Can Dendritic Cell Vaccination Prevent Leukemia Relapse?
Abstract
:1. Introduction
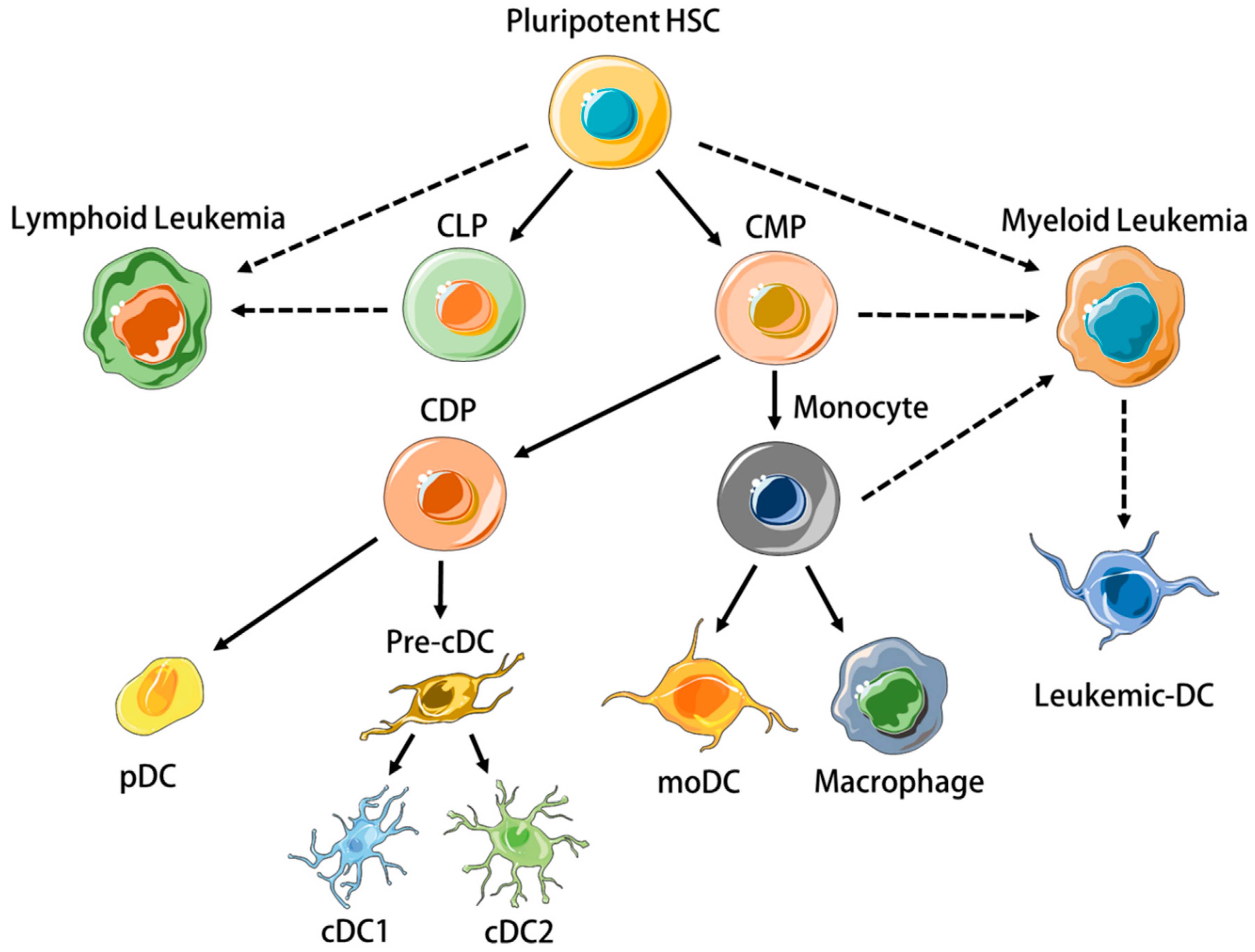
2. Dysregulation of DC Homeostasis and Function in Leukemia
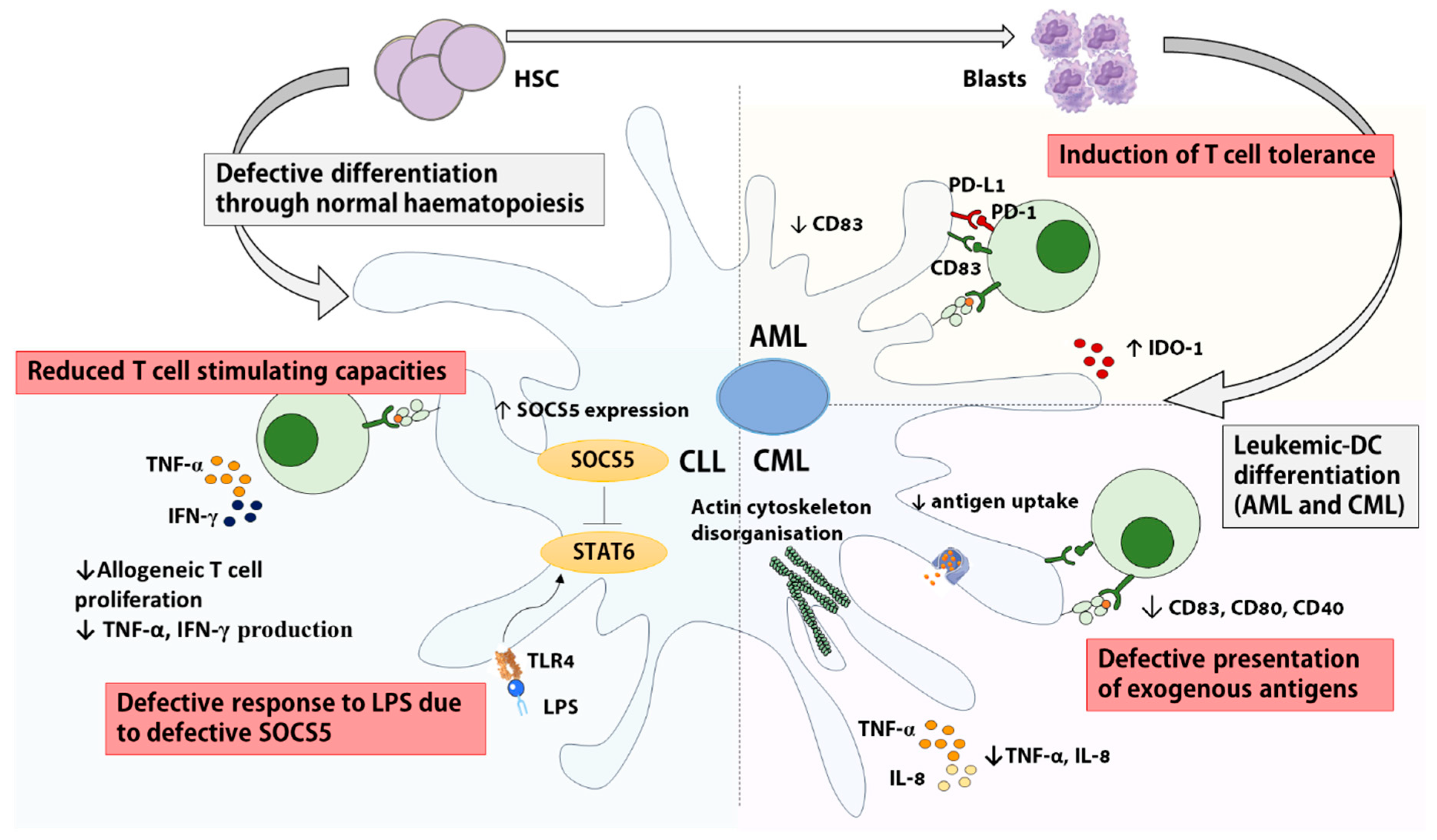
2.1. DCs in Myeloid Leukemias
2.1.1. DCs in AML
2.1.2. DCs in CML
2.2. DCs in Lymphoid Leukemias
2.2.1. DCs in ALL
2.2.2. DCs in CLL
2.3. Concluding Remarks on DCs in Leukemia
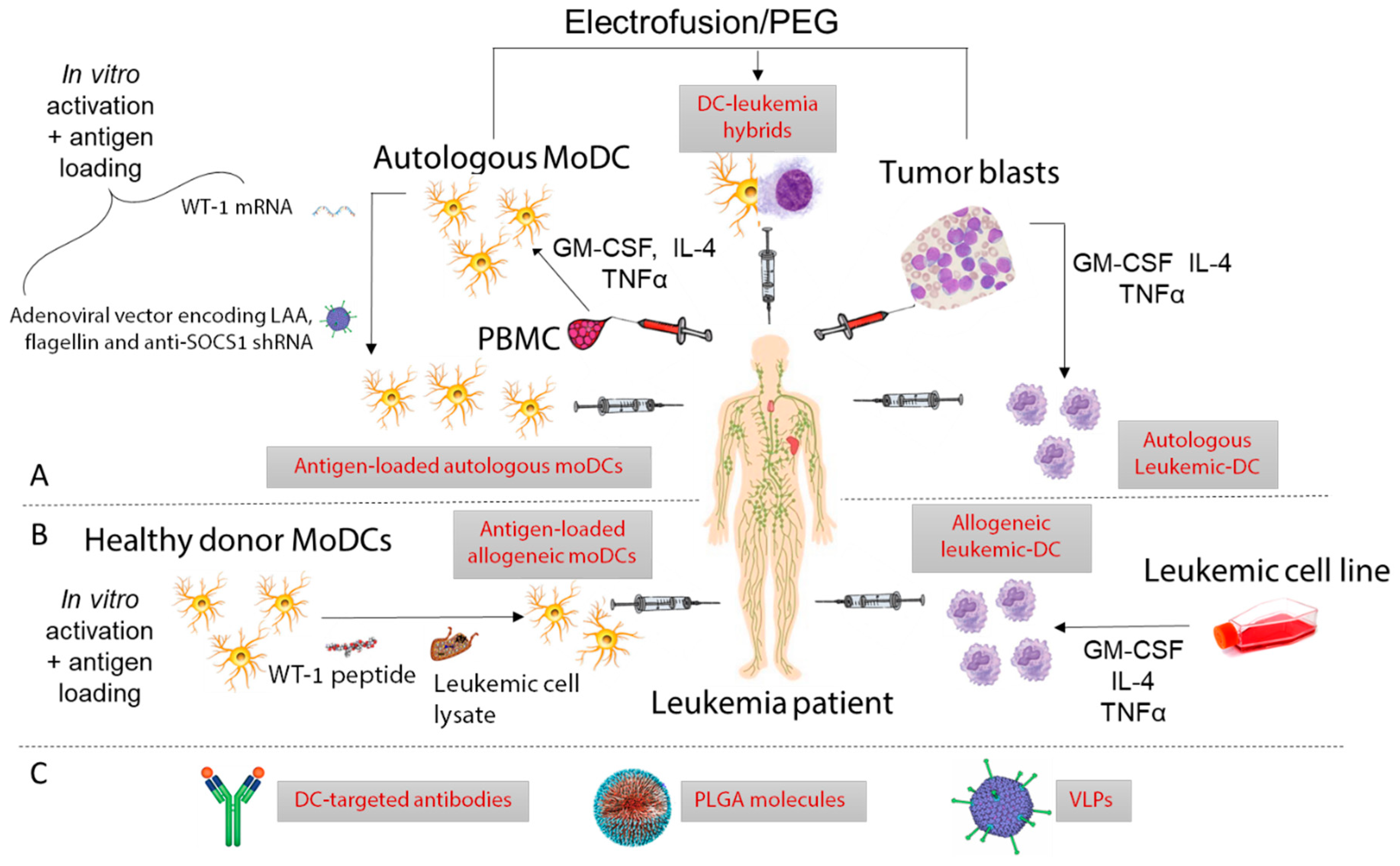
3. DC-Based Immunotherapy in Leukemia
3.1. Ex Vivo Manipulation of DCs for Vaccination
3.1.1. Leukemic-DCs
3.1.2. moDCs
3.1.3. cDCs
3.2. In Vivo Delivery of Antigen and Adjuvants to DCs
Targeting Antibodies
3.3. Considerations for the Design of Future Vaccines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Keeffe, M.; Mok, W.H.; Radford, K.J. Human dendritic cell subsets and function in health and disease. Cell. Mol. Life Sci. 2015, 72, 4309–4325. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudet, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 5, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.-J.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3) + dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef]
- Crozat, K.; Guiton, R.; Contreras, V.; Feuillet, V.; Dutertre, C.-A.; Ventre, E.; Vu Manh, T.-P.; Baranek, T.; Storset, A.K.; Marvel, J.; et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J. Exp. Med. 2010, 207, 1283–1292. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Tang-Huau, T.-L.; Segura, E. Human in vivo-differentiated monocyte-derived dendritic cells. Semin. Cell Dev. Biol. 2019, 86, 44–49. [Google Scholar] [CrossRef]
- Liao, C.-T.; Andrews, R.; Wallace, L.E.; Khan, M.W.A.; Kift-Morgan, A.; Topley, N.; Fraser, D.J.; Taylor, P.R. Peritoneal macrophage heterogeneity is associated with different peritoneal dialysis outcomes. Kidney Int. 2017, 91, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Brinck-Jensen, N.-S.; Zang, M.; Chen, K. Monocyte-derived dendritic cells: Targets as potent antigen-presenting cells for the design of vaccines against infectious diseases. Int. J. Infect. Dis. 2014, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burke, V.P.; Startzell, J.M. The leukemias. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 597–608. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Almaiman, A.A. Proteomic Profile of Lymphoid Leukemia. J. Coll. Physicians Surg. Pak. 2018, 28, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Riether, C.; Schurch, C.M.; Ochsenbein, A.F. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015, 22, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic Cells Create Bone Marrow Niches That Disrupt the Behavior of Normal Hematopoietic Progenitor Cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, C.W.; Shi, J.; Chen, J.; Wang, B.; Yu, Y.H.; Qin, X.; Zhou, X.C.; Cai, Y.J.; Li, Z.Q.; Zhang, F.; et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell 2014, 25, 778–793. [Google Scholar] [CrossRef]
- Mussai, F.; De Santo, C.; Abu-Dayyeh, I.; Booth, S.; Quek, L.; McEwen-Smith, R.M.; Qureshi, A.; Dazzi, F.; Vyas, P.; Cerundolo, V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 2013, 122, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Cutucache, C. Tumor-induced host immunosuppression: Special focus on CLL. Int. Immunopharmacol. 2013, 17, 35–41. [Google Scholar] [CrossRef]
- Isidori, A.; Salvestrini, V.; Ciciarello, M.; Loscocco, F.; Visani, G.; Parisi, S.; Lecciso, M.; Ocadlikova, D.; Rossi, L.; Gabucci, E.; et al. The role of the immunosuppressive microenvironment in acute myeloid leukemia development and treatment. Expert Rev. Hematol. 2014, 7, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, S.; Lu, Y.; Yao, D.; Xu, L.; Zhang, Y.; Yang, L.; Chen, J.; Lai, J.; Yu, Z.; et al. Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chin. J. Cancer Res. = Chung-Kuo Yen Cheng Yen Chiu 2017, 29, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; El-Shanshory, M.R.; Abdou, S.H.; Attia, M.S.; Sobhy, S.M.; Zidan, M.F.; Zidan, A.A. Chemotherapy alters the increased numbers of myeloid-derived suppressor and regulatory T cells in children with acute lymphoblastic leukemia. Immunopharmacol. Immunotoxicol. 2018, 40, 158–167. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Weinberg, J.B.; Yoshizaki, A.; Horikawa, M.; Bryant, J.M.; Iwata, Y.; Matsushita, T.; Matta, K.M.; Chen, Y.; Venturi, G.M.; et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013, 27, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Blobe, G.C. Role of transforming growth factor-beta in hematologic malignancies. Blood 2006, 107, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Folgiero, V.; Goffredo, B.M.; Filippini, P.; Masetti, R.; Bonanno, G.; Caruso, R.; Bertaina, V.; Mastronuzzi, A.; Gaspari, S.; Zecca, M.; et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget 2014, 5, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Cavanagh, L.L.; Bonasio, R.; Mazo, I.B.; Halin, C.; Cheng, G.; van der Velden, A.W.; Cariappa, A.; Chase, C.; Russell, P.; Starnbach, M.N.; et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005, 6, 1029–1037. [Google Scholar] [CrossRef]
- Zirlik, K. MDSCs: The final frontier of the microenvironment in CLL? Blood 2014, 124, 666–668. [Google Scholar] [CrossRef]
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv. Cancer Res. 2015, 128, 95–139. [Google Scholar] [CrossRef]
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Orsini, E.; Calabrese, E.; Maggio, R.; Pasquale, A.; Nanni, M.; Trasarti, S.; Tafuri, A.; Guarini, A.; Foa, R. Circulating myeloid dendritic cell directly isolated from patients with chronic myelogenous leukemia are functional and carry the bcr-abl translocation. Leuk. Res. 2006, 30, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Cwynarski, K.; Entwistle, A.; Marelli-Berg, F.; Dazzi, F.; Simpson, E.; Goldman, J.M.; Melo, J.V.; Lechler, R.I.; Bellantuono, I.; et al. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood 2003, 101, 3560–3567. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Bryant, C.E.; Papadimitrious, M.S.; Kong, B.; Gasiorowski, R.E.; Orellana, D.; McGuire, H.M.; Groth, B.F.S.; Joshua, D.E.; Ho, P.J.; et al. A blood dendritic cell vaccine for acute myeloid leukemia expands anti-tumor T cell responses at remission. Oncoimmunology 2018, 7, e1419114. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Jarrossay, D.; Lafage-Pochitaloff, M.; Zandotti, C.; Briere, F.; de Lamballeri, X.N.; Isnardon, D.; Sainty, D.; Olive, D.; Gaugler, B. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood 2001, 98, 3750–3756. [Google Scholar] [CrossRef] [Green Version]
- Rickmann, M.; Krauter, J.; Stamer, K.; Heuser, M.; Salguero, G.; Mischak-Weissinger, E.; Ganser, A.; Stripecke, R. Elevated frequencies of leukemic myeloid and plasmacytoid dendritic cells in acute myeloid leukemia with the FLT3 internal tandem duplication. Ann. Hematol. 2011, 90, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, D.E.; MacNabb, B.W.; Chen, X.; Chan, W.-C.; Fosco, D.; Kline, J. CD8α+ Dendritic Cells Dictate Leukemia-Specific CD8+ T Cell Fates. J. Immunol. 2018, 201, 3759–3769. [Google Scholar] [CrossRef]
- Meyerson, H.; Osei, E.; Schweitzer, K.; Blidaru, G.; Edinger, A.; Schlegelmilch, J.; Awadallah, A.; Goyal, T. CD1c Myeloid Dendritic Cells in Myeloid Neoplasia. Clin. Cytom. 2016, 90, 337–348. [Google Scholar] [CrossRef]
- Derolf, A.R.; Laane, E.; Bjorklund, E.; Saft, L.; Bjorkholm, M.; Porwit, A. Dendritic cells in bone marrow at diagnosis and after chemotherapy in adult patients with acute myeloid leukaemia. Scand. J. Immunol. 2014, 80, 424–431. [Google Scholar] [CrossRef]
- Lau, C.M.; Nish, S.A.; Yogev, N.; Waisman, A.; Reiner, S.L.; Reizis, B. Leukemia-associated activating mutation of Flt3 expands dendritic cells and alters T cell responses. J. Exp. Med. 2016, 213, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.S.; Richards, S.J.; Master, P.S.; Kendall, J.; Limbert, H.J.; Roberts, B.E. Flow cytometric analysis of membrane CD11b, CD11c and CD14 expression in acute myeloid leukaemia: Relationships with monocytic subtypes and the concept of relative antigen expression. Eur. J. Haematol. 1990, 44, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kremser, A.; Dreyig, J.; Grabrucker, C.; Liepert, A.; Kroell, T.; Scholl, N.; Schmid, C.; Tischer, J.; Kufner, S.; Salih, H.; et al. Dendritic Cells (DCs) Can Be Successfully Generated from Leukemic Blasts in Individual Patients with AML or MDS: An Evaluation of Different Methods. J. Immunother. 2010, 33, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.E.; van der Hoorn, M.; Kluin-Nelemans, H.C.; van Zelderen-Bhola, S.; Willemze, R.; Falkenburg, J.H. The generation of dendritic-like cells with increased allostimulatory function from acute myeloid leukemia cells of various FAB subclasses. Hum. Immunol. 2000, 61, 565–574. [Google Scholar] [CrossRef]
- Narita, M.; Takahashi, M.; Liu, A.; Nikkuni, K.; Furukawa, T.; Toba, K.; Koyama, S.; Takai, K.; Sanada, M.; Aizawa, Y. Leukemia blast-induced T-cell anergy demonstrated by leukemia-derived dendritic cells in acute myelogenous leukemia. Exp. Hematol. 2001, 29, 709–719. [Google Scholar] [CrossRef]
- Harrison, B.D.; Adams, J.A.; Briggs, M.; Brereton, M.L.; Yin, J.A. Stimulation of autologous proliferative and cytotoxic T-cell responses by “leukemic dendritic cells” derived from blast cells in acute myeloid leukemia. Blood 2001, 97, 2764–2771. [Google Scholar] [CrossRef]
- Cignetti, A.; Bryant, E.; Allione, B.; Vitale, A.; Foa, R.; Cheever, M.A. CD34+ Acute Myeloid and Lymphoid Leukemic Blasts Can Be Induced to Differentiate into Dendritic Cells. Blood 1999, 94, 2048–2055. [Google Scholar] [PubMed]
- Roddie, P.H.; Horton, Y.; Turner, M.L. Primary acute myeloid leukaemia blasts resistant to cytokine-induced differentiation to dendritic-like leukaemia cells can be forced to differentiate by the addition of bryostatin-1. Leukemia 2002, 16, 84–93. [Google Scholar] [CrossRef]
- Brady, M.T.; Miller, A.; Sait, S.N.; Ford, L.A.; Minderman, H.; Wang, E.S.; Lee, K.P.; Baumann, H.; Wetzler, M. Down-regulation of signal transducer and activator of transcription 3 improves human acute myeloid leukemia-derived dendritic cell function. Leuk. Res. 2013, 37, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.; Liang, J.C.; Thomas, E.K.; Flores-Romo, L.; Xie, Q.S.; Agusala, K.; Sutaria, S.; Sinha, I.; Champlin, R.E.; Claxton, D.F. Dendritic Cells Derived In Vitro From Acute Myelogenous Leukemia Cells Stimulate Autologous, Antileukemic T-Cell Responses. Blood 1999, 93, 780–786. [Google Scholar]
- Charbonnier, A.; Gaugler, B.; Sainty, D.; Lafage-Pochitaloff, M.; Olive, D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur. J. Immunol. 1999, 29, 2567–2578. [Google Scholar] [CrossRef]
- Ge, W.; Ma, X.; Li, X.; Wang, Y.; Li, C.; Meng, H.; Liu, X.; Yu, Z.; You, S.; Qiu, L. B7-H1 up-regulation on dendritic-like leukemia cells suppresses T cell immune function through modulation of IL-10/IL-12 production and generation of Treg cells. Leuk. Res. 2009, 33, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Curti, A.; Trabanelli, S.; Onofri, C.; Aluigi, M.; Salvestrini, V.; Ocadlikova, D.; Evangelisti, C.; Rutella, S.; De Cristofaro, R.; Ottaviani, E.; et al. Indoleamine 2,3-dioxygenase-expressing leukemic dendritic cells impair a leukemia-specific immune response by inducing potent T regulatory cells. Haematologica 2010, 95, 2022–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spisek, R.; Chevallier, P.; Morineau, N.; Milpied, N.; Avet-Loiseau, H.; Harousseau, J.-L.; Meflah, K.; Gregoire, M. Induction of Leukemia-specific Cytotoxic Response by Cross-Presentation of Late-Apoptotic Leukemic Blasts by Autologous Dendritic Cells of Nonleukemic Origin. Cancer Res. 2002, 62, 2861–2868. [Google Scholar] [PubMed]
- Boissel, N.; Rousselot, P.; Raffoux, E.; Cayuela, J.M.; Maarek, O.; Charron, D.; Degos, L.; Dombret, H.; Toubert, A.; Rea, D. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia 2004, 18, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Isnardon, D.; Vey, N.; Briere, F.; Blaise, D.; Olive, D.; Gaugler, B. Low blood dendritic cells in chronic myeloid leukaemia patients correlates with loss of CD34+/CD38− primitive haematopoietic progenitors. Br. J. Haematol. 2002, 119, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, K.; Lang, A.; Eibl, B.; Nachbaur, D.; Glassl, H.; Fiegl, M.; Thaler, J.; Gastl, G. Phenotypic and functional deficiencies of leukaemic dendritic cells from patients with chronic myeloid leukaemia. Br. J. Haematol. 2003, 120, 63–73. [Google Scholar] [CrossRef]
- Chen, X.; Regn, S.; Raffegerst, S.; Kolb, H.J.; Roskrow, M. Interferon alpha in combination with GM-CSF induces the differentiation of leukaemic antigen-presenting cells that have the capacity to stimulate a specific anti-leukaemic cytotoxic T-cell response from patients with chronic myeloid leukaemia. Br. J. Haematol. 2000, 111, 596–607. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef]
- Zhang, S.; Kipps, T.J. The Pathogenesis of Chronic Lymphocytic Leukemia. Annu. Rev. Pathol. 2014, 9, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Laane, E.; Bjorklund, E.; Mazur, J.; Lonnerholm, G.; Soderhall, S.; Porwit, A. Dendritic cell regeneration in the bone marrow of children treated for acute lymphoblastic leukaemia. Scand. Immunol. 2007, 66, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Mami, N.B.; Mohty, M.; Chambost, H.; Gaugler, B.; Olive, D. Blood dendritic cells in patients with acute lymphoblastic leukaemia. Br. J. Haematol. 2004, 126, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Maecker, B.; Mougiakakos, D.; Zimmermann, M.; Behrens, M.; Hollander, S.; Schrauder, A.; Schrappe, M.; Welte, K.; Klein, C. Dendritic cell deficiencies in pediatric acute lymphoblastic leukemia patients. Leukemia 2006, 20, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, N.; Tamura, Y.; Sahara, H.; Suzuki, N.; Suzuki, K.; Hori, T.; Mizue, N.; Torigoe, T.; Tsutsumi, H.; Sato, N. Induction of autologous CD4- and CD8-mediated T-cell responses against acute lymphocytic leukemia cell line using apoptotic tumor cell loaded dendritic cells. Exp. Hematol. 2006, 34, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, C.J.; Kim, M.J.; Jang, S.; Chi, H.S.; Lee, J.H.; Lee, J.H.; Lee, K.H.; Im, H.J.; Seo, J.J. Generation of lymphocytes potentiated against leukemic lymphoblasts by stimulation using leukemic cell lysate-pulsed dendritic cells in patients with acute lymphoblastic leukemia and measurement of in vitro anti-leukemic cytotoxicity. Hematology 2012, 17, 15–22. [Google Scholar] [CrossRef]
- Mami, N.B.; Mohty, M.; Aurran-Schleinitz, T.; Olive, D.; Gaugler, B. Blood dendritic cells in patients with chronic lymphocytic leukaemia. Immunobiology 2008, 213, 493–498. [Google Scholar] [CrossRef]
- Orsini, E.; Guarini, A.; Chiaretti, S.; Mauro, F.R.; Foa, R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003, 63, 4497–4506. [Google Scholar]
- Vuillier, F.; Maloum, K.; Thomas, E.K.; Jouanne, C.; Dighiero, G.; Scott-Algara, D. Functional monocyte-derived dendritic cells can be generated in chronic lymphocytic leukaemia. Br. J. Haematol. 2001, 115, 831–844. [Google Scholar] [CrossRef]
- Rezvany, M.R.; Jeddi-Tehrani, M.; Biberfeld, P.; Söderlund, J.; Mellstedt, H.; Österborg, A.; Rabbani, H. Dendritic cells in patients with non-progressive B-chronic lymphocytic leukaemia have a normal functional capability but abnormal cytokine pattern. Br. J. Haematol. 2001, 115, 263–271. [Google Scholar] [CrossRef]
- Messmer, D.; Telusma, G.; Wasil, T.; Messmer, B.T.; Allen, S.; Rai, K.R.; Chiorazzi, N. Dendritic Cells from Chronic Lymphocytic Leukemia Patients Are Normal Regardless of Ig V Gene Mutation Status. Mol. Med. 2004, 10, 96–103. [Google Scholar] [CrossRef]
- Goddard, R.V.; Prentice, A.G.; Copplestone, J.A.; Kaminski, E.R. Generation in vitro of B-cell chronic lymphocytic leukaemia-proliferative and specific HLA class-II-restricted cytotoxic T-cell responses using autologous dendritic cells pulsed with tumour cell lysate. Clin. Exp. Immunol. 2001, 126, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, P.A.; Liu, S.; Yeh, J.E.; Ye, D.Q.; Barbuto, J.A.; Frank, D.A. Deregulation of SOCS5 suppresses dendritic cell function in chronic lymphocytic leukemia. Oncotarget 2016, 7, 46301–46314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsini, E.; Pasquale, A.; Maggio, R.; Calabrese, E.; Mauro, F.R.; Giammartini, E.; Guarini, A.; Foa, R. Phenotypic and functional characterization of monocyte-derived dendritic cells in chronic lymphocytic leukaemia patients: Influence of neoplastic CD19+ cells in vivo and in vitro. Br. J. Haematol. 2004, 125, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Liu, J.; Sun, Z.; Huang, S. Acute myeloid leukemia cells inhibit the differentiation and maturation of dendritic cells and induce the generation of regulatory T cells. Chin. Ger. J. Clin. Oncol. 2008, 7, 164–169. [Google Scholar] [CrossRef]
- Mumprecht, S.; Claus, C.; Schürch, C.; Pavelic, V.; Matter, M.S.; Ochsenbein, A.F. Defective homing and impaired induction of cytotoxic T cells by BCR/ABL-expressing dendritic cells. Blood 2009, 113, 4681–4689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.D.; Choi, S.-C.; Noh, Y.-W.; Kim, J.W.; Paik, S.-G.; Yang, Y.; Kim, K.I.; Lim, J.-S. Impaired responses of leukemic dendritic cells derived from a human myeloid cell line to LPS stimulation. Exp. Mol. Med. 2006, 38, 72–84. [Google Scholar] [CrossRef]
- Ni, M.; Hoffmann, J.M.; Schmitt, M.; Schmitt, A. Progress of dendritic cell-based cancer vaccines for patients with hematological malignancies. Expert Opin. Biol. Ther. 2016, 16, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef]
- Nestle, F.O.; Alijagic, S.; Gilliet, M.; Sun, Y.; Grabbe, S.; Dummer, R.; Burg, G.; Schadendorf, D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998, 4, 328–332. [Google Scholar] [CrossRef]
- Rowley, D.A.; Fitch, F.W. The road to the discovery of dendritic cells, a tribute to Ralph Steinman. Cell. Immunol. 2012, 273, 95–98. [Google Scholar] [CrossRef]
- Chen, W.; Peace, D.J.; Rovira, D.K.; You, S.G.; Cheever, M.A. T-cell immunity to the joining region of p210BCR-ABL protein. Proc. Natl. Acad. Sci. USA 1992, 89, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Gajewski, J.L.; Liang, J.C.; Popat, U.; Claxton, D.F.; Kliche, K.-O.; Andreeff, M.; Champlin, R.E. Use of Leukemic Dendritic Cells for the Generation of Antileukemic Cellular Cytotoxicity Against Philadelphia Chromosome-Positive Chronic Myelogenous Leukemia. Blood 1997, 89, 1133–1142. [Google Scholar] [PubMed]
- Westers, T.M.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Dendritic cell-based immunotherapy in acute and chronic myeloid leukaemia. Biomed. Pharmacother. 2007, 61, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Schurch, C.M.; Riether, C.; Ochsenbein, A.F. Dendritic cell-based immunotherapy for myeloid leukemias. Front. Immunol. 2013, 4, 496. [Google Scholar] [CrossRef]
- Anguille, S.; Willemen, Y.; Lion, E.; Smits, E.L.; Berneman, Z.N. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy 2012, 14, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Shimizu, K.; Fujimoto, K.; Kiyokawa, T.; Shimomura, T.; Kinoshita, M.; Kawano, F. Analysis of a chronic myelogenous leukemia patient vaccinated with leukemic dendritic cells following autologous peripheral blood stem cell transplantation. Jpn. J. Cancer Res. Gann 1999, 90, 1117–1129. [Google Scholar] [CrossRef]
- Ossenkoppele, G.J.; Stam, A.G.; Westers, T.M.; de Gruijl, T.D.; Janssen, J.J.; van de Loosdrecht, A.A.; Scheper, R.J. Vaccination of chronic myeloid leukemia patients with autologous in vitro cultured leukemic dendritic cells. Leukemia 2003, 17, 1424–1426. [Google Scholar] [CrossRef]
- Westermann, J.; Kopp, J.; Van Lessen, A.; Hecker, A.-C.; Baskaynak, G.; Le Coutre, P.; Döhner, K.; Döhner, H.; Dörken, B.; Pezzutto, A. Vaccination with autologous non-irradiated dendritic cells in patients with bcr/abl+ chronic myeloid leukaemia. Br. J. Haematol. 2007, 137, 297–306. [Google Scholar] [CrossRef]
- Litzow, M.R.; Dietz, A.B.; Bulur, P.A.; Butler, G.W.; Gastineau, D.A.; Hoering, A.; Fink, S.R.; Letendre, L.; Padley, D.J.; Paternoster, S.F.; et al. Testing the safety of clinical-grade mature autologous myeloid DC in a phase I clinical immunotherapy trial of CML. Cytotherapy 2006, 8, 290–298. [Google Scholar] [CrossRef]
- Li, L.; Giannopoulos, K.; Reinhardt, P.; Tabarkiewicz, J.; Schmitt, A.; Greiner, J.; Rolinski, J.; Hus, I.; Dmoszynska, A.; Wiesneth, M.; et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int. J. Oncol. 2006, 28, 855–861. [Google Scholar] [CrossRef]
- Roddie, H.; Klammer, M.; Thomas, C.; Thomson, R.; Atkinson, A.; Sproul, A.; Waterfall, M.; Samuel, K.; Yin, J.; Johnson, P.; et al. Phase I/II study of vaccination with dendritic-like leukaemia cells for the immunotherapy of acute myeloid leukaemia. Br. J. Haematol. 2006, 133, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Morgan, D.; Pamphilon, D. A rapid culture technique produces functional dendritic-like cells from human acute myeloid leukemia cell lines. J. Biomed. Biotechnol. 2011, 2011, 172965. [Google Scholar] [CrossRef] [PubMed]
- van Loosdrecht, A.; van Wetering, S.; Santegoets, S.J.; Singh, S.K.; Eeltink, C.M.; den Hartog, Y.; Koppes, M.; Kaspers, J.; Ossenkoppele, G.J.; Kruisbeek, A.M.; et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol. Immunother. 2018, 67, 1505–1518. [Google Scholar] [Green Version]
- Draube, A.; Beyer, M.; Wolf, J. Activation of autologous leukemia-specific T cells in acute myeloid leukemia: Monocyte-derived dendritic cells cocultured with leukemic blasts compared with leukemia-derived dendritic cells. Eur. J. Haematol. 2008, 81, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hus, I.; Rolinski, J.; Tabarkiewicz, J.; Wojas, K.; Bojarska-Junak, A.; Greiner, J.; Giannopoulos, K.; Dmoszynska, A.; Schmitt, M. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Hus, I.; Schmitt, M.; Tabarkiewicz, J.; Radej, S.; Wojas, K.; Bojarska-Junak, A.; Schmitt, A.; Giannopoulos, K.; Dmoszynska, A.; Rolinski, J. Vaccination of B-CLL patients with autologous dendritic cells can change the frequency of leukemia antigen-specific CD8+ T cells as well as CD4+CD25+FoxP3+ regulatory T cells toward an antileukemia response. Leukemia 2008, 22, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Bhardwaj, N. Turbocharging vaccines: Emerging adjuvants for dendritic cell based therapeutic cancer vaccines. Curr. Opin. Immunol. 2017, 47, 35–43. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.M.; Figdor, C.G. Dendritic Cell–Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef]
- Decker, W.K.; Xing, D.; Li, S.; Robinson, S.N.; Yang, H.; Yao, X.; Segall, H.; McMannis, J.D.; Komanduri, K.V.; Champlin, R.E.; et al. Double loading of dendritic cell MHC class I and MHC class II with an AML antigen repertoire enhances correlates of T-cell immunity in vitro via amplification of T-cell help. Vaccine 2006, 24, 3203–3216. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8, 368ra171. [Google Scholar] [CrossRef]
- Galea-Lauri, J.; Darling, D.; Mufti, G.; Harrison, P.; Farzaneh, F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: Evaluation of dendritic cell–leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol. Immunother. 2002, 51, 299–310. [Google Scholar] [PubMed]
- Kokhaei, P.; Rezvany, M.R.; Virving, L.; Choudhury, A.; Rabbani, H.; Österborg, A.; Mellstedt, H. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell–tumour hybrids in B-CLL. Leukemia 2003, 17, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Subklewe, M.; Geiger, C.; Lichtenegger, F.S.; Javorovic, M.; Kvalheim, G.; Schendel, D.J.; Bigalke, I. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol. Immunother. 2014, 63, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Huang, X.F.; Hong, B.; Song, X.T.; Hu, L.; Jiang, M.; Zhang, B.; Ning, H.; Li, Y.; Xu, C.; et al. Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Palma, M.; Hansson, L.; Mulder, T.A.; Adamson, L.; Nasman-Glaser, B.; Eriksson, I.; Heimersson, K.; Ryblom, H.; Mozaffari, F.; Svensson, A.; et al. Lenalidomide as immune adjuvant to a dendritic cell vaccine in chronic lymphocytic leukemia patients. Eur. J. Haematol. 2018, 101, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Delluc, S.; Tourneur, L.; Michallet, A.; Boix, C.; Varet, B.; Fradelizi, D.; Guillet, J.; Buzyn, A. Autologous peptides eluted from acute myeloid leukemia cells can be used to generate specific antileukemic CD4 helper and CD8 cytotoxic T lymphocyte responses in vitro. Haematologica 2005, 90, 1050–1062. [Google Scholar]
- Shah, N.N.; Loeb, D.M.; Khuu, H.; Stroncek, D.; Ariyo, T.; Raffeld, M.; Delbrook, C.; Mackall, C.L.; Wayne, A.S.; Fry, T.J. Induction of Immune Response after Allogeneic Wilms’ Tumor 1 Dendritic Cell Vaccination and Donor Lymphocyte Infusion in Patients with Hematologic Malignancies and Post-Transplantation Relapse. Biol. Blood Marrow Transplant. 2016, 22, 2149–2154. [Google Scholar] [CrossRef]
- Longo, D.L. Imatinib Changed Everything. N. Engl. J. Med. 2017, 376, 982–983. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Palma, M.; Hansson, L.; Choudhury, A.; Näsman-Glaser, B.; Eriksson, I.; Adamson, L.; Rossmann, E.; Widén, K.; Horváth, R.; Kokhaei, P.; et al. Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: Effects of various adjuvants and definition of immune response criteria. Cancer Immunol. Immunother. 2012, 61, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Houtenbos, I.; Westers, T.M.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Feasibility of clinical dendritic cell vaccination in acute myeloid leukemia. Immunobiology 2006, 211, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Güttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+/CD141+; cells as homologues of mouse CD8a+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Tullett, K.M.; Lee, Y.S.; Idris, A.; Ding, Y.; McDonald, K.J.; Kassianos, A.; Leal Rojas, I.M.; Jeet, V.; Lahoud, M.H.; et al. Differential uptake and cross-presentation of soluble and necrotic cell antigen by human DC subsets. Eur. J. Immunol. 2016, 46, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, 257–267. [Google Scholar] [CrossRef]
- Schreibelt, G.; Bol, K.F.; Westdorp, H.; Wimmers, F.; Aarntzen, E.H.J.G.; Duiveman-de Boer, T.; van de Rakt, M.W.M.M.; Scharenborg, N.M.; de Boer, A.J.; Pots, J.M.; et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Prue, R.L.; Vari, F.; Radford, K.J.; Tong, H.; Hardy, M.Y.; D’Rozario, R.; Waterhouse, N.J.; Rossetti, T.; Coleman, R.; Tracey, C.; et al. A phase I clinical trial of CD1c (BDCA-1)+ dendritic cells pulsed with HLA-A*0201 peptides for immunotherapy of metastatic hormone refractory prostate cancer. J. Immunother. 2015, 38, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Fromm, P.D.; Papadimitrious, M.S.; Hsu, J.L.; Van Kooten Losio, N.; Verma, N.D.; Lo, T.H.; Silveira, P.A.; Bryant, C.E.; Turtle, C.J.; Prue, R.L.; et al. CMRF-56(+) blood dendritic cells loaded with mRNA induce effective antigen-specific cytotoxic T-lymphocyte responses. Oncoimmunology 2016, 5. [Google Scholar] [CrossRef]
- Freeman, J.L.; Vari, F.; Hart, D.N. CMRF-56 immunoselected blood dendritic cell preparations activated with GM-CSF induce potent antimyeloma cytotoxic T-cell responses. J. Immunother. 2007, 30, 740–748. [Google Scholar] [CrossRef]
- Balan, S.; Arnold-Schrauf, C.; Abbas, A.; Couespel, N.; Savoret, J.; Imperatore, F.; Villani, A.-C.; Vu Manh, T.-P.; Bhardwaj, N.; Dalod, M. Large-Scale Human Dendritic Cell Differentiation Revealing Notch-Dependent Lineage Bifurcation and Heterogeneity. Cell Rep. 2018, 24, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Kirkling, M.E.; Cytlak, U.; Lau, C.M.; Lewis, K.L.; Resteu, A.; Khodadadi-Jamayran, A.; Siebel, C.W.; Salmon, H.; Merad, M.; Tsirigos, A.; et al. Notch Signaling Facilitates Generation of Cross-Presenting Classical Dendritic Cells. Cell Rep. 2018, 23, 3658–3672. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Balan, S.; Roudko, V.; Bhardwaj, N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018, 2, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Elze, M.C.; Ciocarlie, O.; Heinze, A.; Kloess, S.; Gardlowski, T.; Esser, R.; Klingebiel, T.; Bader, P.; Huenecke, S.; Serban, M.; et al. Dendritic cell reconstitution is associated with relapse-free survival and acute GVHD severity in children after allogeneic stem cell transplantation. Bone Marrow Transplant. 2014, 50, 266. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Gartlan, K.H.; Kuns, R.D.; Lane, S.W.; Hill, G.R. Conventional dendritic cells are required for the cross-presentation of leukemia-specific antigen in a model of AML relapse post-BMT. Bone Marrow Transplant. 2018, 53, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Torensma, R.; Figdor, C.G. Targeting antigens to dendritic cells in vivo. Immunobiology 2006, 211, 599–608. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Bonifaz, L.C.; Bonnyay, D.P.; Charalambous, A.; Darguste, D.I.; Fujii, S.-I.; Soares, H.; Brimnes, M.K.; Moltedo, B.; Moran, T.M.; Steinman, R.M. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J. Exp. Med. 2004, 199, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638. [Google Scholar] [CrossRef]
- Tran, T.H.; Tran, T.T.P.; Nguyen, H.T.; Phung, C.D.; Jeong, J.-H.; Stenzel, M.H.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Kim, J.O. Nanoparticles for dendritic cell-based immunotherapy. Int. J. Pharm. 2018, 542, 253–265. [Google Scholar] [CrossRef]
- Lahoud, M.H.; Ahmet, F.; Kitsoulis, S.; Wan, S.S.; Vremec, D.; Lee, C.-N.; Phipson, B.; Shi, W.; Smyth, G.K.; Lew, A.M.; et al. Targeting Antigen to Mouse Dendritic Cells via Clec9A Induces Potent CD4 T Cell Responses Biased toward a Follicular Helper Phenotype. J. Immunol. 2011, 187, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Liu, X.; Sun, Y.; Zhou, P.; Wang, Y.; Zhang, Y. Dendritic cell targeted vaccines: Recent progresses and challenges. Hum. Vaccines Immunother. 2015, 12, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhodapkar, M.V.; Sznol, M.; Zhao, B.; Wang, D.; Carvajal, R.D.; Keohan, M.L.; Chuang, E.; Sanborn, R.E.; Lutzky, J.; Powderly, J.; et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014, 6, 232ra251. [Google Scholar] [CrossRef] [PubMed]
- Tullett, K.M.; Leal Rojas, I.M.; Minoda, Y.; Tan, P.S.; Zhang, J.-G.; Smith, C.; Khanna, R.; Shortman, K.; Caminschi, I.; Lahoud, M.H.; et al. Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition. JCI Insight 2016, 1, e87102. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Pots, J.M.; Torensma, R.; Buschow, S.I.; Figdor, C.G. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials 2012, 33, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Hartung, E.; Becker, M.; Bachem, A.; Reeg, N.; Jäkel, A.; Hutloff, A.; Weber, H.; Weise, C.; Giesecke, C.; Henn, V.; et al. Induction of Potent CD8 T Cell Cytotoxicity by Specific Targeting of Antigen to Cross-Presenting Dendritic Cells In Vivo via Murine or Human XCR1. J. Immunol. 2015, 194, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Srivastava, P.; Matsuzaki, J.; Brumberger, Z.; Wang, E.S.; Kocent, J.; Miller, A.; Roloff, G.W.; Wong, H.Y.; Paluch, B.E.; et al. NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin. Cancer Res. 2017, 24, 1019–1029. [Google Scholar] [CrossRef]
- Kato, M.; McDonald, K.J.; Khan, S.; Ross, I.L.; Vuckovic, S.; Chen, K.; Munster, D.; MacDonald, K.P.; Hart, D.N. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int. Immunol. 2006, 18, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Caminschi, I.; Proietto, A.I.; Ahmet, F.; Kitsoulis, S.; Shin Teh, J.; Lo, J.C.Y.; Rizzitelli, A.; Wu, L.; Vremec, D.; van Dommelen, S.L.H.; et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008, 112, 3264–3273. [Google Scholar] [CrossRef]
- Sancho, D.; Joffre, O.P.; Keller, A.M.; Rogers, N.C.; Martínez, D.; Hernanz-Falcón, P.; Rosewell, I.; Sousa, C.R.e. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009, 458, 899–903. [Google Scholar] [CrossRef]
- Tullett, K.M.; Lahoud, M.H.; Radford, K.J. Harnessing Human Cross-Presenting CLEC9A(+)XCR1(+) Dendritic Cells for Immunotherapy. Front. Immunol. 2014, 5, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, S.; Duan, J.; Hu, Y.; Gu, N.; Xu, H.; Yang, X.-D. Enhancement of DC-mediated anti-leukemic immunity in vitro by WT1 antigen and CpG co-encapsulated in PLGA microparticles. Protein Cell 2013, 4, 887–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyzer, A.R.; Avigan, D.E.; Rosenblatt, J. Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum. Vaccines Immunother. 2014, 10, 3125–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fucikova, J.; Kralikova, P.; Fialova, A.; Brtnicky, T.; Rob, L.; Bartunkova, J.; Špíšek, R. Human Tumor Cells Killed by Anthracyclines Induce a Tumor-Specific Immune Response. Cancer Res. 2011, 71, 4821–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panaretakis, T.; Joza, N.; Modjtahedi, N.; Tesniere, A.; Vitale, I.; Durchschlag, M.; Fimia, G.M.; Kepp, O.; Piacentini, M.; Froehlich, K.U.; et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008, 15, 1499–1509. [Google Scholar] [CrossRef] [Green Version]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2018, 93, 442–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, K.R.; Jain, P. Chronic lymphocytic leukemia (CLL)-Then and now. Am. J. Hematol. 2016, 91, 330–340. [Google Scholar] [CrossRef]
- Pulte, D.; Redaniel, M.T.; Jansen, L.; Brenner, H.; Jeffreys, M. Recent trends in survival of adult patients with acute leukemia: Overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica 2013, 98, 222–229. [Google Scholar] [CrossRef]
- Brenner, H.; Gondos, A.; Pulte, D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood 2008, 111, 4916–4921. [Google Scholar] [CrossRef] [Green Version]
- Van Willigen, W.W.; Bloemendal, M.; Gerritsen, W.R.; Schreibelt, G.; de Vries, I.J.M.; Bol, K.F. Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time. Front. Immunol. 2018, 9, 2265. [Google Scholar] [CrossRef]
- Filley, A.C.; Henriquez, M.; Dey, M. CART Immunotherapy: Development, Success, and Translation to Malignant Gliomas and Other Solid Tumors. Front. Oncol. 2018, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Akahori, Y.; Wang, L.; Yoneyama, M.; Seo, N.; Okumura, S.; Miyahara, Y.; Amaishi, Y.; Okamoto, S.; Mineno, J.; Ikeda, H.; et al. Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination. Blood 2018, 132, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Grady, D. F.D.A. Approves First Gene-Altering Leukemia Treatment, Costing $475,000; New York Times: New York, NY, USA, 30 August 2017. [Google Scholar]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2018, 9, 3176. [Google Scholar] [CrossRef]
- Zhong, R.K.; Loken, M.; Lane, T.A.; Ball, E.D. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy 2006, 8, 3–12. [Google Scholar] [CrossRef]
- Pen, J.J.; Keersmaecker, B.D.; Heirman, C.; Corthals, J.; Liechtenstein, T.; Escors, D.; Thielemans, K.; Breckpot, K. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther. 2014, 21, 262–271. [Google Scholar] [CrossRef]
- Hobo, W.; Maas, F.; Adisty, N.; de Witte, T.; Schaap, N.; van der Voort, R.; Dolstra, H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood 2010, 116, 4501–4511. [Google Scholar] [CrossRef]
- Kourie, H.R.; Klastersky, J. Immune checkpoint inhibitors side effects and management. Immunotherapy 2016, 8, 799–807. [Google Scholar] [CrossRef]
- Garg, A.D.; Coulie, P.G.; Van den Eynde, B.J.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Brusa, D.; Serra, S.; Coscia, M.; Rossi, D.; D’Arena, G.; Laurenti, L.; Jaksic, O.; Fedele, G.; Inghirami, G.; Gaidano, G.; et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013, 98, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, J.; Liu, Q.; Li, J.; Xi, Y. Expression and significance of CD47, PD1 and PDL1 in T-cell acute lymphoblastic lymphoma/leukemia. Pathol. Res. Pract. 2019, 215, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kronig, H.; Kremmler, L.; Haller, B.; Englert, C.; Peschel, C.; Andreesen, R.; Blank, C.U. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur. J. Haematol. 2014, 92, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Christiansson, L.; Soderlund, S.; Svensson, E.; Mustjoki, S.; Bengtsson, M.; Simonsson, B.; Olsson-Stromberg, U.; Loskog, A.S. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE 2013, 8, e55818. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, L.J.; Guillerey, C.; Radford, K.J. Can Dendritic Cell Vaccination Prevent Leukemia Relapse? Cancers 2019, 11, 875. https://doi.org/10.3390/cancers11060875
O’Brien LJ, Guillerey C, Radford KJ. Can Dendritic Cell Vaccination Prevent Leukemia Relapse? Cancers. 2019; 11(6):875. https://doi.org/10.3390/cancers11060875
Chicago/Turabian StyleO’Brien, Liam J., Camille Guillerey, and Kristen J. Radford. 2019. "Can Dendritic Cell Vaccination Prevent Leukemia Relapse?" Cancers 11, no. 6: 875. https://doi.org/10.3390/cancers11060875




