Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion
Abstract
:1. Introduction
2. Results
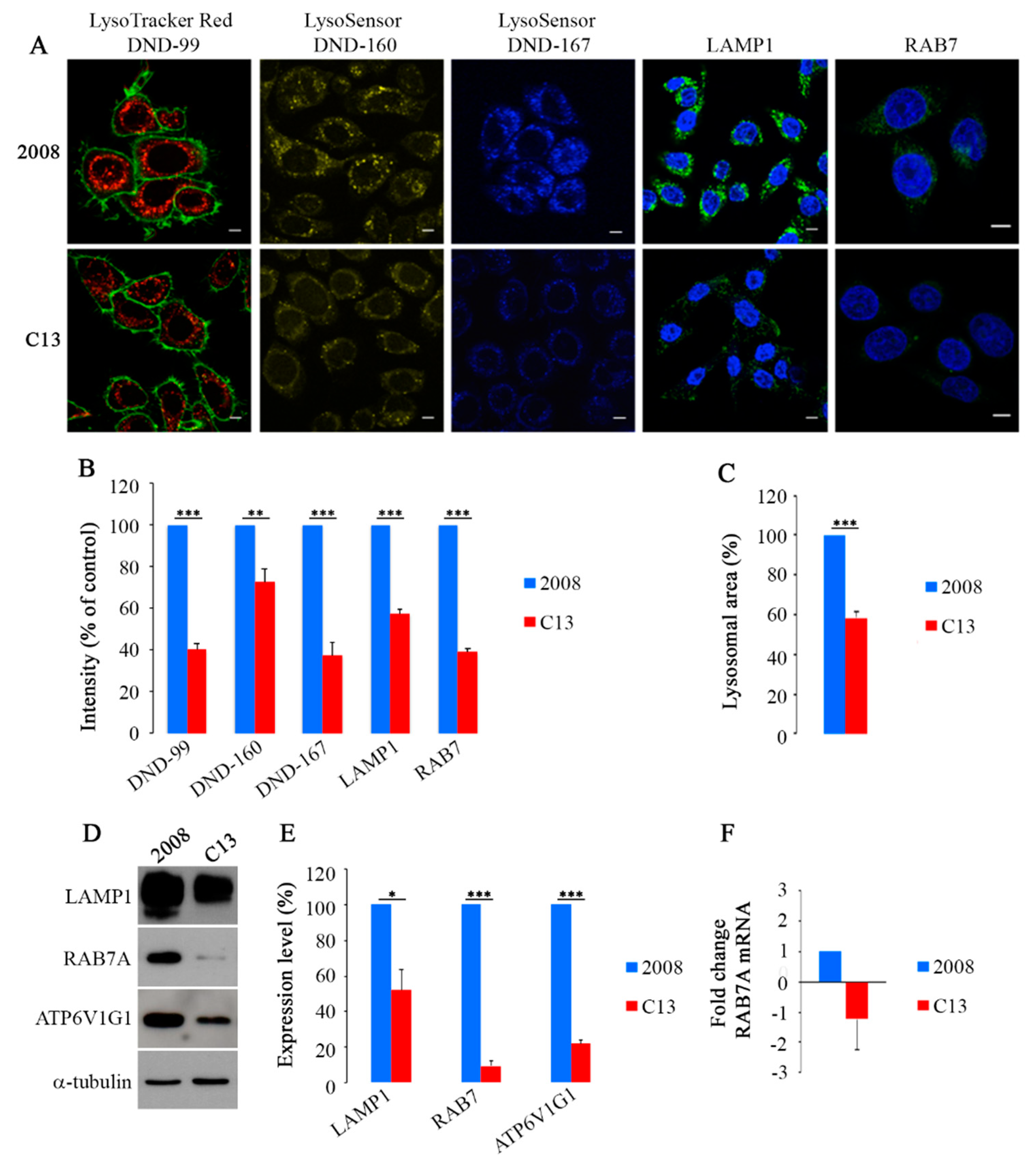
2.1. CDDP-Resistant Cells Display Alterations of Late Endocytic Pathway
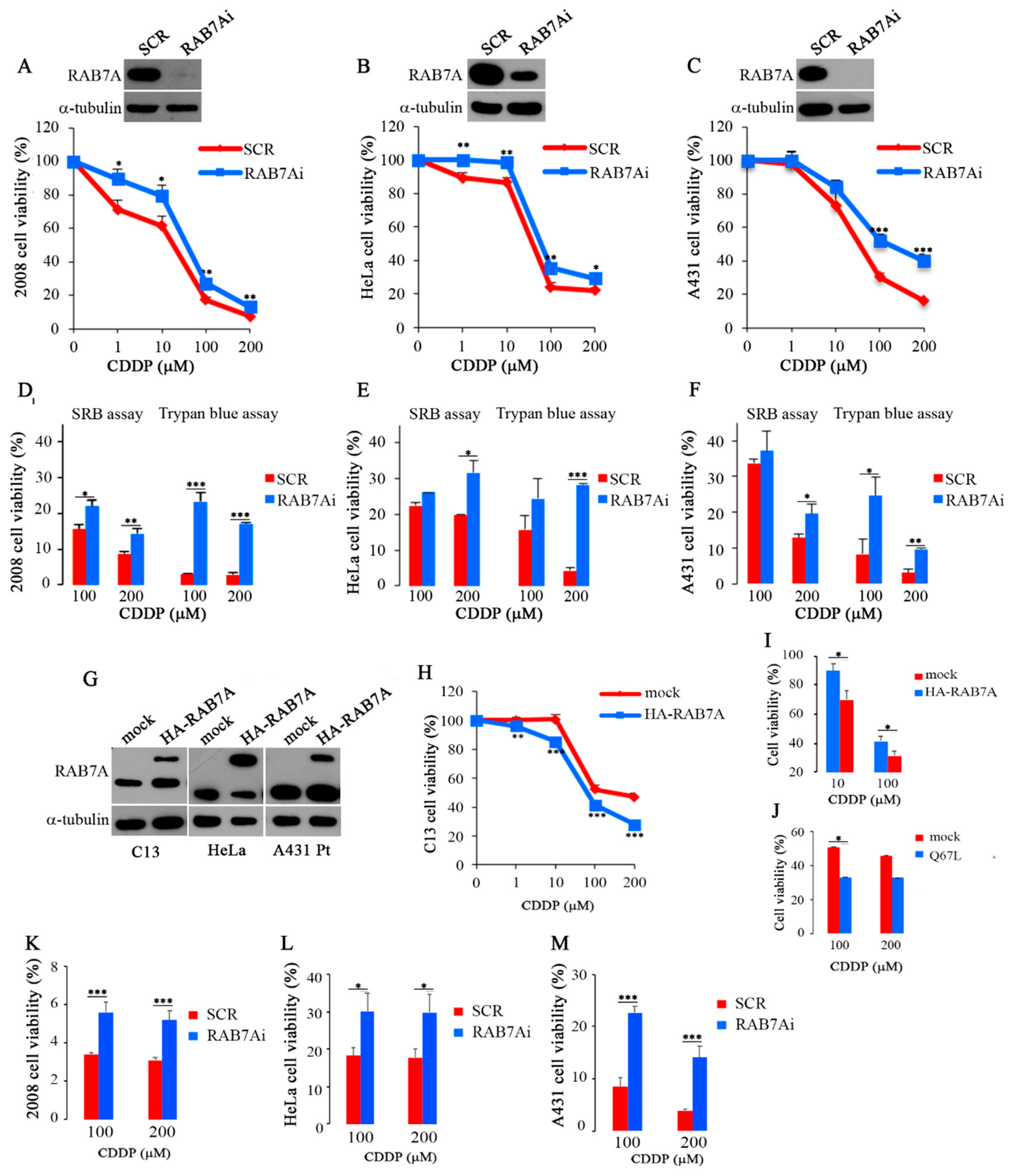
2.2. Modulation of RAB7A Abundance Influences the Chemoresistant Phenotype
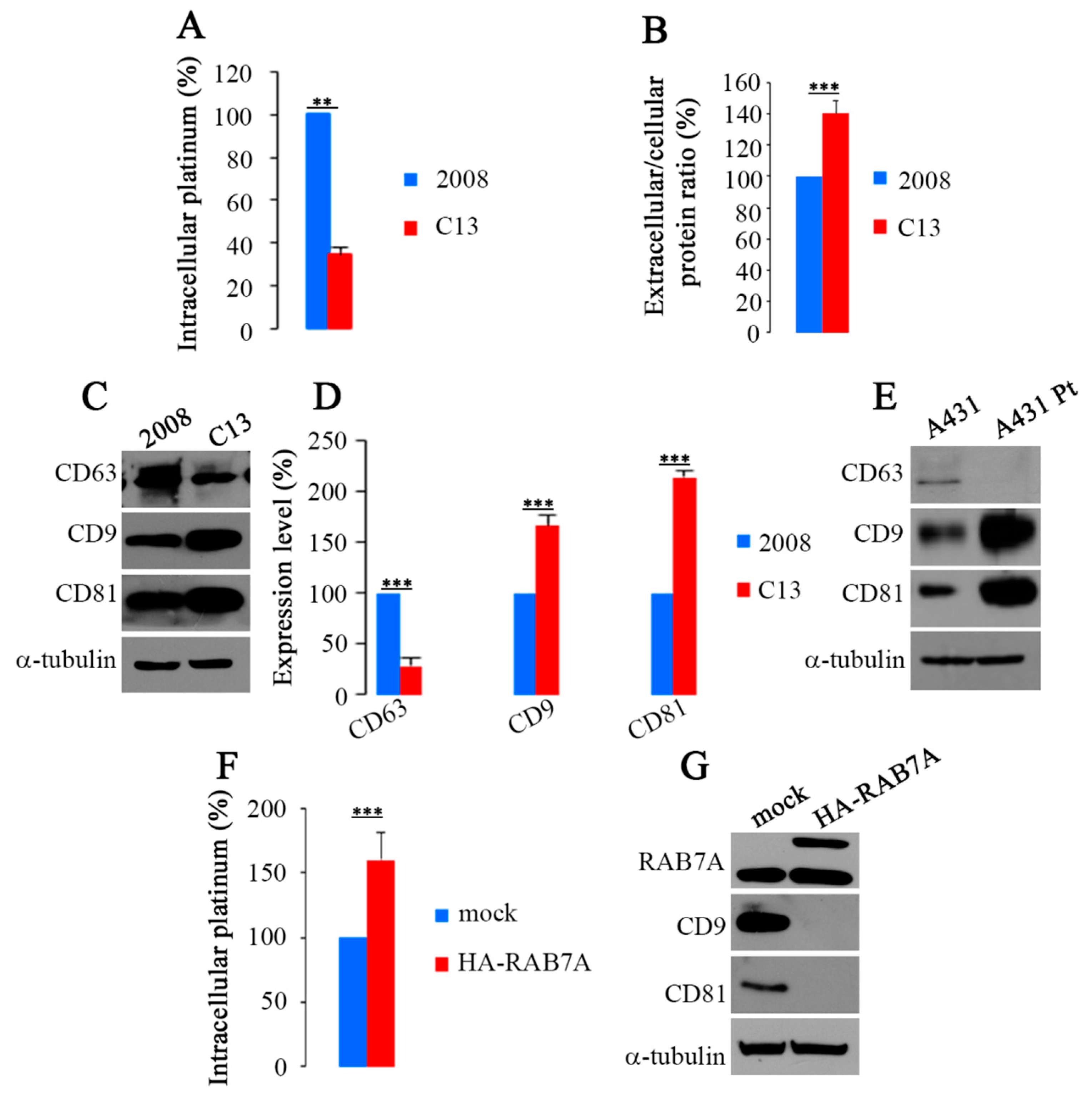
2.3. RAB7A Depletion Determines CDDP Efflux in Extracellular Vesicles
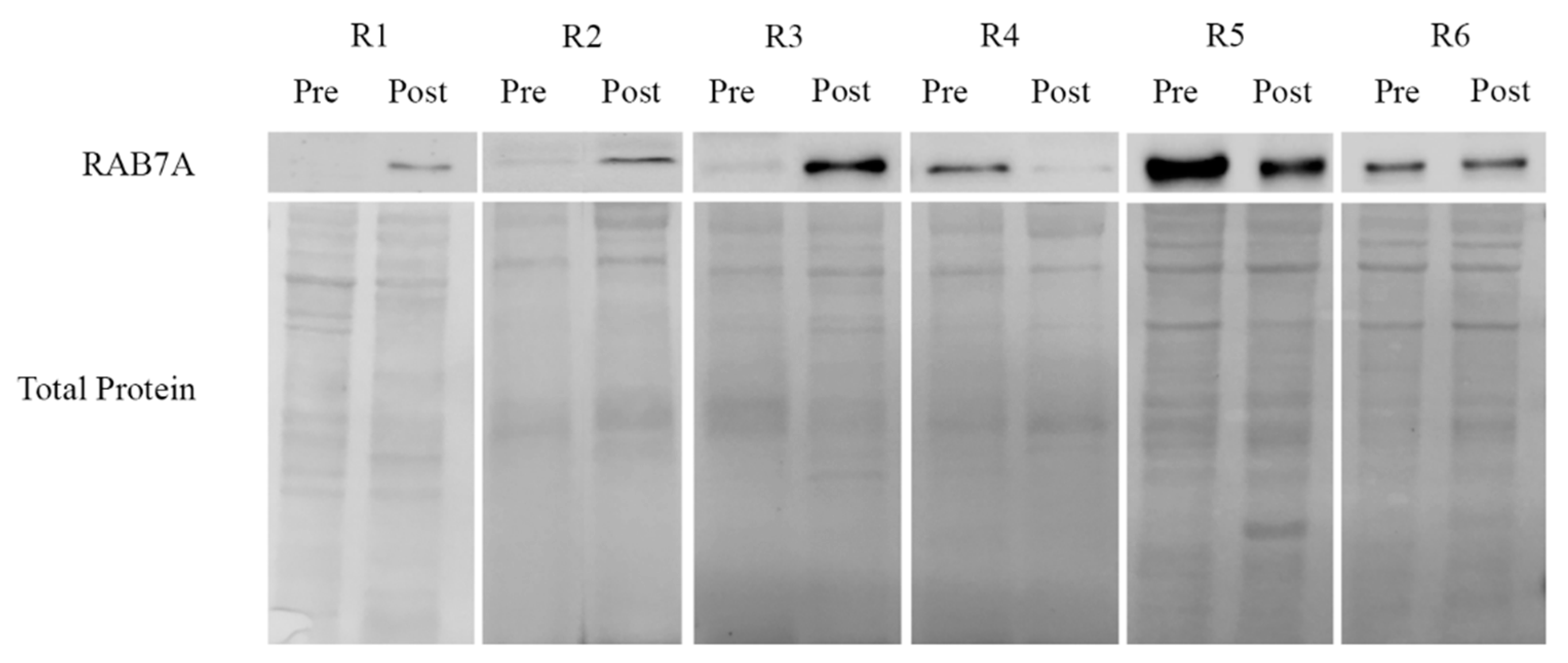
2.4. Rab7A Expression and Response to Chemotherapy in Ovarian Cancer Patient Tissues
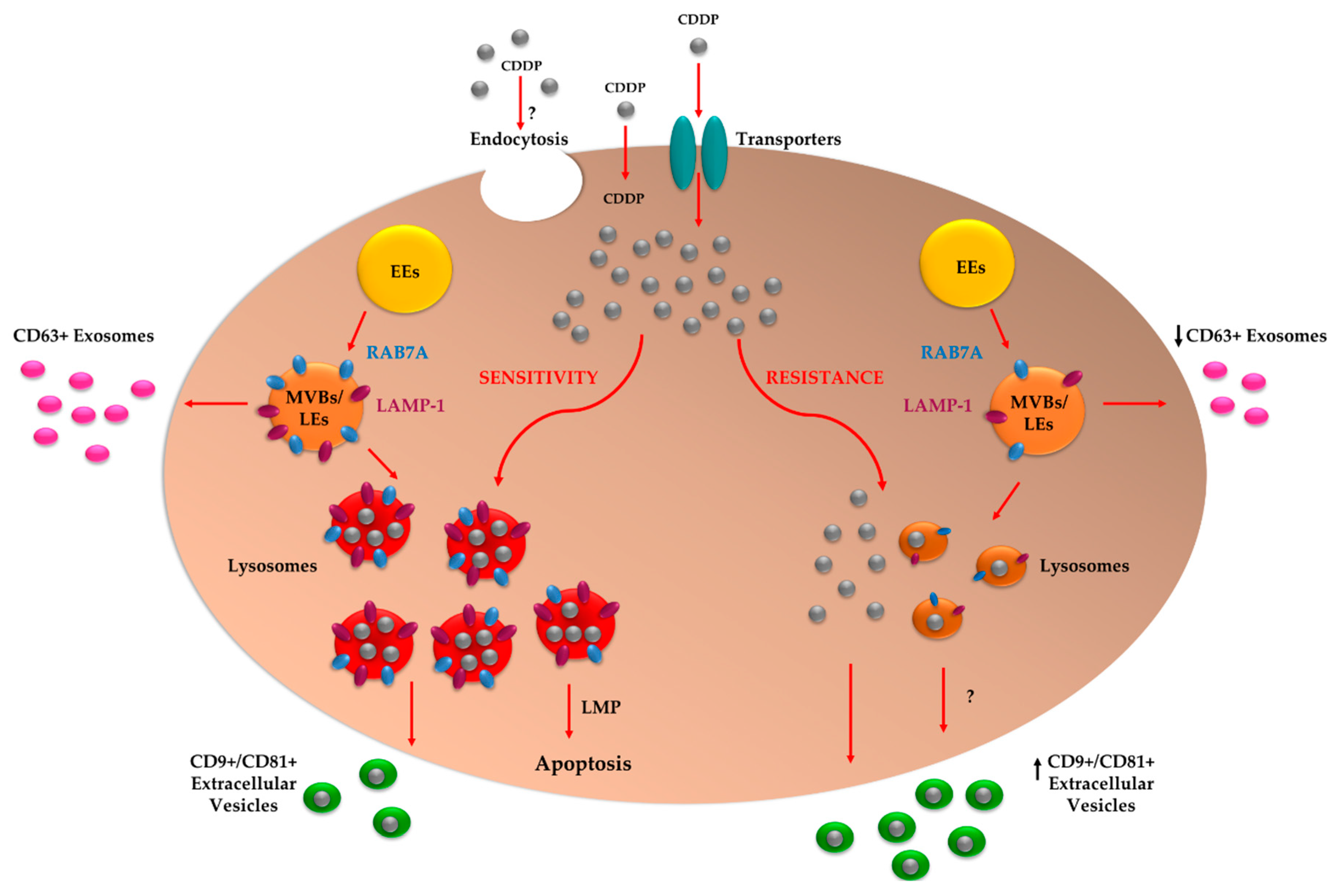
3. Discussion
4. Materials and Methods
4.1. Cells Lines
4.2. Case Series
4.3. Tumors Specimens
4.4. Induction of Chemoresistance in HeLa Cells
4.5. Transfection and RNA Interference
4.6. Western Blotting
4.7. Quantitative Real Time PCR
4.8. Immunofluorescence and Live Microscopy
4.9. Cell viability Measurements
4.10. Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) Analysis
4.11. Extracellular Vesicles (EVs) Purification
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spreckelmeyer, S.; Orvig, C.; Casini, A. Cellular transport mechanisms of cytotoxic metallodrugs: An overview beyond cisplatin. Molecules 2014, 19, 15584–15610. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Okabe, M.; Shen, D.W.; Liang, X.J.; Gottesman, M.M. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 495–535. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.L.; Loos, W.J.; Eechoute, K.; Verweij, J.; Mathijssen, R.H.J.; Wiemer, E.A.C. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist. Updates 2011, 14, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.N.; Nagatani, Y.; Tsukimoto, M.; Harada, H.; Miwa, M.; Takagi, K. Sodium-dependent glucose transporter reduces peroxynitrite and cell injury caused by cisplatin in renal tubular epithelial cells. Biochim. Biophys. Acta Biomembr. 2005, 1717, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, V.K.; Krieger, K.L.; Bendas, G.; Jaehde, U.; Kalayda, G.V. Contribution of intracellular ATP to cisplatin resistance of tumor cells. J. Biol. Inorg. Chem. 2013, 18, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.G.; Larson, C.A.; Adams, P.L.; Abada, P.B.; Pesce, C.E.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol. Pharmacol. 2011, 79, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Su, A.; Liang, X.J.; Pai-Panandiker, A.; Gottesman, M.M. Reduced expression of small gtpases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br. J. Cancer 2004, 91, 270–276. [Google Scholar] [CrossRef]
- Shen, D.W.; Liang, X.J.; Gawinowicz, M.A.; Gottesman, M.M. Identification of cytoskeletal [14c]carboplatin-binding proteins reveals reduced expression and disorganization of actin and filamin in cisplatin-resistant cell lines. Mol. Pharmacol. 2004, 66, 789–793. [Google Scholar] [PubMed]
- Safaei, R.; Katano, K.; Larson, B.J.; Samimi, G.; Holzer, A.K.; Naerdemann, W.; Tomioka, M.; Goodman, M.; Howell, S.B. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin. Cancer Res. 2005, 11, 756–767. [Google Scholar] [PubMed]
- Safaei, R.; Larson, B.J.; Cheng, T.C.; Gibson, M.A.; Otani, S.; Naerdemann, W.; Howell, S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005, 4, 1595–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Xu, Y.; Su, J.; Yu, H.; Kang, J.; Li, H.; Li, X.; Xie, Q.; Yu, C.; Sun, L.; et al. Autophagic flux promotes cisplatin resistance in human ovarian carcinoma cells through atp-mediated lysosomal function. Int. J. Oncol. 2015, 47, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Liang, X.J.; Su, A.W.; Pai-Panandiker, A.; Shen, D.W.; Hanover, J.A.; Gottesman, M.M. Reduced endocytosis and altered lysosome function in cisplatin-resistant cell lines. Br. J. Cancer 2003, 88, 1327–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennukul, K.; Numkliang, S.; Leardkamolkarn, V. Melatonin attenuates cisplatin-induced hepg2 cell death via the regulation of mtor and ercc1 expressions. World J. Hepatol. 2014, 6, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. RAB GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 2013, 25, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Gottesman, M.M. Rab8 enhances tmem205-mediated cisplatin resistance. Pharm. Res. 2012, 29, 643–650. [Google Scholar] [CrossRef]
- Guerra, F.; Bucci, C. Multiple roles of the small GTPase Rab7. Cells 2016, 5, E34. [Google Scholar] [CrossRef]
- Bucci, C.; Thomsen, P.; Nicoziani, P.; McCarthy, J.; van Deurs, B. Rab7: A key to lysosome biogenesis. Mol. Biol. Cell 2000, 11, 467–480. [Google Scholar] [CrossRef]
- Vitelli, R.; Santillo, M.; Lattero, D.; Chiariello, M.; Bifulco, M.; Bruni, C.; Bucci, C. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 1997, 272, 4391–4397. [Google Scholar] [CrossRef]
- Meresse, S.; Gorvel, G.P.; Chavrier, P. The Rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci. 1995, 108, 3349–3358. [Google Scholar] [PubMed]
- Jager, S.; Bucci, C.; Tanida, I.; Ueno, T.; Kominami, E.; Saftig, P.; Eskelinen, E.L. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 2004, 117, 4837–4848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlandingham, P.A.; Ceresa, B.P. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 2009, 284, 12110–12124. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Vonderheit, A.; Helenius, A. Rab7 associates with early endo—Somes to mediate sorting and transport of semliki forest virus to late endosomes. PLoS Biol. 2005, 3, e233. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, G.; Alifano, P.; Roberti, V.; Bruni, C.B.; Bucci, C. Rab-interacting lysosomal protein (RILP): The Rab7 effector required for transport to lysosomes. EMBO J. 2001, 20, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Jordens, I.; Fernandez-Borja, M.; Marsman, M.; Dusseljee, S.; Janssen, L.; Calafat, J.; Janssen, H.; Wubbolts, R.; Neefjes, J. The Rab7 effector protein rilp controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001, 11, 1680–1685. [Google Scholar] [CrossRef]
- Johansson, M.; Lehto, M.; Tanhuanpaa, K.; Cover, T.L.; Olkkonen, V.M. The oxysterol-binding protein homologue orp1l interacts with rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 2005, 16, 5480–5492. [Google Scholar] [CrossRef]
- De Luca, M.; Cogli, L.; Progida, C.; Nisi, V.; Pascolutti, R.; Sigismund, S.; Di Fiore, P.P.; Bucci, C. Rilp regulates vacuolar ATPase through interaction with the V1G1 subunit. J. Cell Sci. 2014, 127, 2697–2708. [Google Scholar] [CrossRef]
- De Luca, M.; Bucci, C. A new v-atpase regulatory mechanism mediated by the Rab interacting lysosomal protein (RILP). Commun. Integr. Biol. 2014, 7, 1–4. [Google Scholar] [CrossRef]
- Johnson, D.E.; Ostrowski, P.; Jaumouillé, V.; Grinstein, S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 2016, 212, 677–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, M.; Munafó, D.; Berón, W.; Colombo, M. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004, 117, 2687–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, R.E.; Brumell, J.H.; Khandani, A.; Bucci, C.; Scott, C.C.; Jiang, X.; Finlay, B.B.; Grinstein, S. Salmonella impairs RILP recruitment to rab7 during maturation of invasion vacuoles. Mol. Biol. Cell 2004, 15, 3146–3154. [Google Scholar] [CrossRef]
- Yamano, K.; Fogel, A.I.; Wang, C.; van der Bliek, A.M.; Youle, R.J. Mitochondrial Rab gaps govern autophagosome biogenesis during mitophagy. Elife 2014, 3, e01612. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Cinalli, R.M.; Thompson, C.B. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev. Cell 2003, 5, 571–582. [Google Scholar] [CrossRef]
- Saxena, S.; Howe, C.L.; Cosgaya, J.M.; Steiner, P.; Hirling, H.; Chan, J.R.; Weis, J.; Kruttgen, A. Differential endocytic sorting of p75NTR and TrKA in response to NGF: A role for late endosomes in TrKA trafficking. Mol. Cell. Neurosci. 2005, 28, 571–587. [Google Scholar] [CrossRef]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Reversi, A.; Berninghausen, O.; Hopkins, C.R.; Schiavo, G. Neurotrophins redirect p75NTR from a clathrin-independent to a clathrin-dependent endocytic pathway coupled to axonal transport. Traffic 2007, 8, 1736–1749. [Google Scholar] [CrossRef]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013, 20, 3–11. [Google Scholar] [CrossRef]
- Haugland, R.P. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Molecular Probes, Invitrogen Corp: Carlsbad, CA, USA, 2005; p. 390. [Google Scholar]
- Progida, C.; Spinosa, M.; De Luca, A.; Bucci, C. RILP interacts with the VPS22 component of the ESCRT-II complex. Biochem. Biophys. Res. Commun. 2006, 347, 1074–1079. [Google Scholar] [CrossRef]
- Progida, C.; Malerød, L.; Stuffers, S.; Brech, A.; Bucci, C.; Stenmark, H. RILP is required for proper morphology and function of late endosomes. J. Cell Sci. 2007, 120, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, A.; Progida, C.; Bakke, O.; Bucci, C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim. Biophys. Acta 2017, 1864, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Bhatnagar, S. Exosome function: From tumor immunology to pathogen biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T.J. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Cho, K.R.; Shih, I. Ovarian cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Fink, G.; Wright, A.A.; Keating, N.L.; Gockley, A.A.; Del Carmen, M.G.; Schorge, J.O.; Rauh-Hain, J.A. Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: Quasi-experimental study. BMJ 2018, 360, j5463. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676–677. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N. Quantitative prognostic indicators of peritoneal surface malignancy: Carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg. Oncol. Clin. N. Am. 2003, 12, 649–671. [Google Scholar] [CrossRef]
- Böhm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy response score: Development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, M.; Krise, J.P. Intracellular drug sequestration events associated with the emergence of multidrug resistance: A mechanistic review. Front. Biosci. 2005, 10, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget 2015, 6, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Howell, S.B. Copper transporters regulate the cellular pharmacology and sensitivity to PT drugs. Crit. Rev. Oncol. Hematol. 2005, 53, 13–23. [Google Scholar] [CrossRef]
- Nilsson, C.; Roberg, K.; Grafstrom, R.C.; Ollinger, K. Intrinsic differences in cis-platin sensitivity of head and neck cancer cell lines: Correlation to lysosomal pH. Head Neck 2010, 32, 1185–1194. [Google Scholar] [CrossRef]
- Miller, D.K.; Griffiths, E.; Lenard, J.; Firestone, R.A. Cell killing by lysosomotropic detergents. J. Cell Biol. 1983, 97, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Kalayda, G.V.; Wagner, C.H.; Buss, I.; Reedijk, J.; Jaehde, U. Altered localisation of the copper efflux transporters atp7a and atp7b associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 2008, 8, 175. [Google Scholar] [CrossRef]
- Song, P.; Trajkovic, K.; Tsunemi, T.; Krainc, D. Parkin modulates endosomal organization and function of the endo-lysosomal pathway. J. Neurosci. 2016, 36, 2425–2437. [Google Scholar] [CrossRef]
- Kazmi, F.; Hensley, T.; Pope, C.; Funk, R.S.; Loewen, G.J.; Buckley, D.B.; Parkinson, A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug Metab. Dispos. 2013, 41, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Sundler, R. Lysosomal and cytosolic pH as regulators of exocytosis in mouse macrophages. Acta Physiol. Scand. 1997, 161, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through met. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Jaé, N.; McEwan, D.G.; Manavski, Y.; Boon, R.A.; Dimmeler, S. Rab7a and Rab27b control secretion of endothelial microrna through extracellular vesicles. FEBS Lett. 2015, 589, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-alix regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking Stat3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Rappa, G.; Santos, M.F.; Green, T.M.; Karbanová, J.; Hassler, J.; Bai, Y.; Barsky, S.H.; Corbeil, D.; Lorico, A. Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-associated late endosomes. Oncotarget 2017, 8, 14443–14461. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Bristow, R.E.; Eisenhauer, E.L.; Santillan, A.; Chi, D.S. Delaying the primary surgical effort for advanced ovarian cancer: A systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol. Oncol. 2007, 104, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Gasparre, G.; Kurelac, I.; Capristo, M.; Iommarini, L.; Ghelli, A.; Ceccarelli, C.; Nicoletti, G.; Nanni, P.; De Giovanni, C.; Scotlandi, K.; et al. A mutation threshold distinguishes the antitumorigenic effects of the mitochondrial gene MTND1, an oncojanus function. Cancer Res. 2011, 71, 6220–6229. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Perrone, A.M.; Kurelac, I.; Santini, D.; Ceccarelli, C.; Cricca, M.; Zamagni, C.; De Iaco, P.; Gasparre, G. Mitochondrial DNA mutation in serous ovarian cancer: Implications for mitochondria-coded genes in chemoresistance. J. Clin. Oncol. 2012, 30, e373–e378. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Curbelo, D.; Riveiro-Falkenbach, E.; Pérez-Guijarro, E.; Cifdaloz, M.; Karras, P.; Osterloh, L.; Megías, D.; Cañón, E.; Calvo, T.G.; Olmeda, D.; et al. Rab7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 2014, 26, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.A.; Albright, K.D. Mitochondrial defects in cis-diamminedichloroplatinum(II)-resistant human ovarian carcinoma cells. Cancer Res. 1992, 52, 1895–1901. [Google Scholar]
- Catanzaro, D.; Gaude, E.; Orso, G.; Giordano, C.; Guzzo, G.; Rasola, A.; Ragazzi, E.; Caparrotta, L.; Frezza, C.; Montopoli, M. Inhibition of glucose-6-phosphate dehydrogenase sensitizes cisplatin-resistant cells to death. Oncotarget 2015, 6, 30102–30114. [Google Scholar] [CrossRef] [Green Version]
- Del Mercato, L.L.; Guerra, F.; Lazzari, G.; Nobile, C.; Bucci, C.; Rinaldi, R. Biocompatible multilayer capsules engineered with a graphene oxide derivative: Synthesis, characterization and cellular uptake. Nanoscale 2016, 8, 7501–7512. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Girolimetti, G.; Guerra, F.; Iommarini, L.; Kurelac, I.; Vergara, D.; Maffia, M.; Vidone, M.; Amato, L.B.; Leone, G.; Dusi, S.; et al. Platinum-induced mitochondrial DNA mutations confer lower sensitivity to paclitaxel by impairing tubulin cytoskeletal organization. Hum. Mol. Genet. 2017, 26, 2961–2974. [Google Scholar] [CrossRef]
| Cell Lines | EC50 (µM) |
|---|---|
| C13 mock C13 HA-RAB7A | 137 μM ± 0.064 (***) 67 μM ± 0.057 |
| 2008 SCR 2008 RAB7Ai | 47 μM ± 0.046 (*) 88 μM ± 0.063 |
| A431 Pt mock A431 Pt HA-RAB7A | 94 μM ± 0.06 (**) 54 μM ± 0.056 |
| A431 SCR A431 RAB7Ai | 36μM ± 0.138 (*) 100 μM ± 0.118 |
| HeLa mock HeLa HA-RAB7A | 46 μM ± 0.049 (*) 28 μM ± 0.072 |
| HeLa SCR HeLa RAB7Ai | 45 μM ± 0.046 (***) 78 μM ± 0.039 |
| Patients | Age at Diagnosis | Stage (FIGO) | Chemotherapy (No. of Cycles) | SCS [51] | CC [52] | CRS [53] | PFS | OS | Follow-up Status |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 32 | IIIC | 6 | 10 | 2 | NA | 14 | 32 | DOD |
| R2 | 77 | IIIC | 6 | 2 | 1 | NA | 45 | 69 | DOD |
| R3 | 75 | IIC | 7 | 2 | 2 | NA | 15 | 32 | DOD |
| R4 | 61 | IVA | 6 | 3 | 0 | NA | 71 | 71 | AWOD |
| R5 | 68 | IVA | 3 | 10 | 0 | 2 | 10 | 20 | DOD |
| R6 | 44 | IVA | 6 | 10 | 0 | 1 | 25 | 25 | AWOD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.; Paiano, A.; Migoni, D.; Girolimetti, G.; Perrone, A.M.; De Iaco, P.; Fanizzi, F.P.; Gasparre, G.; Bucci, C. Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion. Cancers 2019, 11, 52. https://doi.org/10.3390/cancers11010052
Guerra F, Paiano A, Migoni D, Girolimetti G, Perrone AM, De Iaco P, Fanizzi FP, Gasparre G, Bucci C. Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion. Cancers. 2019; 11(1):52. https://doi.org/10.3390/cancers11010052
Chicago/Turabian StyleGuerra, Flora, Aurora Paiano, Danilo Migoni, Giulia Girolimetti, Anna Myriam Perrone, Pierandrea De Iaco, Francesco Paolo Fanizzi, Giuseppe Gasparre, and Cecilia Bucci. 2019. "Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion" Cancers 11, no. 1: 52. https://doi.org/10.3390/cancers11010052
APA StyleGuerra, F., Paiano, A., Migoni, D., Girolimetti, G., Perrone, A. M., De Iaco, P., Fanizzi, F. P., Gasparre, G., & Bucci, C. (2019). Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion. Cancers, 11(1), 52. https://doi.org/10.3390/cancers11010052









