Fabrication of a Metal Micro Mold by Using Pulse Micro Electroforming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Methods
2.2.1. Preparation of the SU-8 Mold
2.2.2. Micro Electroforming Process
3. Results and Discussion
3.1. Effect of Additive on the Surface Quality of the Electroforming Deposit
3.2. Effect of Chemical Etching on the Bonding Strength
3.3. Parameters Optimization for Improving the Surface Roughness of Metal Micro Molds
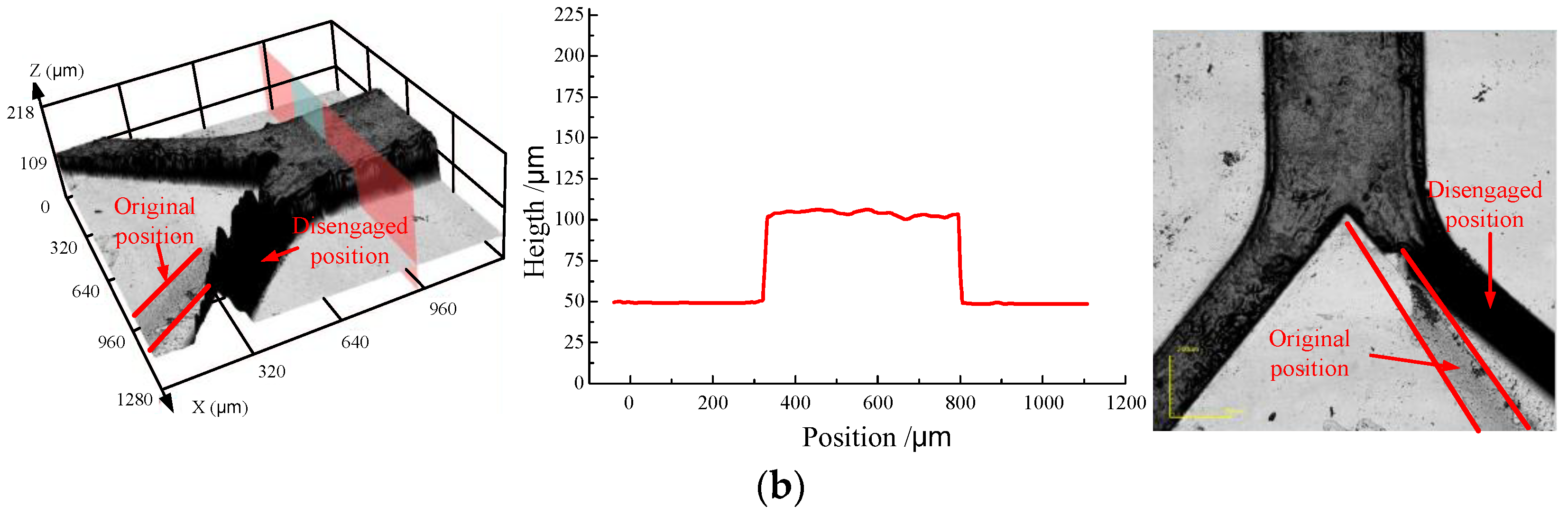
3.4. Hot Embossing Experiment with the Micro Metal Mold
- The PMMA substrate with a thickness of 2 mm was placed in the system and heated in vacuum to a temperature of 110 °C, which was above the glass transition temperature (Tg = 104 °C)
- The micro metal mold was also heated to the same temperature
- The micro structure was pressed into the PMMA substrate with a pressure of 0.5 MPa, and the holding time was about 10 min
- Metal mold and PMMA substrate were isothermally cooled to the temperature of 50 °C and then separated (demolding).
4. Conclusions
- For electroforming micro molds, the additive was beneficial for removing the pits and improving the surface quality of the deposit. NaC12H25SO3 with a concentration of 0.08 g/L was used as the additive.
- The pretreatment of substrate with chemical etching was helpful for enhancing the bonding strength between the metal micro mold and substrate. The adhesion strength reached 325 N, and it was about 2.6 times stronger than substrate without chemical etching pretreatment.
- By using orthogonal experiments, the parameters with a pulse frequency of 4 kHz, pulse duty cycle of 20%, current density of 4 A/dm2 and a temperature of 46 °C were optimized for electroforming a micro mold with good surface quality. Also, metal micro molds with different structures were well prepared with the minimum roughness of 0.56 μm.
- The embossing experiment also indicated that by using the method proposed in this paper, there was no deposited micro structure falling off from the metal substrate after the embossing experiment, and the quality of micro metal mold was much improved.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron. Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Zhang, S.J.; Shin, Y.C. Effective methods for fabricating trapezoidal shape microchannel of arbitrary dimensions on polymethyl methacrylate (PMMA) substrate by a CO2 laser. Int. J. Adv. Manuf. Technol. 2017, 93, 1079–1094. [Google Scholar] [CrossRef]
- Su, Q.; Xu, J.; Wang, C.; Shan, D.; Guo, B. The fabrication of micro-array channels with the ultrafine-grained LZ91 Mg-Li alloy by micro-embossing. Micromachines 2018, 9, 55. [Google Scholar] [CrossRef]
- Shan, C.; Chen, F.; Yang, Q.; Jiang, Z.; Hou, X. 3D multi-microchannel helical mixer fabricated by femtosecond laser inside fused silica. Micromachines 2018, 9, 29. [Google Scholar] [CrossRef]
- Gardeniers, J.G.E.; Oosterbroek, R.; Berg, A.V.D. Silicon and glass micromachining for μTAS. Lab Chip 2003, 37–64. [Google Scholar] [CrossRef]
- Hong, T.F.; Ju, W.J.; Wu, M.C.; Tai, C.H.; Tsai, C.H.; Fu, L.M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid Nanofluid 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Pan, C.W.; Lee, W.C.; Li, K.M. An experimental study of micromilling parameters to manufacture microchannels on a PMMA substrate. Int. J. Adv. Manuf. Technol. 2014, 71, 1623–1630. [Google Scholar] [CrossRef]
- Xu, B.Y.; Yan, X.N.; Zhang, J.D.; Xu, J.J.; Chen, H.Y. Glass etching to bridge micro- and nanofluidics. Lab. Chip 2012, 12, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Chen, P.C.; You, B.H.; Kim, N.; Park, T.; Lee, T.Y.; Datta, P.; Desta, Y.; Soper, S.A.; Nikitopoulos, D.E.; et al. Titer-plate formatted continuous flow thermal reactors for high through put applications: Fabrication and test. J. Micromech. Microeng. 2010, 20, 055003. [Google Scholar] [CrossRef]
- Juang, Y.J.; Lee, L.J.; Koelling, K.W. Rheological characterization and process analysis. Polym. Eng. Sci. 2002, 42, 551–566. [Google Scholar] [CrossRef]
- Rowland, H.D.; King, W.P. Polymer deformation and filling modes during microembossing. J. Micromech. Microeng. 2004, 14, 1625–1632. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xu, X. Excimer laser fabrication of polymer microfluidic devices. J. Laser Appl. 2003, 15, 255–260. [Google Scholar] [CrossRef]
- Jensen, M.F.; McCormack, J.E.; Helbo, B.; Christensen, L.H.; Christensen, T.R.; Geschke, O. Rapid prototyping of polymer microsystems via excimer laser ablation of polymeric moulds. Lab Chip 2004, 4, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, R.K.; Bouwstra, S. Electroplated compliant metal microactuators with small feature sizes using a removable SU-8 mold. Microsyst. Technol. 2000, 6, 214–217. [Google Scholar] [CrossRef]
- Steigert, J.; Haeberle, S.; Brenner, T.; Müller, C.; Steinert, C.P.; Koltay, P.; Gottschlich, N.; Reinecke, H.; Rühe, J.; Zengerle, R.; et al. Rapid prototyping of microfluidic chips in COC. J. Micromech. Microeng. 2007, 17, 333–341. [Google Scholar] [CrossRef]
- Galhotra, V.; Sangishetty, V.; Ma, E.; Kelly, K.W. Electroplated nickel mold insert for LIGA. Proc. SPIE 1995, 2639, 158–163. [Google Scholar]
- Guan, X.J.; Zhong, M.E.; Xiao, Y.K. Study on the morphology and corrosion resistance of the electrodeposition of PD Co alloy. Mater. Prot. 2007, 40, 1–3. [Google Scholar]
- Zhang, Y.B.; Gao, X.L.; Wang, D.Z.; Liu, H.D.; Li, C.R. Analysis of mechanism and dynamics for pulse electrodeposition and its verification. Mater. Prot. 2011, 6, 18–21. [Google Scholar]

















| Composition and Process Condition | Value |
|---|---|
| Pulse duty cycle | 20%, 40%, 60% |
| Frequency (kHz) | 2, 3, 4 |
| Ni(NH2·SO3)2·6H2O (g/L) | 400 |
| H3BO3 (g/L) | 35 |
| NiCl2 (g/L) | 15 |
| NaC12H25SO3 (g/L) | 0.08 |
| pH | 3.8 |
| Temperature (°C) | 45 |
| Parameter Factors | Parameter Levels | ||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |
| A: Pulse frequency (kHz) | 4 | 3 | 2 |
| B: Pulse duty cycle (%) | 60 | 40 | 20 |
| C: Current density (A/dm2) | 5 | 4 | 3 |
| D: Temperature (°C) | 50 | 48 | 46 |
| Exp. No. | A | B | C | D | Surface Roughness (Ra/μm) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 0.7 |
| 2 | 1 | 2 | 2 | 2 | 0.8 |
| 3 | 1 | 3 | 3 | 3 | 1.5 |
| 4 | 2 | 3 | 2 | 1 | 1 |
| 5 | 2 | 1 | 3 | 2 | 1.1 |
| 6 | 2 | 2 | 1 | 3 | 1.2 |
| 7 | 3 | 2 | 3 | 1 | 1.6 |
| 8 | 3 | 1 | 1 | 2 | 2.1 |
| 9 | 3 | 3 | 2 | 3 | 0.6 |
| k1 | 1 | 1.3 | 1.33 | 1.1 | - |
| k2 | 1.116 | 1.2 | 0.8 | 1.33 | - |
| k3 | 1.43 | 1.03 | 1.4 | 1.1 | - |
| Range | 0.43 | 0.27 | 0.6 | 0.23 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, L.; He, J.; Zuo, F.; Guo, Z. Fabrication of a Metal Micro Mold by Using Pulse Micro Electroforming. Micromachines 2018, 9, 203. https://doi.org/10.3390/mi9050203
Chen X, Liu L, He J, Zuo F, Guo Z. Fabrication of a Metal Micro Mold by Using Pulse Micro Electroforming. Micromachines. 2018; 9(5):203. https://doi.org/10.3390/mi9050203
Chicago/Turabian StyleChen, Xiaolei, Li Liu, Junfeng He, Fei Zuo, and Zhongning Guo. 2018. "Fabrication of a Metal Micro Mold by Using Pulse Micro Electroforming" Micromachines 9, no. 5: 203. https://doi.org/10.3390/mi9050203




