A Mini-System Integrated with Metal-Oxide-Semiconductor Sensor and Micro-Packed Gas Chromatographic Column
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
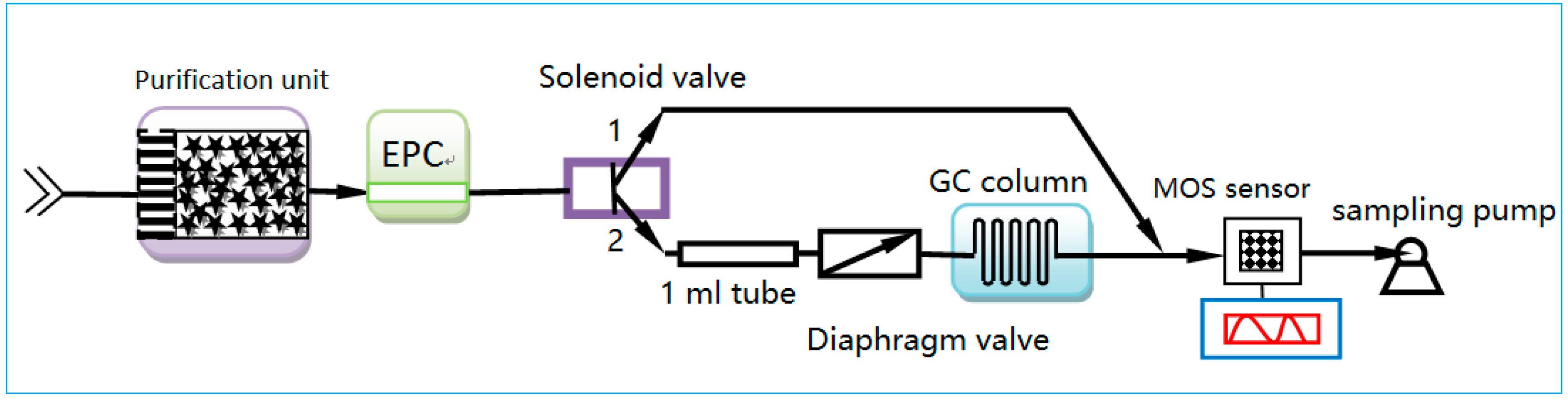
2.2. Experimental Setup
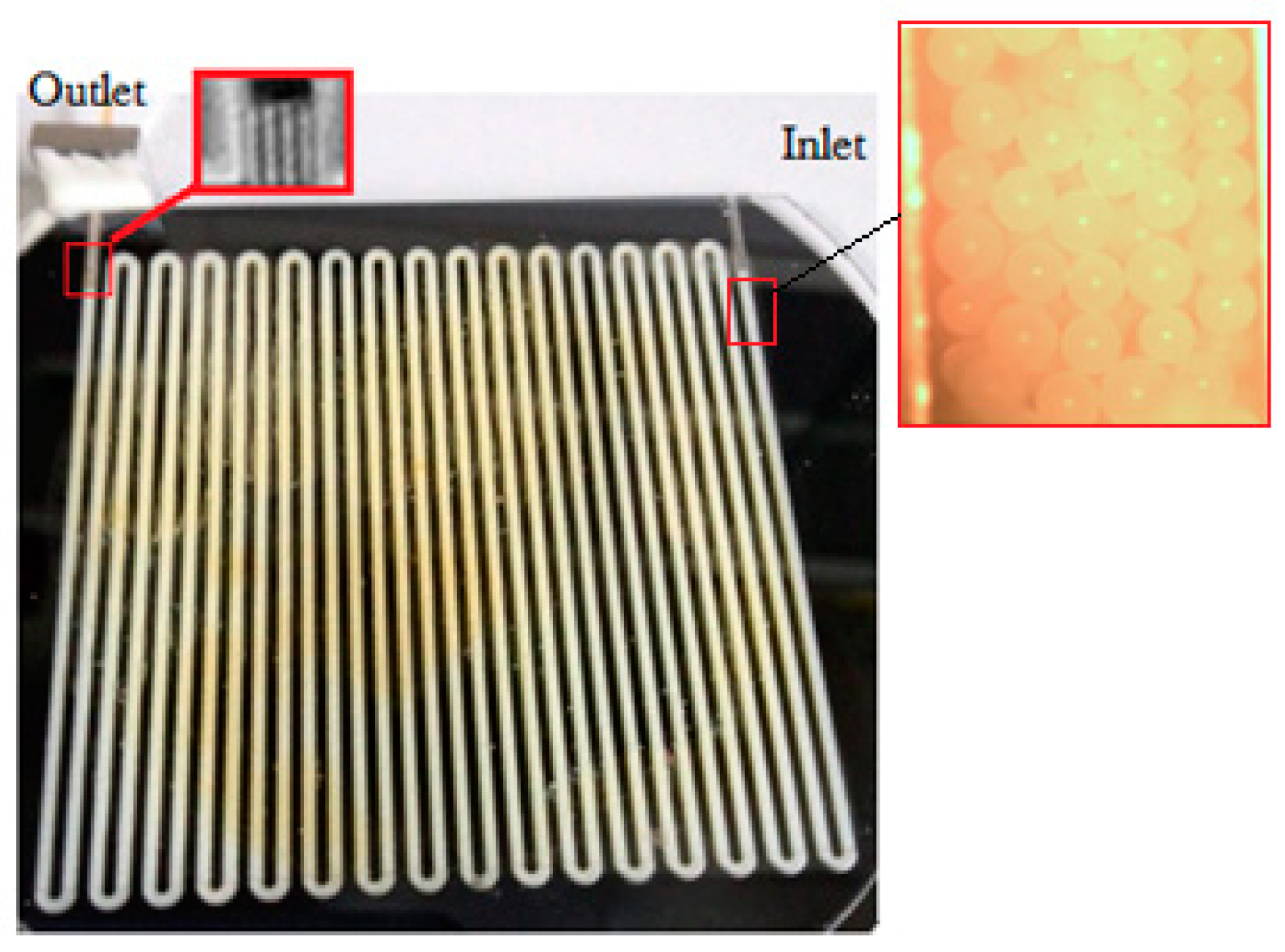
2.3. Fabrication of MOS Array Sensor
3. Results
3.1. Gas Sensing Characteristics of the MOS Array Sensors
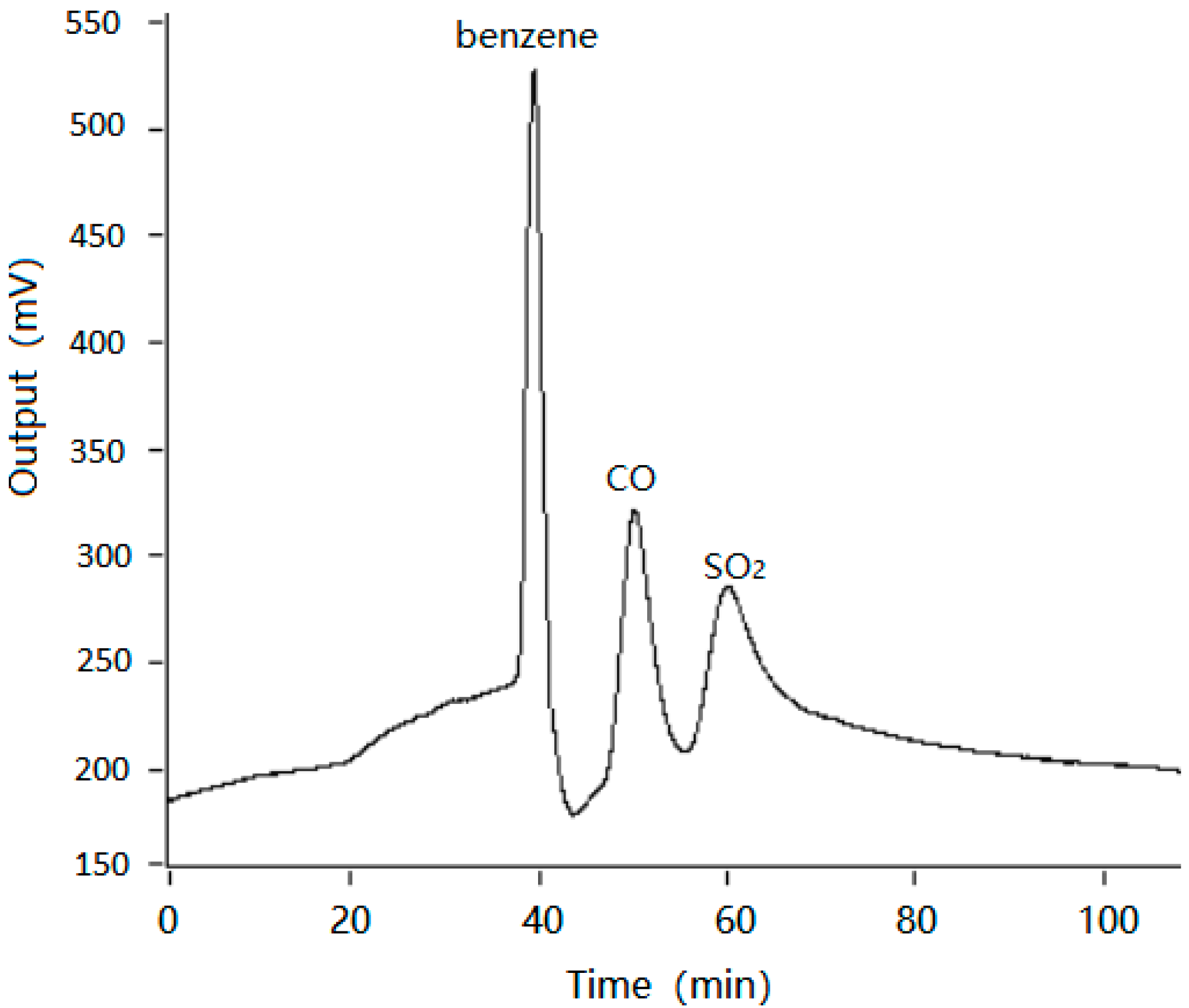
3.2. Rapid Detection of Polluted Gases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pekkanen, J.; Peters, A.; Hoek, G.; Tiittanen, P.; Brunekreef, B.; de Hartog, J.; Heinrich, J.; Ibald-Mulli, A.; Kreyling, W.G.; Lanki, T.; et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease. Circulation 2002, 106, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infaction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Parkin, I.; Binions, R. Gas sensing studies of an N-N hetero-junction array based on SnO2 and ZnO composites. Chemosensors 2016, 4, 3. [Google Scholar] [CrossRef]
- Nicoletti, S.; Zampolli, S.; Elmi, I.; Dori, L.; Severi, M. Use of different sensing materials and deposition techniques for thin-film sensors to increase sensitivity and selectivity. IEEE Sens. J. 2003, 3, 454–459. [Google Scholar] [CrossRef]
- Elmi, I.; Zampolli, S.; Cardinali, G.C. Optimization of a wafer-level process for the fabrication of highly reproducible thin-film MOX sensors. Sens. Actuators B Chem. 2008, 131, 548–555. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sabhajeet, S.R.; Singh, S.; Yadav, B.C. Synthesis of ZnO nanopetals and its application as NO2 gas sensor. Mater. Lett. 2015, 152, 189–191. [Google Scholar] [CrossRef]
- Leonard, C.; Liu, H.F.; Brewer, S.; Sacks, R.D. High-speed gas extraction of volatile and semivolatile organic compounds from aqueous samples. Anal. Chem. 1998, 70, 3498–3504. [Google Scholar] [CrossRef]
- Lee, D.S.; Jung, J.K.; Lim, J.W.; Huh, J.S.; Lee, D.D. Recognition of volatile organic compounds using SnO2 sensors array and pattern recognition analysis. Sens. Actuators B Chem. 2001, 77, 228–236. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Groppelli, S.; Nelli, P.; Camanzi, A. A new technique for growing large surface area SnO2 thin film (RGTO technique). Semicond. Sci. Technol. 1990, 5, 1231–1233. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Kim, J.H.; Zheng, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and selective sensing properties of rGO/metal-coloaded SnO2 nanofibers. J. Electron. Mater. 2017, 46, 3531–3541. [Google Scholar] [CrossRef]
- Fan, H.; Xu, S.; Cao, X.; Liu, D.; Yin, Y.; Hao, H.; Wei, D.; Shen, Y. Ultra-long Zn2SnO4-ZnO microwires based gas sensor for hydrogen detection. Appl. Surf. Sci. 2017, 400, 440–445. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2(n)-NiO(p)composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X.; Wang, Y.; Liu, G.; Zhu, X. Study on gas sensing of reduced graphene oxide/ZnO thin film at room temperature. Sens. Actuators B Chem. 2017, 240, 870–880. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Y.; Ren, H.; Gao, C.; Zhou, Y. Enhancing the NO2 gas sensing properties of rGO/SnO2 nanocomposite films by using microporous substrates. Sens. Actuators B Chem. 2017, 248, 560–570. [Google Scholar] [CrossRef]
- Niu, X.S.; Du, W.P.; Du, W.M. Preparation and gas sensing properties of ZnM2O4 (M = Fe, Co, Cr). Sens. Actuators B Chem. 2004, 99, 405–409. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Jadhav, H.S.; Shimizu, F.M.; Suman, P.H.; M’Peko, J.C.; Orlandi, M.O.; Seo, J.G.; Mastelaro, V.R.; Oliveira, O.N., Jr. Yolk-shelled ZnCo2O4 microspheres: Surface properties and gas sensing application. Sens. Actuators B Chem. 2018, 257, 906–915. [Google Scholar] [CrossRef]
- Sun, F.J.; Li, X.G.; Liu, L.P.; Wang, J. Novel Zn–M–O (M = Sn, Co) sensing electrodes for selective mixed potential CO/C3H8 sensors. Sens. Actuators B Chem. 2013, 184, 220–227. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Guo, B.; Chen, J. MCo2O4 (M = Ni, Cu, Zn) nanotubes: Template synthesis and application in gas sensors. Sens. Actuators B Chem. 2006, 114, 402–409. [Google Scholar] [CrossRef]
- Song, Z.; Liu, J.; Liu, Q.; Yu, H.; Zhang, W. Enhanced H2S gas sensing properties based on SnO2 quantum wire/reduced graphene oxide nanocomposites: Equilibrium and kinetics modeling. Sens. Actuators B Chem. 2017, 249, 632–638. [Google Scholar] [CrossRef]
- Huang, J.; Du, Y.; Wang, Q.; Zhang, H.; Geng, Y.; Li, X.; Tian, X. UV-enhanced ethanol sensing properties of RF magnetron-sputtered ZnO film. Sensors 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, E.J.; Meglen, R.M.; Peterson, D.; Sluiter, J. Metal oxide sensor arrays for the detection, differentiation, and quantification of volatile organic compounds at sub-parts-per-million concentration levels. Sens. Actuators B Chem. 2006, 115, 322–329. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Reddy, K.; Jing, L.; Oo, M.; Fan, X. Integrated separation columns and Fabry-Perot sensors for micro gas chromatography systems. IEEE J. Microelectromech. Syst. 2013, 22, 1174–1179. [Google Scholar] [CrossRef]
- Sun, J.H.; Guan, F.Y.; Cui, D.F.; Chen, X.; Zhang, L.L. An improved photoionization detector with a micro gas chromatography column for portable rapid gas chromatography system. Sens. Actuators B Chem. 2013, 188, 513–518. [Google Scholar] [CrossRef]
- Ali, S.; Ashraf-Khorassani, M.; Taylor, L.T.; Agah, M. MEMS-based semi-packed gas chromatography columns. Sens. Actuators B Chem. 2009, 14, 309–315. [Google Scholar] [CrossRef]
- Sun, J.H.; Guan, F.Y.; Zhu, X.F.; Ning, Z.W.; Ma, T.J.; Liu, J.H.; Deng, T. Micro-fabricated packed gas chromatography column based on laser etching technology. J. Chromatogr. A 2016, 1429, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kohl, D. The role of noble metals in the chemistry of solid state gas sensors. Sens. Actuators B. Chem. 1990, 21, 158–165. [Google Scholar] [CrossRef]



| Average Response | MOS Sensor (Coated with SnO/SnO2) | MOS Sensor (Coated with SnO2 Only) |
|---|---|---|
| The average response of benzene (mV) | 290.0 | 165.0 |
| The average response of CO (mV) | 120.0 | 75.0 |
| The average response of SO2 (mV) | 85.0 | 48.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Geng, Z.; Xue, N.; Liu, C.; Ma, T. A Mini-System Integrated with Metal-Oxide-Semiconductor Sensor and Micro-Packed Gas Chromatographic Column. Micromachines 2018, 9, 408. https://doi.org/10.3390/mi9080408
Sun J, Geng Z, Xue N, Liu C, Ma T. A Mini-System Integrated with Metal-Oxide-Semiconductor Sensor and Micro-Packed Gas Chromatographic Column. Micromachines. 2018; 9(8):408. https://doi.org/10.3390/mi9080408
Chicago/Turabian StyleSun, Jianhai, Zhaoxin Geng, Ning Xue, Chunxiu Liu, and Tianjun Ma. 2018. "A Mini-System Integrated with Metal-Oxide-Semiconductor Sensor and Micro-Packed Gas Chromatographic Column" Micromachines 9, no. 8: 408. https://doi.org/10.3390/mi9080408
APA StyleSun, J., Geng, Z., Xue, N., Liu, C., & Ma, T. (2018). A Mini-System Integrated with Metal-Oxide-Semiconductor Sensor and Micro-Packed Gas Chromatographic Column. Micromachines, 9(8), 408. https://doi.org/10.3390/mi9080408






