Glancing Angle Deposition Effect on Structure and Light-Induced Wettability of RF-Sputtered TiO2 Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. RF Reactive Magnetron Sputtering Depositions
2.2. TiO2 Thin Films Characterization
3. Results and Discussion
3.1. Effect of the Glancing Angle Deposition on TiO2 Structure and Morphology
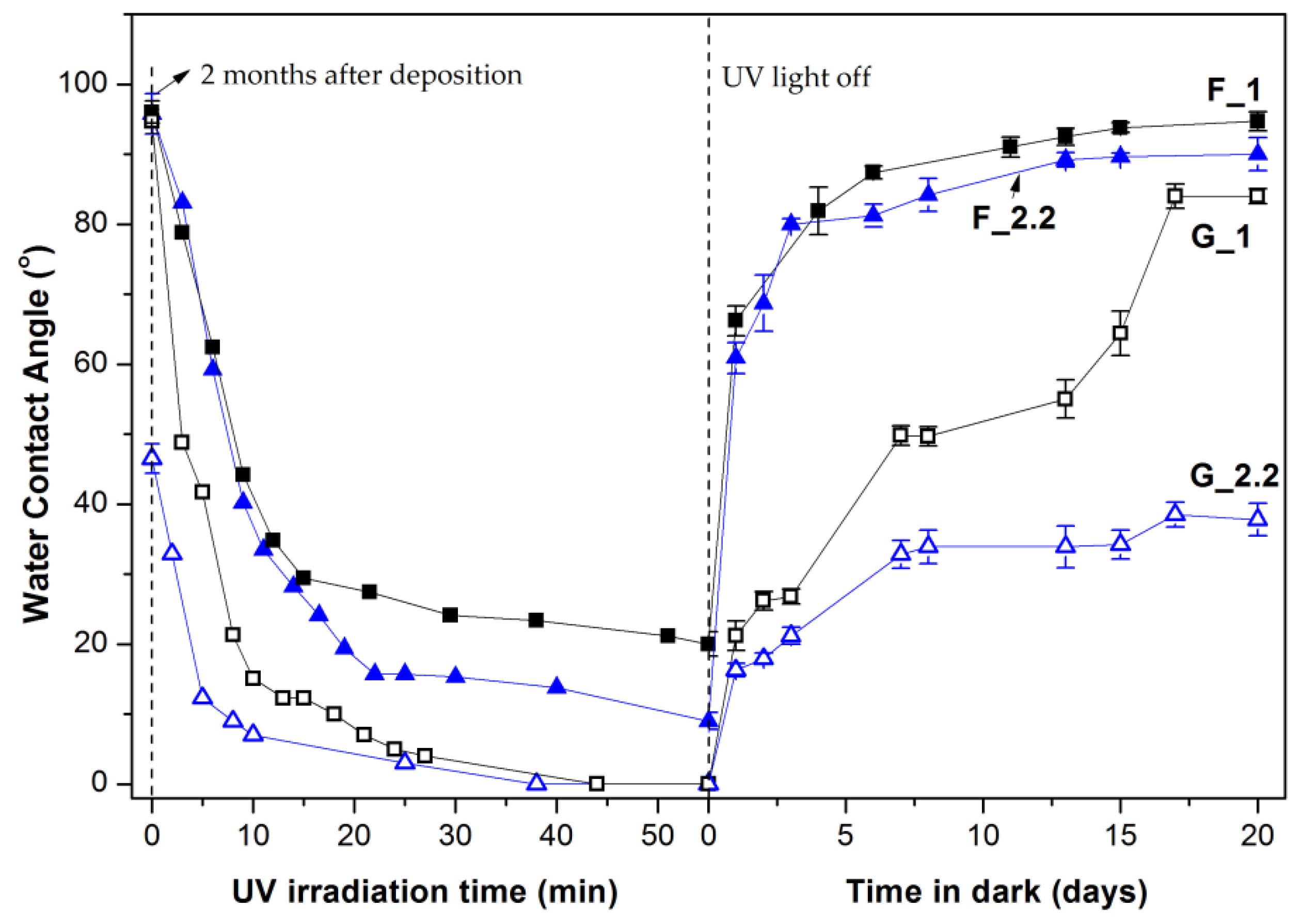
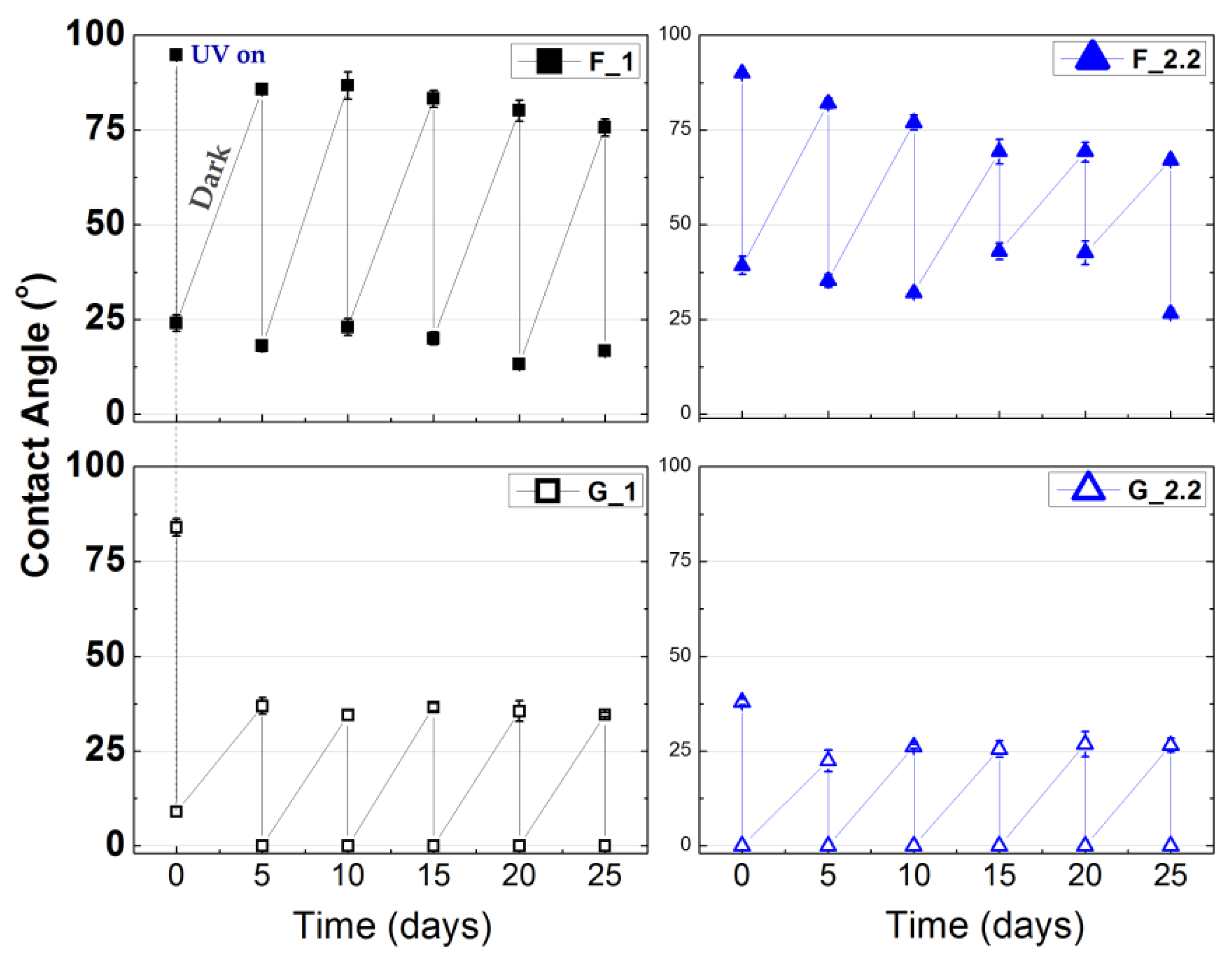
3.2. Effect of Glancing Angle Deposition on Wetting Properties of the TiO2 Thin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dervaux, J.; Cormier, P.-A.; Konstantinidis, S.; Di Ciuccio, R.; Coulembier, O.; Dubois, P.; Snyders, R. Deposition of porous titanium oxide thin films as anode material for dye sensitized solar cells. Vacuum 2015, 114, 213–220. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinés, R.; Lévy, F. TiO2 anatase thin films as gas sensors. Sens. Actuators B Chem. 1995, 26, 71–75. [Google Scholar] [CrossRef]
- Miyauchi, M.; Kieda, N.; Hishita, S.; Mitsuhashi, T.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Reversible wettability control of TiO2 surface by light irradiation. Surf. Sci. 2002, 511, 401–407. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Phase, D.; Sathe, V.; Rodríguez-Carvajal, J.; Choudhary, R.J.; Kulkarni, S.K. Relation between crystallinity and chemical nature of surface on wettability: A study on pulsed laser deposited TiO2 thin films. J. Appl. Phys. 2011, 109, 123512. [Google Scholar] [CrossRef]
- Borras, A.; González-Elipe, A.R. Wetting Properties of Polycrystalline TiO2 Surfaces: A Scaling Approach to the Roughness Factors. Langmuir 2010, 26, 15875–15882. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qi, H.; Chai, Y.; Li, M.; Guo, M.; Pan, M.; Wang, H.; Cui, Y.; Shao, J. Alteration of titanium dioxide material properties by glancing angle deposition plus annealing treatment. Superlattices Microstruct. 2016, 90, 87–95. [Google Scholar] [CrossRef]
- Vrakatseli, V.E.; Pagonis, E.; Amanatides, E.; Mataras, D. Photoinduced superhydrophilicity of amorphous TiOx-like thin films by a simple room temperature sol-gel deposition and atmospheric plasma jet treatment. J. Phys. Conf. Ser. 2014, 550, 012034. [Google Scholar] [CrossRef] [Green Version]
- Musil, J.; Heřman, D.; Šícha, J. Low-temperature sputtering of crystalline TiO2 films. J. Vac. Sci. Technol. Vac. Surf. Films 2006, 24, 521. [Google Scholar] [CrossRef]
- Vrakatseli, V.E.; Amanatides, E.; Mataras, D. Comparative study of RF reactive magnetron sputtering and sol-gel deposition of UV induced superhydrophilic TiOx thin films. J. Phys. Conf. Ser. 2016, 700, 012039. [Google Scholar] [CrossRef] [Green Version]
- Naik, V.M.; Haddad, D.; Naik, R.; Benci, J.; Auner, G.W. Optical Properties of Anatase, Rutile and Amorphous Phases of TiO2 Thin Films Grown at Room Temperature by RF Magnetron Sputtering. MRS Online Proc. Lib. Arch. 2003, 755, 413–418. [Google Scholar] [CrossRef]
- Barnes, M.C.; Kumar, S.; Green, L.; Hwang, N.-M.; Gerson, A.R. The mechanism of low temperature deposition of crystalline anatase by reactive DC magnetron sputtering. Surf. Coat. Technol. 2005, 190, 321–330. [Google Scholar] [CrossRef]
- Hasan, M.M.; Haseeb, A.S.M.A.; Saidur, R.; Masjuki, H.H. Effects of Annealing Treatment on Optical Properties of Anatase TiO2 Thin Films. Int. J. Chem. Biol. Eng. 2008, 2, 92–96. [Google Scholar]
- Zeman, P.; Takabayashi, S. Effect of total and oxygen partial pressures on structure of photocatalytic TiO2 films sputtered on unheated substrate. Surf. Coat. Technol. 2002, 153, 93–99. [Google Scholar] [CrossRef]
- Okimura, K.; Maeda, N.; Shibata, A. Characteristics of rutile TiO2 films prepared by RF magnetron sputtering at a low temperature. Thin Solid Films 1996, 281–282, 427–430. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. TiO2 coatings obtained by reactive sputtering at room temperature: Physical properties as a function of the sputtering pressure and film thickness. Thin Solid Films 2017, 636, 193–199. [Google Scholar] [CrossRef]
- Mbarek, I.B.; Chaabouni, F.; Selmi, M.; Abaab, M.; Rezig, B. Effect of the substrate temperature on the properties of the RF sputtered TiO2 thin films. Phys. Status Solidi C 2010, 7, 2311–2315. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; González-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Yasuda, Y.; Kitahara, N.; Hoshi, Y. Glancing Angle Sputter-Deposition of Titanium Dioxide Films with Rotating Substrate Holder for Photocatalytic Application. Meet. Abstr. 2012, 2, 93. [Google Scholar]
- Pihosh, Y.; Turkevych, I.; Ye, J.; Goto, M.; Kasahara, A.; Kondo, M.; Tosa, M. Photocatalytic Properties of TiO2 Nanostructures Fabricated by Means of Glancing Angle Deposition and Anodization. J. Electrochem. Soc. 2009, 156, K160–K165. [Google Scholar] [CrossRef]
- Birgin, E.G.; Chambouleyron, I.; Martínez, J.M. Estimation of the Optical Constants and the Thickness of Thin Films Using Unconstrained Optimization. J. Comput. Phys. 1999, 151, 862–880. [Google Scholar] [CrossRef] [Green Version]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Alhomoudi, I.A.; Newaz, G. Residual stresses and Raman shift relation in anatase TiO2 thin film. Thin Solid Films 2009, 517, 4372–4378. [Google Scholar] [CrossRef]
- Matthews, A. The crystallization of anatase and rutile from amorphous titanium dioxide under hydrothermal conditions. Am. Mineral. 1976, 61, 419–424. [Google Scholar]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Kinetics of Crystallization and Crystal Growth of Nanocrystalline Anatase in Nanometer-Sized Amorphous Titania. Chem. Mater. 2002, 14, 4145–4154. [Google Scholar] [CrossRef]
- Zhou, Y.; Fichthorn, K.A. Microscopic View of Nucleation in the Anatase-to-Rutile Transformation. J. Phys. Chem. C 2012, 116, 8314–8321. [Google Scholar] [CrossRef]
- Satoh, N.; Nakashima, T.; Yamamoto, K. Metastability of anatase: Size dependent and irreversible anatase-rutile phase transition in atomic-level precise titania. Sci. Rep. 2013, 3, 1959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 2000, 15, 437–448. [Google Scholar] [CrossRef]
- Amores, J.M.G.; Escribano, V.S.; Busca, G. Anatase crystal growth and phase transformation to rutile in high-area TiO2, MoO3–TiO2 and other TiO2-supported oxide catalytic systems. J. Mater. Chem. 1995, 5, 1245–1249. [Google Scholar] [CrossRef]
- Lobl, P.; Huppertz, M.; Mergel, D. Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Films 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Thornton, J.A. Structure-Zone Models Of Thin Films. Int. Soc. Opt. Photonics 1988, 0821, 95–106. [Google Scholar]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 2000, 11, 666. [Google Scholar] [CrossRef]
- Windischmann, H. Intrinsic stress in sputter-deposited thin films. Crit. Rev. Solid State Mater. Sci. 1992, 17, 547–596. [Google Scholar] [CrossRef]
- Wang, S.; Xia, G.; He, H.; Yi, K.; Shao, J.; Fan, Z. Structural and optical properties of nanostructured TiO2 thin films fabricated by glancing angle deposition. J. Alloys Compd. 2007, 431, 287–291. [Google Scholar] [CrossRef]
- González-Elipe, A.R.; Yubero, F.; Sanz, J.M. Low Energy Ion Assisted Film Growth; Imperial College Press: London, UK, 2003. [Google Scholar]
- Yoldas, B.E.; Partlow, D.P. Formation of broad band antireflective coatings on fused silica for high power laser applications. Thin Solid Films 1985, 129, 1–14. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantitative Evaluation of the Photoinduced Hydrophilic Conversion Properties of TiO2 Thin Film Surfaces by the Reciprocal of Contact Angle. J. Phys. Chem. B 2003, 107, 1028–1035. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Barabási, A.-L.; Stanley, H.E. Fractal Concepts in Surface Growth; Cambridge University Press: Cambridge, UK, 1995; p. 386. [Google Scholar]
- Morgan, B.J.; Watson, G.W. Intrinsic n-type Defect Formation in TiO2: A Comparison of Rutile and Anatase from GGA+U Calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Miyamura, A.; Kaneda, K.; Sato, Y.; Shigesato, Y. Effects of internal stress on photocatalytic properties of TiO2 films. Thin Solid Films 2008, 516, 4603–4608. [Google Scholar] [CrossRef]
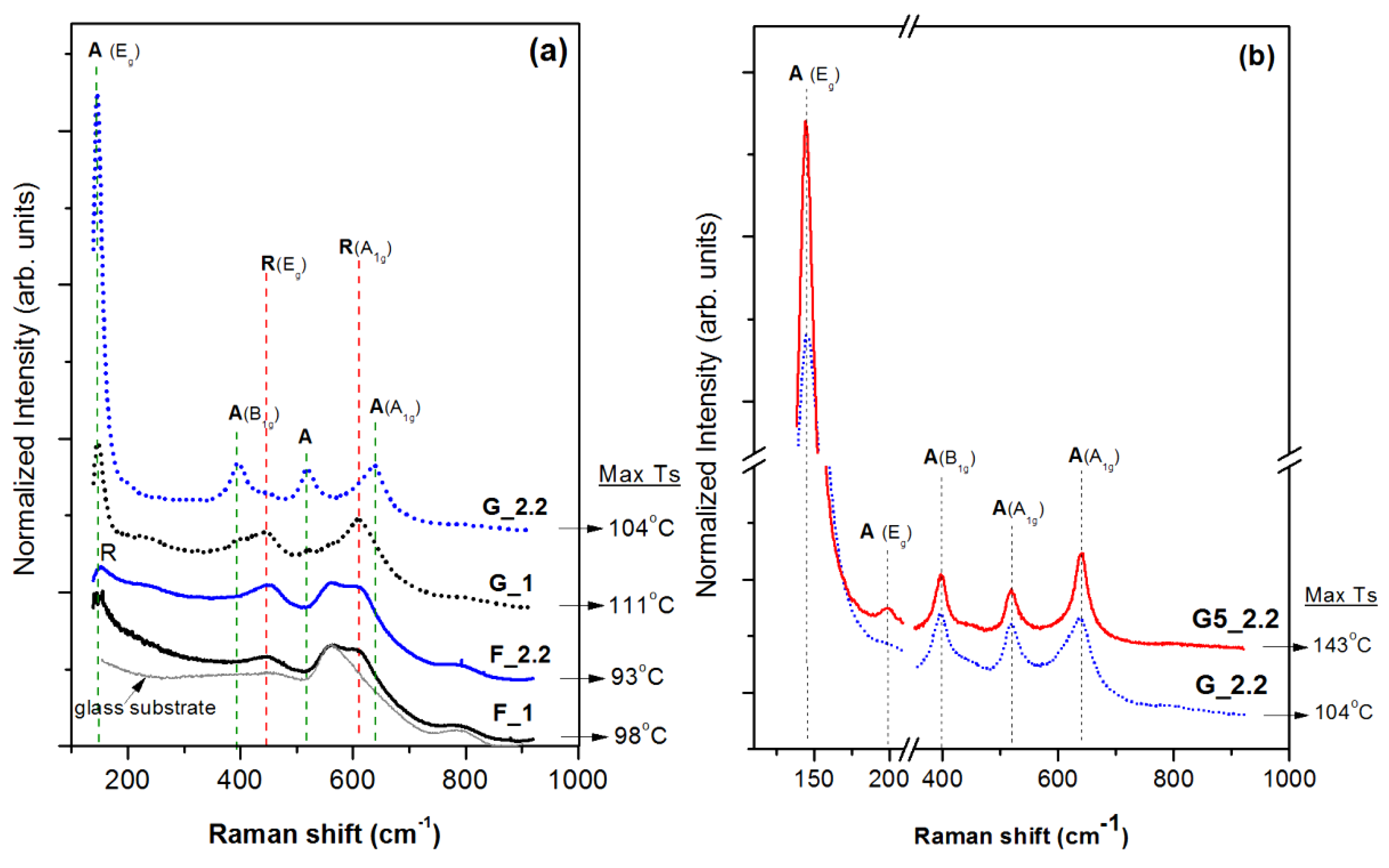
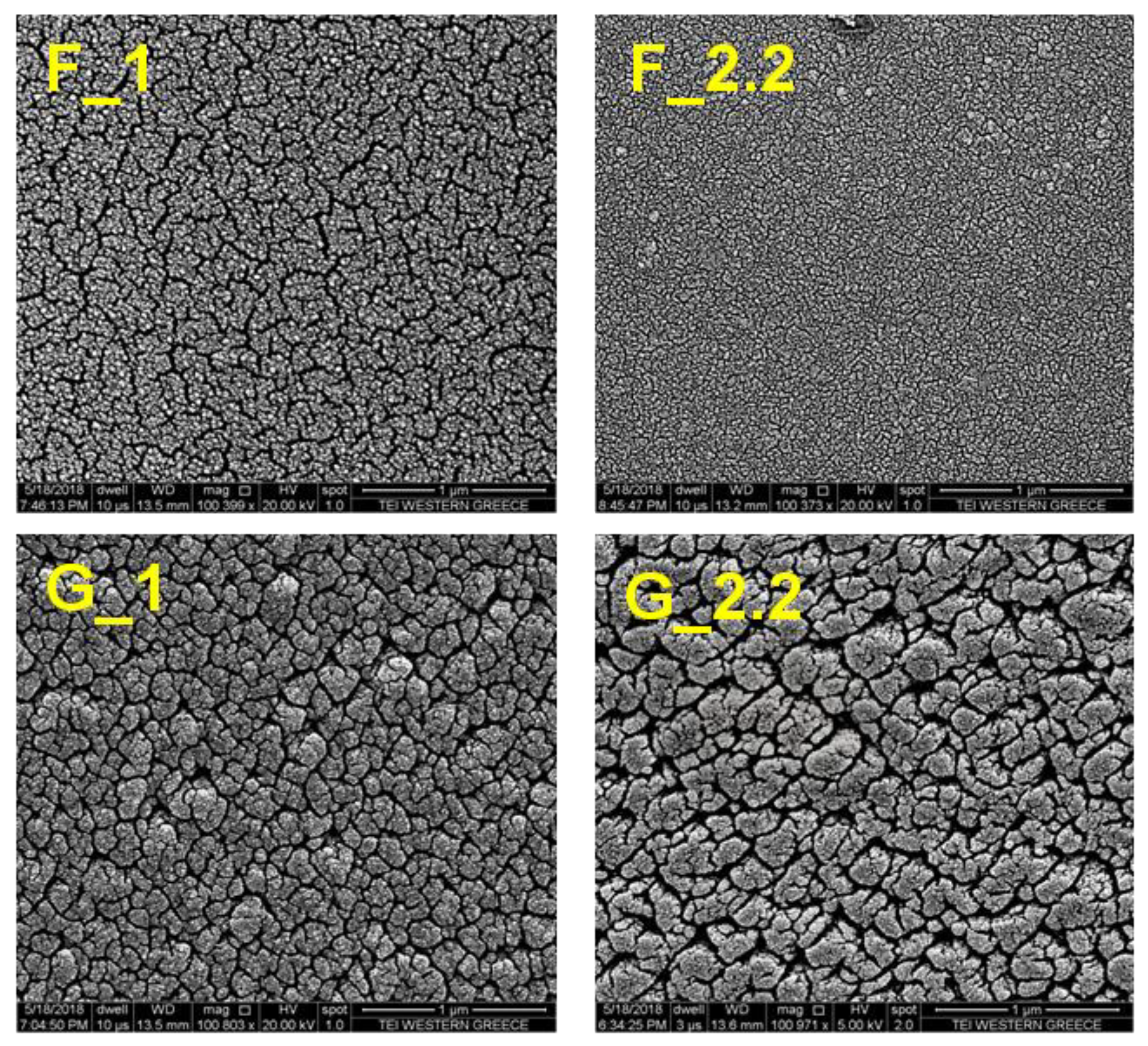
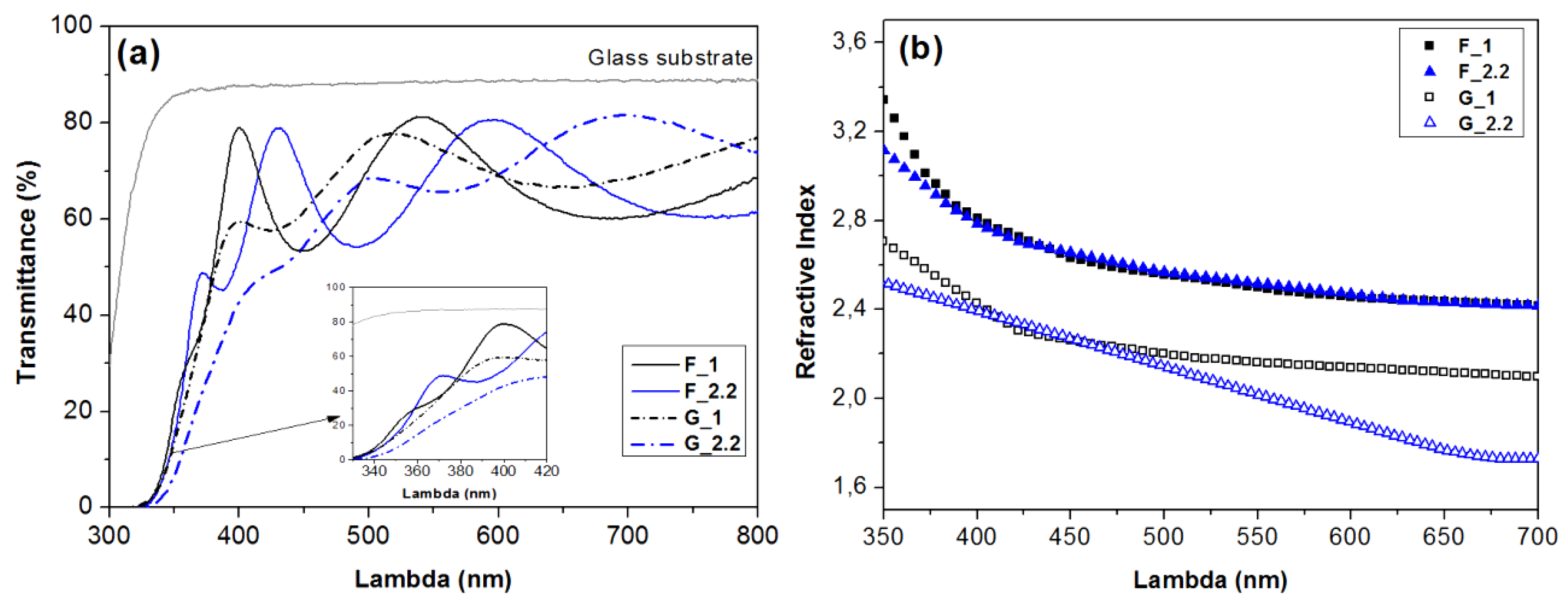


| Substrate Configuration | Sample | Target-to-Substrate Distance (cm) | Pressure (mTorr) | Dep. Rate (nm/min) | SbstrTmax (°C) | Film Thickness (±5 nm) | Crystalline Phase | Porosity (%) |
|---|---|---|---|---|---|---|---|---|
| Normal a = 0° | F_1 | 10 | 1 | 3.7 | 98 | 240 | Rutile | 19 |
| F_2.2 | 10 | 2.2 | 3.67 | 93 | 210 | Rutile | 20 | |
| GLAD a = 87° | G_1 | 10 | 1 | 0.65 | 111 | 240 | Rutile/Anatase | 34 |
| G_2.2 | 10 | 2.2 | 0.75 | 104 | 225 | Anatase | 52.6 | |
| G5_2.2 | 5 | 2.2 | 2.24 | 143 | 245 | Anatase | n.a. |
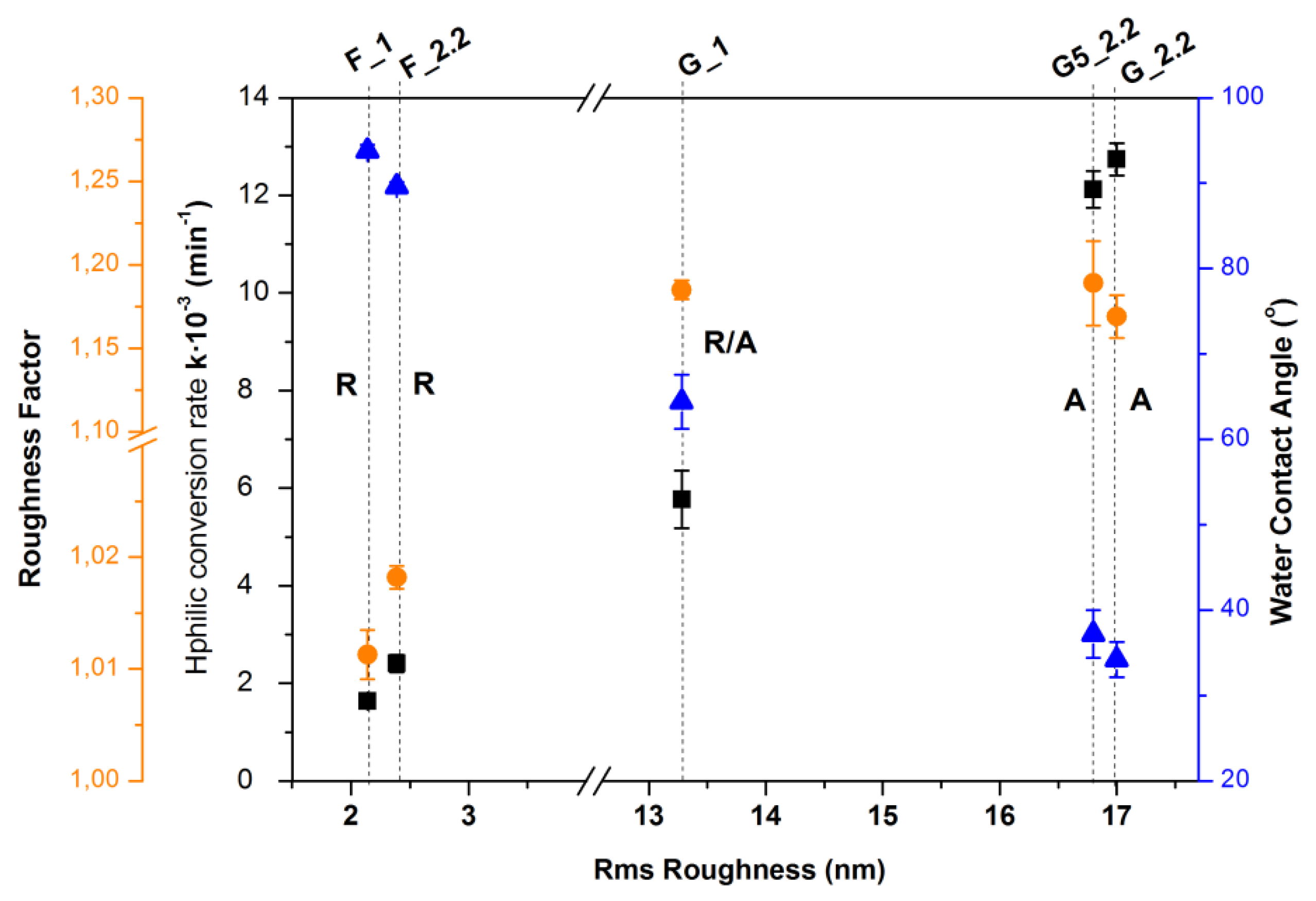
| Sample | Crystalline Phase | RMS Roughness (nm) | Roughness Factor | Hydrophilic Conversion Rate k × 10−3 (min−1) | WCA after 15 Days in Dark (Degrees) |
|---|---|---|---|---|---|
| F_1 | Rutile | 2.14 | 1.0113 | 1.64 | 93.8 |
| F_2.2 | Rutile | 2.39 | 1.0182 | 2.41 | 89.6 |
| G_1 | Rutile/Anatase | 13.28 | 1.185 | 5.77 | 64.4 |
| G5_2.2 | Anatase | 16.81 | 1.189 | 12.12 | 37.2 |
| G_2.2 | Anatase | 17.00 | 1.169 | 12.74 | 34.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrakatseli, V.E.; Kalarakis, A.N.; Kalampounias, A.G.; Amanatides, E.K.; Mataras, D.S. Glancing Angle Deposition Effect on Structure and Light-Induced Wettability of RF-Sputtered TiO2 Thin Films. Micromachines 2018, 9, 389. https://doi.org/10.3390/mi9080389
Vrakatseli VE, Kalarakis AN, Kalampounias AG, Amanatides EK, Mataras DS. Glancing Angle Deposition Effect on Structure and Light-Induced Wettability of RF-Sputtered TiO2 Thin Films. Micromachines. 2018; 9(8):389. https://doi.org/10.3390/mi9080389
Chicago/Turabian StyleVrakatseli, Vasiliki E., Alexandros N. Kalarakis, Angelos G. Kalampounias, Eleftherios K. Amanatides, and Dimitrios S. Mataras. 2018. "Glancing Angle Deposition Effect on Structure and Light-Induced Wettability of RF-Sputtered TiO2 Thin Films" Micromachines 9, no. 8: 389. https://doi.org/10.3390/mi9080389




