Curcuminoids as EBV Lytic Activators for Adjuvant Treatment in EBV-Positive Carcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Chemicals, Plasmids, and Antibodies
2.3. Cytotoxicity and Cell Viability Assays
2.4. Immunoblot Analysis
2.5. FACS Analysis, Immunofluorescence Assay and Measurement of Percentage of Cells Induced into Lytic Cycle
2.6. Quantitative RT-PCR Assay for the Detection of Specific mRNA Related to EBV Lytic Reactivation
3. Results
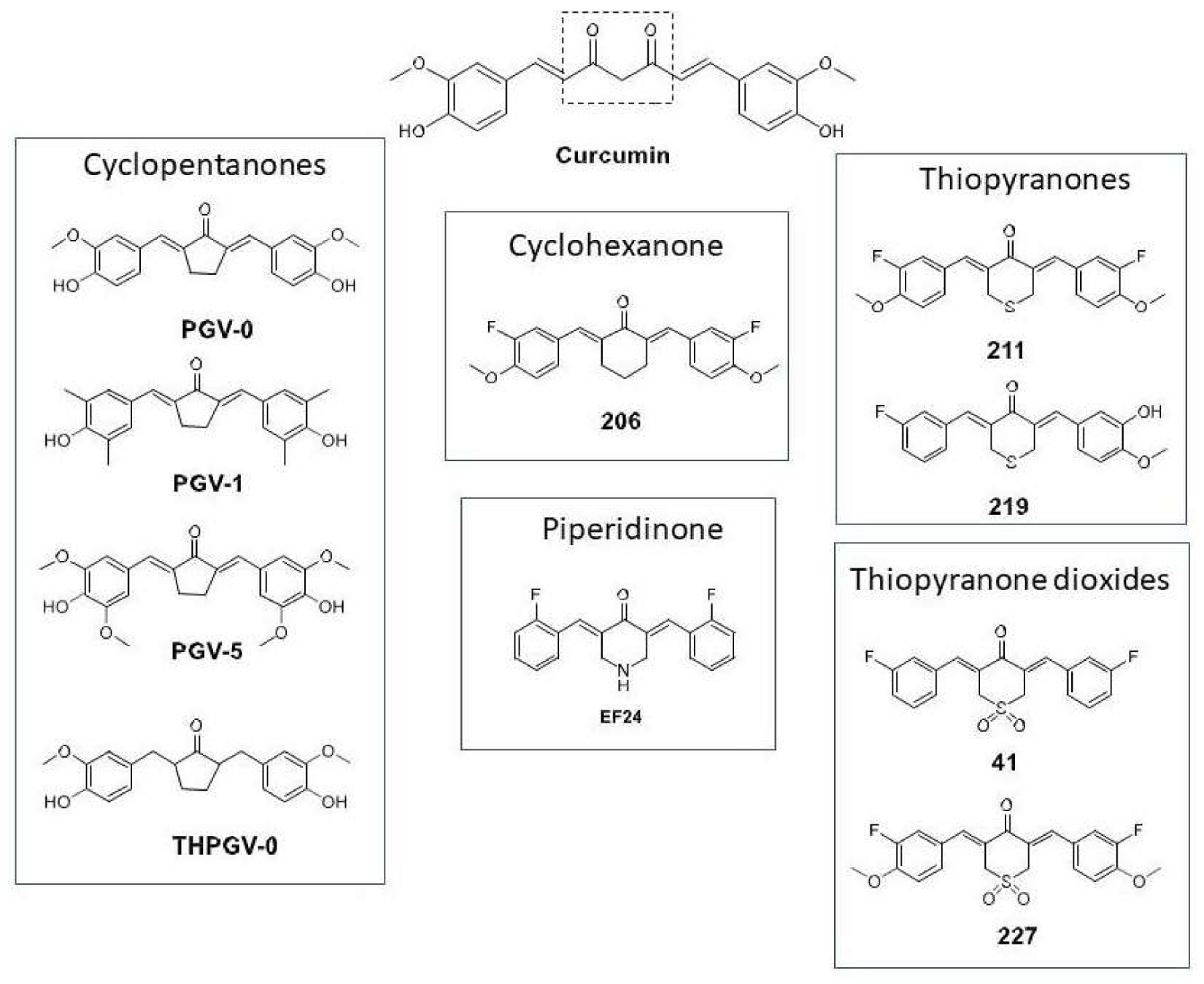
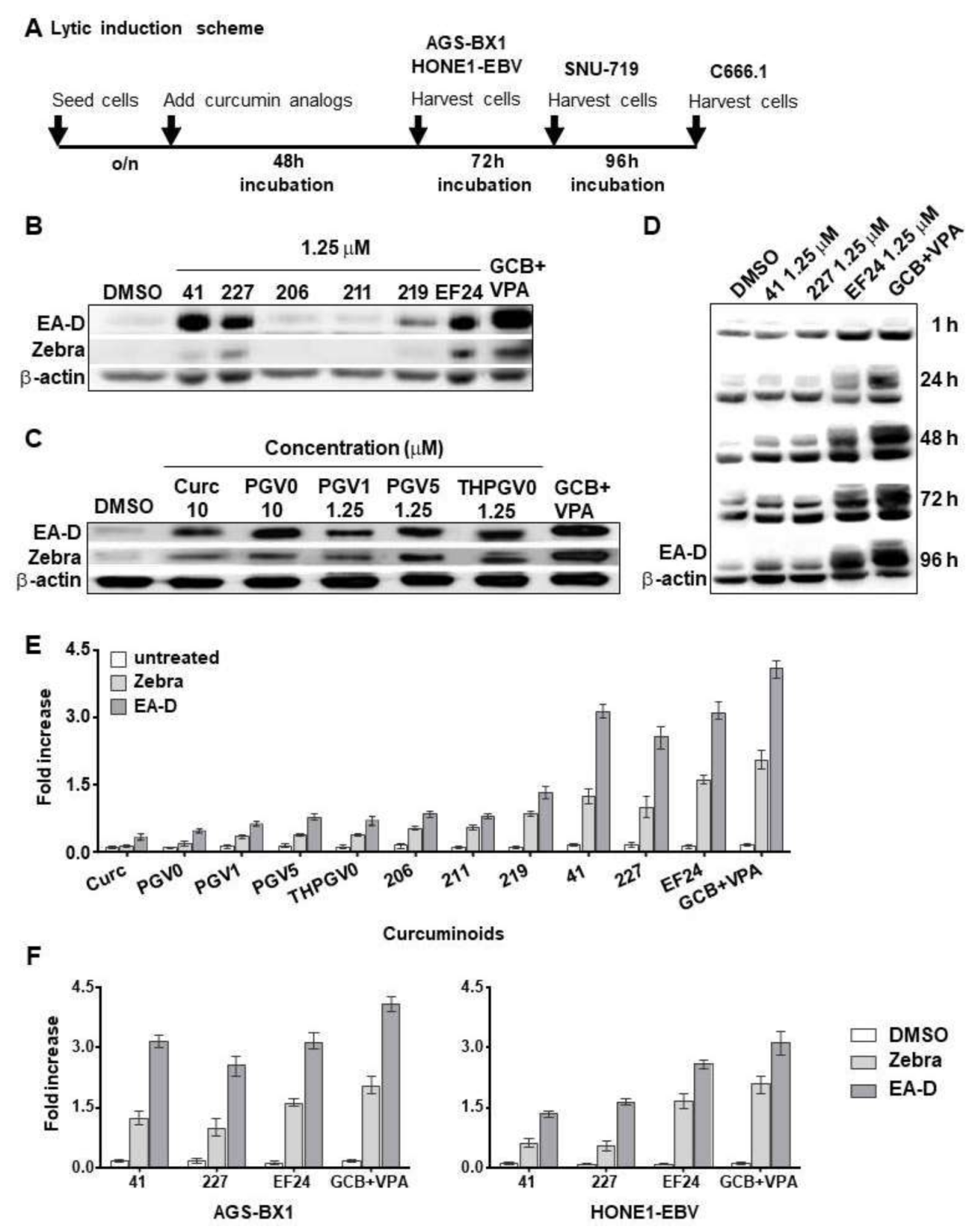
3.1. Comparison of Curcumin and Curcuminoids as EBV Lytic Inducer Agents
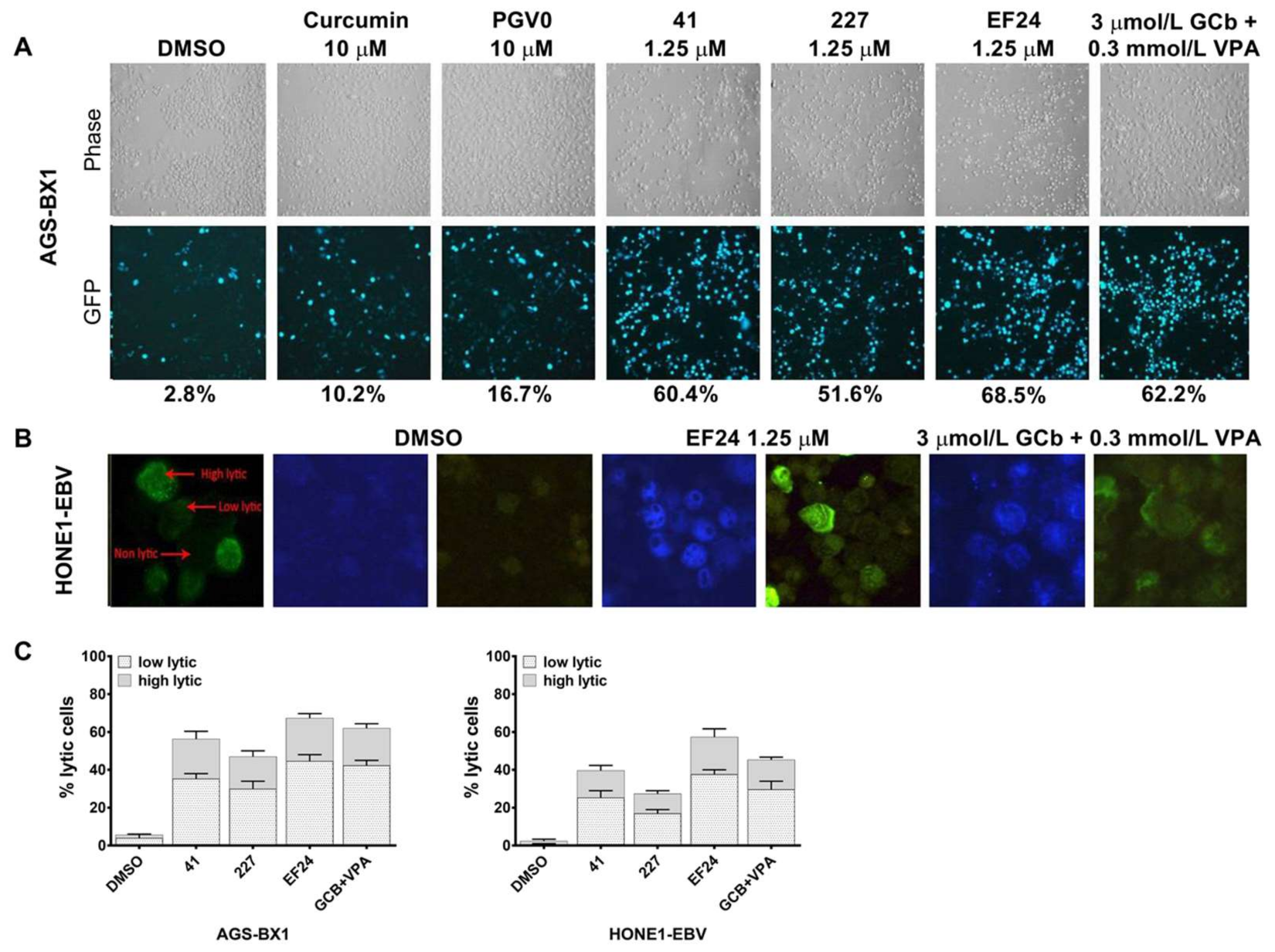
3.2. Different Lytic Induction Effect of Curcuminoids in Gastric and Nasopharyngeal Carcinoma Cell Line Carrying a Recombinant EBV Genome
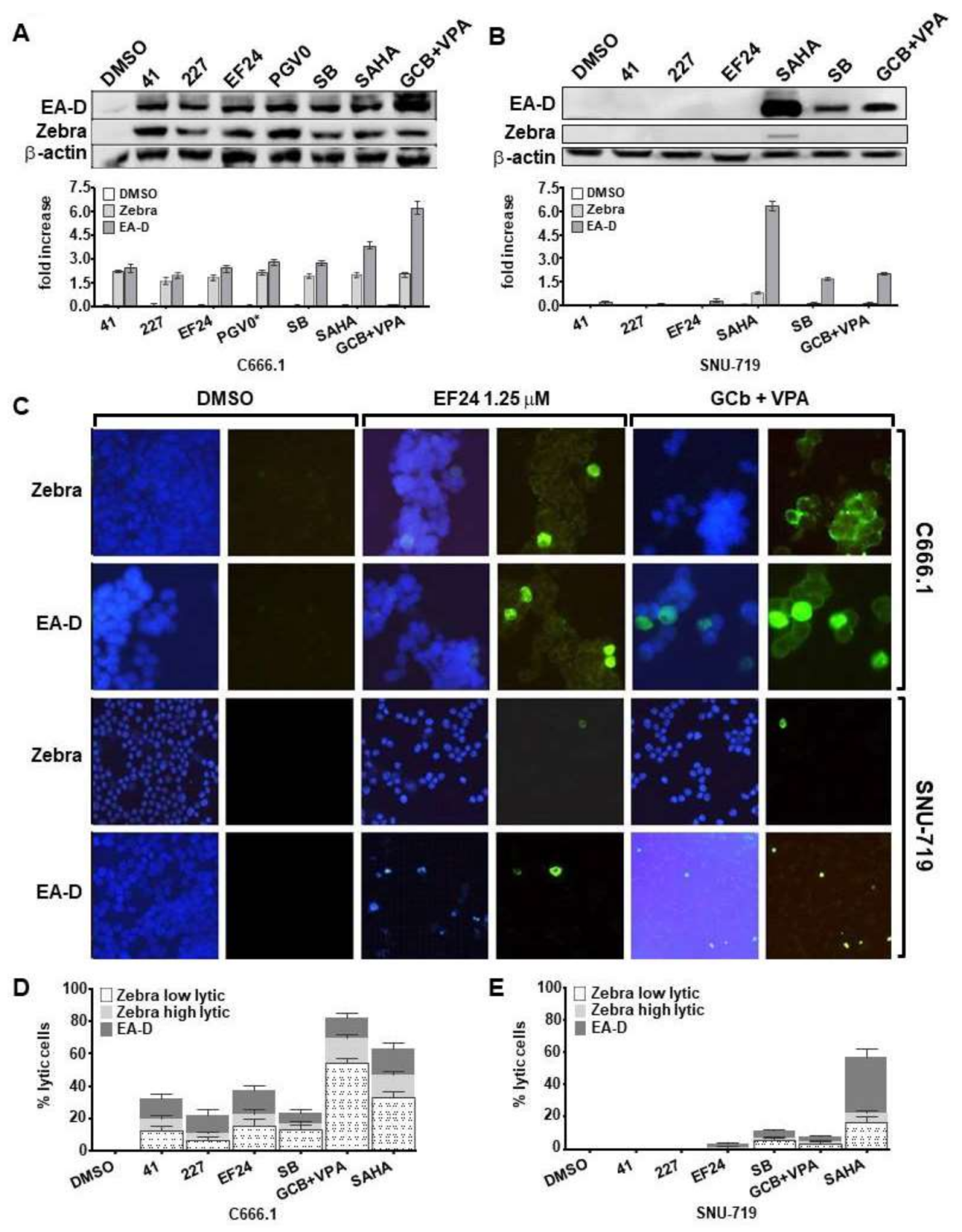
3.3. Curcuminoids Induce EBV Reactivation Better in Carcinoma Cells Carrying Recombinant EBV Compared to a Natural EBV Genome
3.4. Analysis of Cell Viability at Different Concentrations of EBV Lytic Inducers
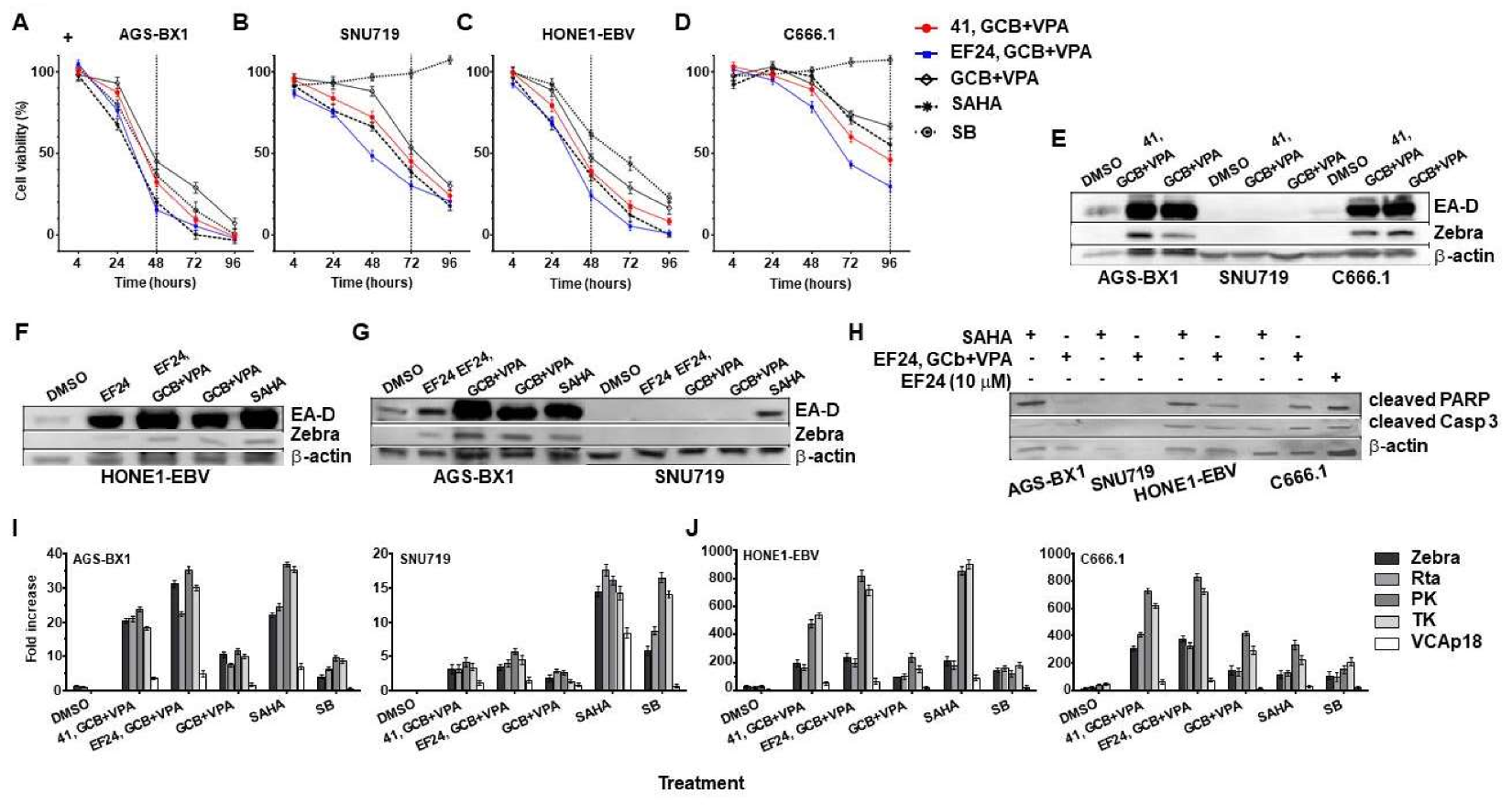
3.5. Synergistic Effects of Curcuminoids in Combination with CLVA Regimen
3.6. Effect of Combined EF24 and CLVA Treatment on Apoptosis and EBV Lytic Protein Expression in GC and NPC Cells Carrying Artificial EBV Genomes
3.7. EBV Immediate-Early and Early Rather Than Late Lytic Gene Expression Is Induced by Combination Curcuminoid and CLVA Regimen Treatment in EBV-Positive Carcinomas
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elgui de Oliveira, D.; Müller-Coan, B.G.; Pagano, J.S. Viral carcinogenesis beyond malignant transformation: EBV in the progression of human cancers. Trends Microbiol. 2016, 24, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Niller, H.H.; Banati, F.; Minarovits, J. Epigenetic alterations in nasopharyngeal carcinoma and Epstein-Barr virus (EBV) associated gastric carcinoma: A lesson in contrasts. J. Nasopharyngeal Carcinoma 2014, 1. [Google Scholar] [CrossRef]
- Abe, H.; Kaneda, A.; Fukayama, M. Epstein-Barr virus-associated gastric carcinoma: Use of host cell machineries and somatic gene mutations. Pathobiology 2015, 82, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Perrine, S.P.; Faller, D.V. Advances in virus-directed therapeutics against Epstein-Barr Virus-associated malignancies. Adv. Virol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hutajulu, S.H.; Kurnianda, J.; Tan, I.B.; Middeldorp, J.M. Therapeutic implications of Epstein-Barr virus infection for the treatment of nasopharyngeal carcinoma. Ther. Clin. Risk Manag. 2014, 10, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Wildeman, M.A.; Novalic, Z.; Verkuijlen, S.A.; Juwana, H.; Huitema, A.D.; Tan, I.B.; Middeldorp, J.M.; de Boer, J.P.; Greijer, A.E. Cytolytic virus activation therapy for Epstein-Barr virus-driven tumors. Clin. Cancer Res. 2012, 18, 5061–5070. [Google Scholar] [CrossRef] [PubMed]
- Stoker, S.D.; Novalić, Z.; Wildeman, M.A.; Huitema, A.D.; Verkuijlen, S.A.; Juwana, H.; Greijer, A.E.; Tan, I.B.; Middeldorp, J.M.; de Boer, J.P. Epstein-Barr virus-targeted therapy in nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.; El-Guindy, A. Epstein-Barr virus lytic cycle reactivation. Curr. Top. Microbiol. Immunol. 2015, 391, 237–261. [Google Scholar] [PubMed]
- Novalic, Z.; van Rossen, T.M.; Greijer, A.E.; Middeldorp, J.M. Agents and approaches for lytic induction therapy of Epstein-Barr Virus associated malignancies. Med. Chem. 2016, 6, 449–466. [Google Scholar]
- Liu, S.F.; Wang, H.; Lin, X.C.; Xiang, H.; Deng, X.Y.; Li, W.; Tang, M.; Cao, Y. NF-κB inhibitors induce lytic cytotoxicity in Epstein-Barr virus-positive nasopharyngeal carcinoma cells. Cell Biol. Int. 2008, 32, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Tikhmyanova, N.; Schultz, D.C.; Lee, T.; Salvino, J.M.; Lieberman, P.M. Identification of a new class of small molecules that efficiently reactivate latent Epstein-Barr virus. ACS Chem. Biol. 2014, 9, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Ho, D.N.; Hui, K.F.; Kao, R.Y.; Chiang, A.K.S. Identification of novel small organic compounds with diverse structures for the induction of Epstein-Barr virus (EBV) lytic cycle in EBV-positive epithelial malignancies. PLoS ONE 2015, 10, e0145994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, W.; Li, P.; Zheng, Z.; Tu, Y.; Zhang, Y.; You, T. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Inducing Gastric Cancer Cell Apoptosis Both In Vitro and In Vivo. Oncol. Res. 2016, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, G.; Xiao, J.; Su, B.; Zhou, F.; Wei, Y. A novel curcuminoid exhibits enhanced antitumor activity in nasopharyngeal carcinoma. Int. J. Oncol. 2016, 48, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Koh, S.B.; Ee, R.P.; Khan, M.; Go, M.L. Curcuminoids with potent and selective anti-proliferative activity on acute promyelocytic leukemia: Involvement of accumulated misfolded nuclear receptor co-repressor (N-CoR) protein as a basis for selective activity. Chem. Med. Chem. 2012, 7, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Ali, A.; Du, Y.; Fu, H.; Jin, H.X.; Chin, T.M.; Khan, M.; Go, M.L. Synthesis and evaluation of bisbenzylidenedioxotetrahydrothiopranones as activators of endoplasmic reticulum (ER) stress signaling pathways and apoptotic cell death in acute promyelocytic leukemic cells. J. Med. Chem. 2014, 57, 5904–5918. [Google Scholar] [CrossRef] [PubMed]
- Sardjiman, S.S.; Reksohadiprodjo, M.S.; Hakim, L.; van der Goot, H.; Timmerman, H. 1,5-Diphenyl-1,4-pentadiene-3-ones and cyclic analogues as antioxidative agents. Synthesis and structure-activity relationship. Eur. J. Med. Chem. 1997, 32, 625–630. [Google Scholar] [CrossRef]
- Putri, D.D.; Susidarti, R.A.; Murwanti, R.; Sardjiman, F.A.; Husnaa, U.; Purnomo, H.; Kawaichi, M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-κB activation. Asian Pac. J. Cancer Prev. 2014, 15, 179–184. [Google Scholar]
- Zou, P.; Xia, Y.; Chen, W.; Chen, X.; Ying, S.; Feng, Z.; Chen, T.; Ye, Q.; Wang, Z.; Qiu, C.; et al. EF24 induces ROS-mediated apoptosis via targeting thioredoxin reductase 1 in gastric cancer cells. Oncotarget 2016, 7, 18050–18064. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.Y.; Khuri, F.R.; Wang, C.Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IκB Kinase-Nuclear Factor-κB Signaling Pathway by 3,5-Bis(2-flurobenzylidene) piperidin-4-one (EF24), a Novel Monoketone Analog of Curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yue, C.; Sims, M.; Pfeffer, L.M. The curcumin analog EF24 targets NF-κB and miRNA-21 and has potent anticancer activity in vitro and in vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.F.; Ho, D.N.; Tsang, C.M.; Middeldorp, J.M.; Tsao, G.S.; Chiang, A.K. Activation of lytic cycle of Epstein-Barr virus by suberoylanilide hydroxamic acid hits to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int. J. Cancer 2012, 131, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yu, J.; Du, L.; Tang, J.; Feng, W.H. Cordycepin enhances Epstein-Barr virus lytic infection and Epstein-Barr virus-positive tumor treatment efficacy by doxorubicin. Cancer Lett. 2016, 376, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Giunco, S.; Dolcetti, R.; Keppel, S.; Celeghin, A.; Indraccolo, S.; Dal Col, J.; Mastorci, K.; De Rossi, A. hTERT inhibition triggers Epstein-Barr virus lytic cycle and apoptosis in immortalized and transformed B cells: A basis for new therapies. Clin. Cancer Res. 2013, 19, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Kim, D.H.; Roh, J.K.; Middeldorp, J.M.; Kim, Y.S.; Kim, S.; Han, S.; Kim, C.W.; Lee, B.L.; Kim, W.H.; et al. Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric carcinoma cells through regulation of NF-κB. J. Virol. 2013, 87, 10515–10523. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.F.; Chiang, A.K. Suberoylanilide hydroxamic acid induces viral lytic cycle in Epstein-Barr virus-positive epithelial malignancies and mediates enhanced cell death. Int. J. Cancer 2010, 126, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, M.A.; Holthaus, A.M.; Johannsen, E. The Epstein-Barr virus LF2 protein inhibits viral replication. J. Virol. 2008, 82, 8509–8519. [Google Scholar] [CrossRef] [PubMed]
- Greijer, A.E.; Ramayanti, O.; Verkuijlen, S.A.; Novalić, Z.; Juwana, H.; Middeldorp, J.M. Quantitative multi-target RNA profiling in Epstein-Barr virus infected tumor cells. J. Virol. Methods 2017, 241, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.M.; Deng, W.; Jia, L.; Yang, J.; Tsurumi, T.; Chiang, A.K.; Huen, M.S.Y.; Tsao, S.W. Role of ATM in the formation of the replication compartment during lytic replication of Epstein-Barr virus in nasopharyngeal epithelial cells. J. Virol. 2015, 89, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Ramayanti, O.; Juwana, H.; Verkuijlen, S.A.; Adham, M.; Pegtel, M.D.; Greijer, A.E.; Middeldorp, J.M. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer 2017, 140, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Remick, J.; Zeichner, S.L. Activation of human herpesvirus replication by apoptosis. J. Virol. 2013, 87, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Y.; Wang, L.; Tian, L.; Song, R.; Han, T.; Pan, S.; Liu, L. In vivo and in vitro suppression of hepatocellular carcinoma by EF24, a curcuminoid. PLoS ONE 2012, 7, e48075. [Google Scholar]
- Sivachandran, N.; Wang, X.; Frappier, L. Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. J. Virol. 2012, 86, 6146–6158. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.T.; Fernandez, S.G.; Somberg, J.J.; Keck, K.M.; Miranda, J.L. Epstein-Barr virus latency type and spontaneous reactivation predict lytic induction levels. Biochem. Biophys. Res. Commun. 2016, 474, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Seo, J.S.; Moon, U.Y.; Kang, K.H.; Shin, D.J.; Yoon, S.K.; Kim, W.H.; Park, J.G.; Lee, S.K. A naturally derived gastric cancer cell line shows latency I Epstein-Barr virus infection closely resembling EBV-associated gastric cancer. Virology 2004, 320, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Lee, M.; Ryu, E.; Moon, A.; Jeong, C.S.; Jung, Y.W.; Park, G.H.; Sung, G.H.; Cho, H.; Kang, H. Genipin as a novel chemical activator of EBV lytic cycle. J. Microbiol. 2015, 53, 155–165. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt’s lymphoma cells by affecting multiple molecular targets. Antivir. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chuang, H.Y.; Lin, C.Y.; Chen, Y.J.; Tsai, W.H.; Fang, C.Y.; Huang, S.Y.; Chuang, F.Y.; Lin, S.F.; Chang, Y.; et al. Inhibition of Epstein-Barr virus reactivation in nasopharyngeal carcinoma cells by dietary sulforaphane. Mol. Carcinog. 2013, 52, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Fang, C.Y.; Hsu, H.Y.; Chen, Y.J.; Chou, S.P.; Huang, S.Y.; Cheng, Y.J.; Lin, S.F.; Chang, Y.; Tsai, C.H.; et al. Luteolin inhibits Epstein-Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antivir. Res. 2016, 132, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Tierney, R.J.; Shannon-Lowe, C.D.; Fitzsimmons, L.; Bell, A.I.; Rowe, M. Unexpected patterns of Epstein-Barr virus transcription revealed by a high throughput PCR array for absolute quantification of viral mRNA. Virology 2015, 474, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.F.; Cheung, A.K.; Choi, C.K.; Yeung, P.L.; Middeldorp, J.M.; Lung, M.L.; Tsao, S.W.; Chiang, A.K. Inhibition of class I histone deacetylases by romidepsin potently induces Epstein-Barr virus lytic cycle and mediates enhanced cell death with ganciclovir. Int. J. Cancer 2016, 138, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, W.H.; Wang, J.J.; Hsueh, S.P.; Hsu, P.J.; Chang, L.C.; Hsu, C.S.; Hsu, K.Y. In vitro and in vivo studies of pharmacokinetics and antitumor efficacy of D07001-F4, an oral gemcitabine formulation. Cancer Chemother. Pharmacol. 2013, 71, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Pratt, S.E.; Durland-Busbice, S.; Shepard, R.L.; Donoho, G.P.; Starling, J.J.; Wickremsinhe, E.R.; Perkins, E.J.; Dantzig, A.H. Efficacy of low-dose oral metronomic dosing of the prodrug of gemcitabine, LY2334737, in human tumor xenografts. Mol. Cancer Ther. 2013, 12, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Wildeman, M.A.; Fles, R.; Herdini, C.; Indrasari, R.S.; Vincent, A.D.; Tjokronagoro, M.; Stoker, S.; Kurnianda, J.; Karakullukcu, B.; Taroeno-Hariadi, K.W.; et al. Primary treatment results of Nasopharyngeal Carcinoma (NPC) in Yogyakarta, Indonesia. PLoS ONE 2013, 8, e63706. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramayanti, O.; Brinkkemper, M.; Verkuijlen, S.A.W.M.; Ritmaleni, L.; Go, M.L.; Middeldorp, J.M. Curcuminoids as EBV Lytic Activators for Adjuvant Treatment in EBV-Positive Carcinomas. Cancers 2018, 10, 89. https://doi.org/10.3390/cancers10040089
Ramayanti O, Brinkkemper M, Verkuijlen SAWM, Ritmaleni L, Go ML, Middeldorp JM. Curcuminoids as EBV Lytic Activators for Adjuvant Treatment in EBV-Positive Carcinomas. Cancers. 2018; 10(4):89. https://doi.org/10.3390/cancers10040089
Chicago/Turabian StyleRamayanti, Octavia, Mitch Brinkkemper, Sandra A. W. M. Verkuijlen, Leni Ritmaleni, Mei Lin Go, and Jaap M. Middeldorp. 2018. "Curcuminoids as EBV Lytic Activators for Adjuvant Treatment in EBV-Positive Carcinomas" Cancers 10, no. 4: 89. https://doi.org/10.3390/cancers10040089




