869. [Google Scholar] [CrossRef]
- Pace, L.; Nicolai, E.; Luongo, A.; Aiello, M.; Catalano, O.A.; Soricelli, A.; Salvatore, M. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: Lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur. J. Radiol. 2014, 83, 289–296. [Google Scholar] [CrossRef]
- Veronesi, U.; Zurrida, S.; Viale, G.; Galimberti, V.; Arnone, P.; Nole, F. Rethinking TNM: A Breast Cancer Classification to Guide to Treatment and Facilitate Research. Breast J. 2009, 15, 291–295. [Google Scholar] [CrossRef]
- Inglese, M.; Cavaliere, C.; Monti, S.; Forte, E.; Incoronato, M.; Nicolai, E.; Salvatore, M.; Aiello, M. A multi-parametric PET/MRI study of breast cancer: Evaluation of DCE-MRI pharmacokinetic models and correlation with diffusion and functional parameters. NMR Biomed. 2018, e4026. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Aiello, M.; Incoronato, M.; Grimaldi, A.M.; Moscarino, M.; Mirabelli, P.; Ferbo, U.; Cavaliere, C.; Salvatore, M. DCE-MRI Pharmacokinetic-Based Phenotyping of Invasive Ductal Carcinoma: A Radiomic Study for Prediction of Histological Outcomes. Contrast Media Mol. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Incoronato, M.; Coppola, L.; Infante, T.; Parente, C.A.; Nicolai, E.; Soricelli, A.; Salvatore, M. SDN biobank: Bioresource of human samples associated with functional and/or morphological bioimaging results for the study of oncological, cardiological, neurological, and metabolic diseases. Open J. Bioresour. 2017, 4. [Google Scholar] [CrossRef]
- Bellingham, S.A.; Coleman, B.M.; Hill, A.F. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012, 40, 10937–10949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44. [Google Scholar] [CrossRef] [PubMed]



| Healthy Control Samples (n = 78) | |
| Age range | 28–84 years |
| Mean ± S.D. | 51.05 ± 11.26 |
| Breast Cancer Samples (n = 77) | |
| Age range | 28–82 years |
| Mean ± S.D. | 53.68 ± 12.02 |
| Subtype | |
| Luminal A | 16 |
| Luminal B | 50 |
| HER2+ | 7 |
| Triple negative | 4 |
| Ki67 | |
| Low (1–20%) | 21 |
| High (21–100%) | 56 |
| Grade | |
| G1 | 1 |
| G2 | 41 |
| G3 | 35 |
| Stage | |
| Stage I | 1 |
| Stage II | 12 |
| Stage III | 42 |
| Stage IV | 22 |
| Circulating Biomarkers | Estimate | Std. Error | p-Value |
|---|---|---|---|
| miR-125b-5p * | 9.018 | 2.401 | 0.000248 |
| miR-143-3p * | 1.017 × 101 | 2.644 | 0.000179 |
| CA15-3 * | 2.123 × 10−3 | 8.984 × 10−4 | 0.01941 |
| CEA | 4.866 × 10−3 | 7.095 × 10−3 | 0.4939 |
| miR-125b-5p | Disease + | Disease − | Total | Sensitivity | Specificity | Diagnostic Accuracy |
| Test + | 61 (TP) | 16 (FP) | 77 | 0.79 (CI 0.68–0.88) | 0.79 (CI 0.69–0.88) | 0.79 (CI 0.72–0.85) |
| Test − | 16 (FN) | 62 (TN) | 78 | |||
| Total | 77 | 78 | 155 | |||
| miR-143-3p | Disease + | Disease − | Total | Sensitivity | Specificity | Diagnostic accuracy |
| Test + | 53 (TP) | 20 (FP) | 73 | 0.69 (CI 0.57–0.79) | 0.74 (CI 0.63–0.84) | 0.72 (CI 0.64–0.79) |
| Test − | 24 (FN) | 58 (TN) | 82 | |||
| Total | 77 | 78 | 155 | |||
| CA15-3 | Disease + | Disease − | Total | Sensitivity | Specificity | Diagnostic accuracy |
| Test + | 29 (TP) | 1 (FP) | 30 | 0.38 (CI 0.27–0.50) | 0.99 (CI 0.93–1.00) | 0.68 (CI 0.60–0.76) |
| Test − | 47 (FN) | 75 (TN) | 122 | |||
| Total | 76 | 76 | 152 | |||
| CEA | Disease + | Disease − | Total | Sensitivity | Specificity | Diagnostic accuracy |
| Test + | 25 (TP) | 8 (FP) | 73 | 0.33 (CI 0.23–0.45) | 0.89 (CI 0.80–0.95) | 0.61 (CI 0.53–0.69) |
| Test − | 51 (FN) | 68 (TN) | 82 | |||
| Total | 76 | 76 | 152 |
| Stage II | Stage III | Stage IV | ||||||
|---|---|---|---|---|---|---|---|---|
| iAUCmean | Ki67 | Kepmean | SUVmax | iAUCmean | Ki67 | Ki67 | Ktransmean | |
| miR-125-5p | ns | ns | ns | ns | ns | ns | −0.513 p-value 0.029 | −0.421 p-value 0.040 |
| miR-143-3p | 0.943 p-value 0.005 | ns | 0.943 p-value 0.005 | 0.829 p-value 0.042 | 0.938 p-value 0.044 | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incoronato, M.; Grimaldi, A.M.; Mirabelli, P.; Cavaliere, C.; Parente, C.A.; Franzese, M.; Staibano, S.; Ilardi, G.; Russo, D.; Soricelli, A.; et al. Circulating miRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study. Cancers 2019, 11, 876. https://doi.org/10.3390/cancers11060876
Incoronato M, Grimaldi AM, Mirabelli P, Cavaliere C, Parente CA, Franzese M, Staibano S, Ilardi G, Russo D, Soricelli A, et al. Circulating miRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study. Cancers. 2019; 11(6):876. https://doi.org/10.3390/cancers11060876
Chicago/Turabian StyleIncoronato, Mariarosaria, Anna Maria Grimaldi, Peppino Mirabelli, Carlo Cavaliere, Chiara Anna Parente, Monica Franzese, Stefania Staibano, Gennaro Ilardi, Daniela Russo, Andrea Soricelli, and et al. 2019. "Circulating miRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study" Cancers 11, no. 6: 876. https://doi.org/10.3390/cancers11060876





