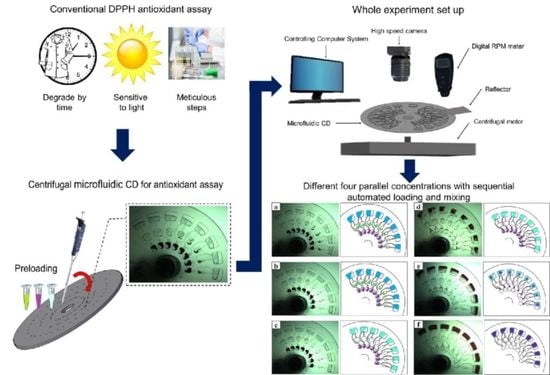
A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials Preparation
2.2. DPPH Conventional Antioxidant Activity Test
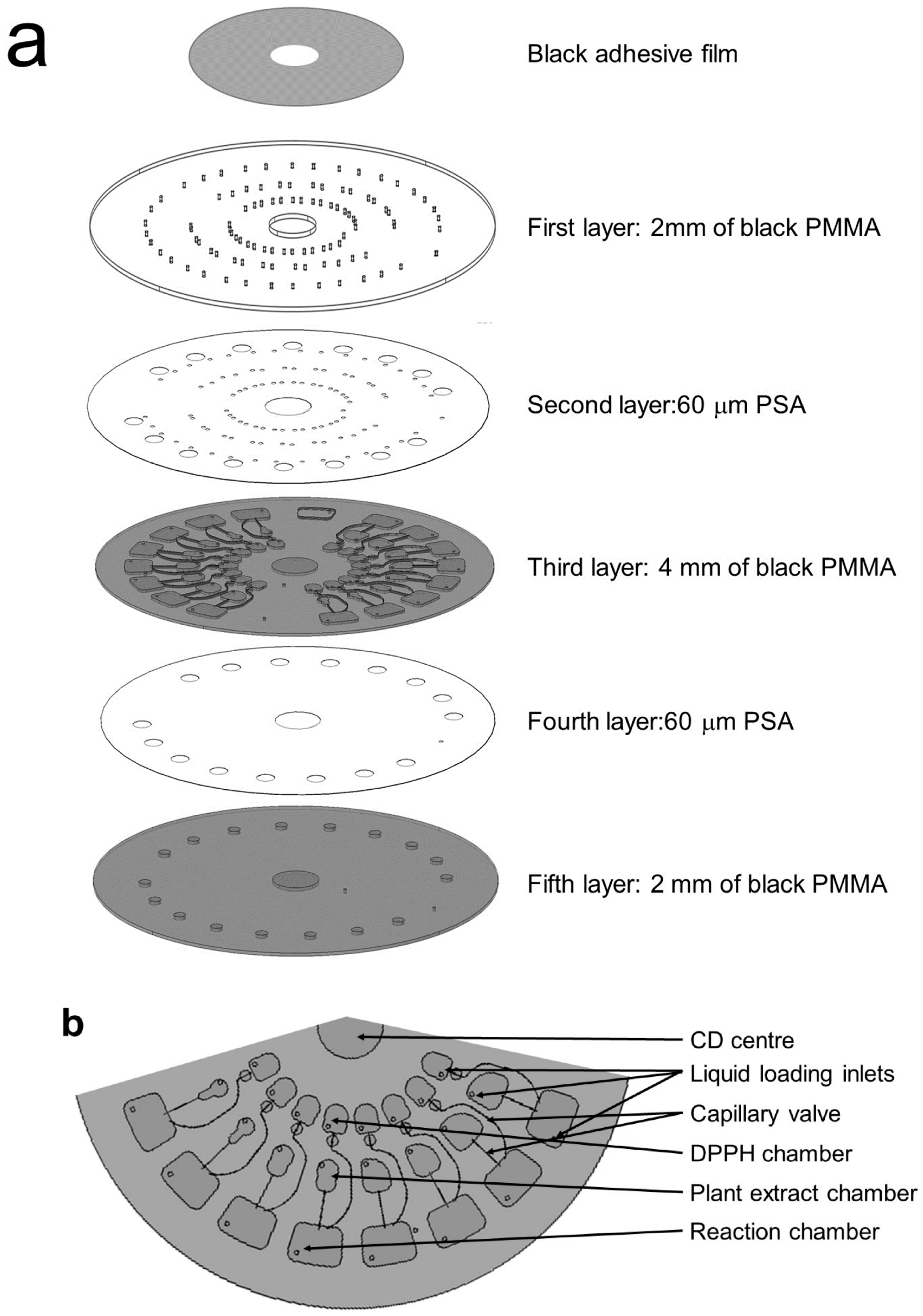
2.3. Microfluidic Compact Disc (CD) Design and Fabrication
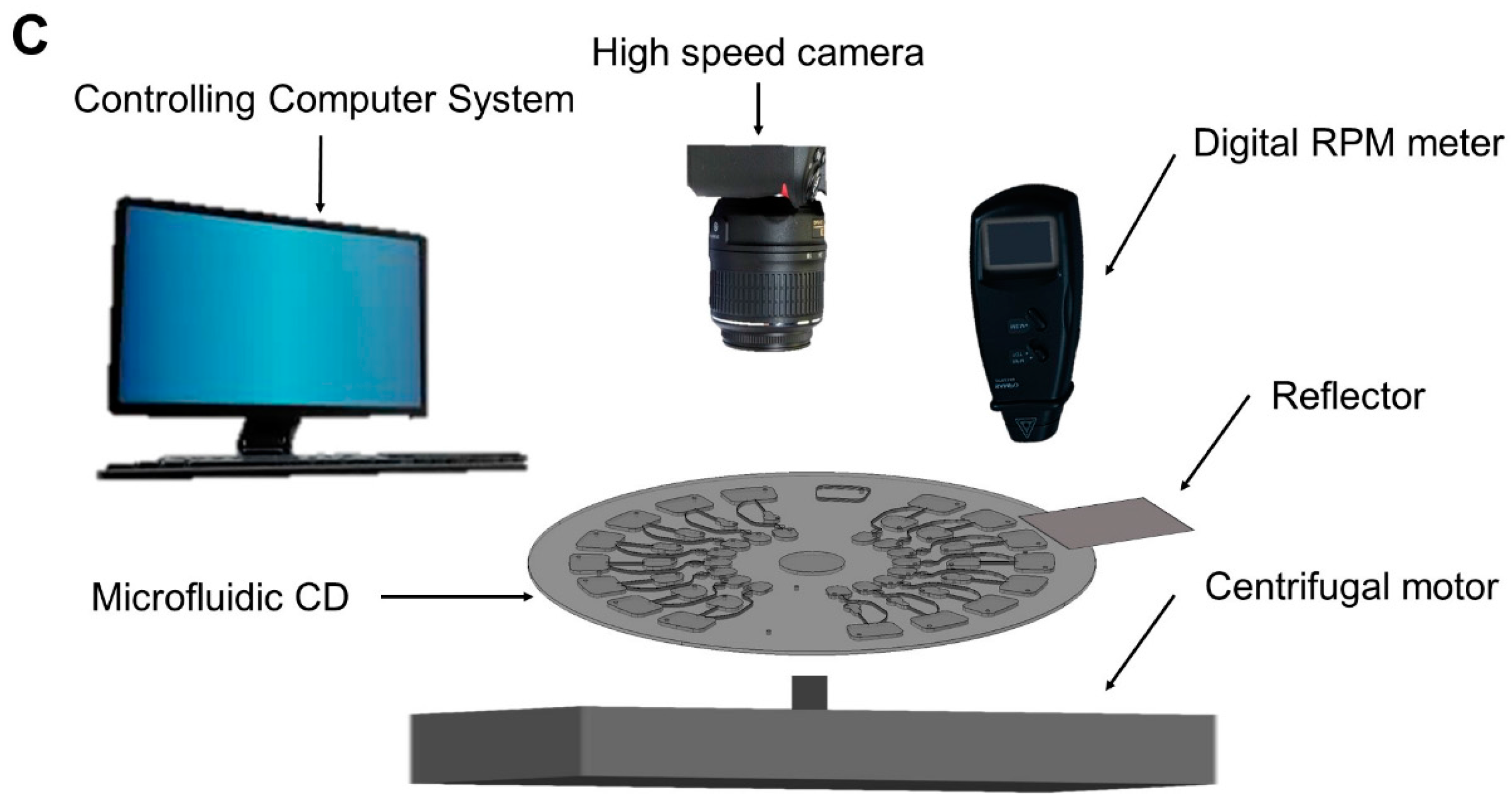
2.4. Integrated Microfluidic DPPH CD Operations
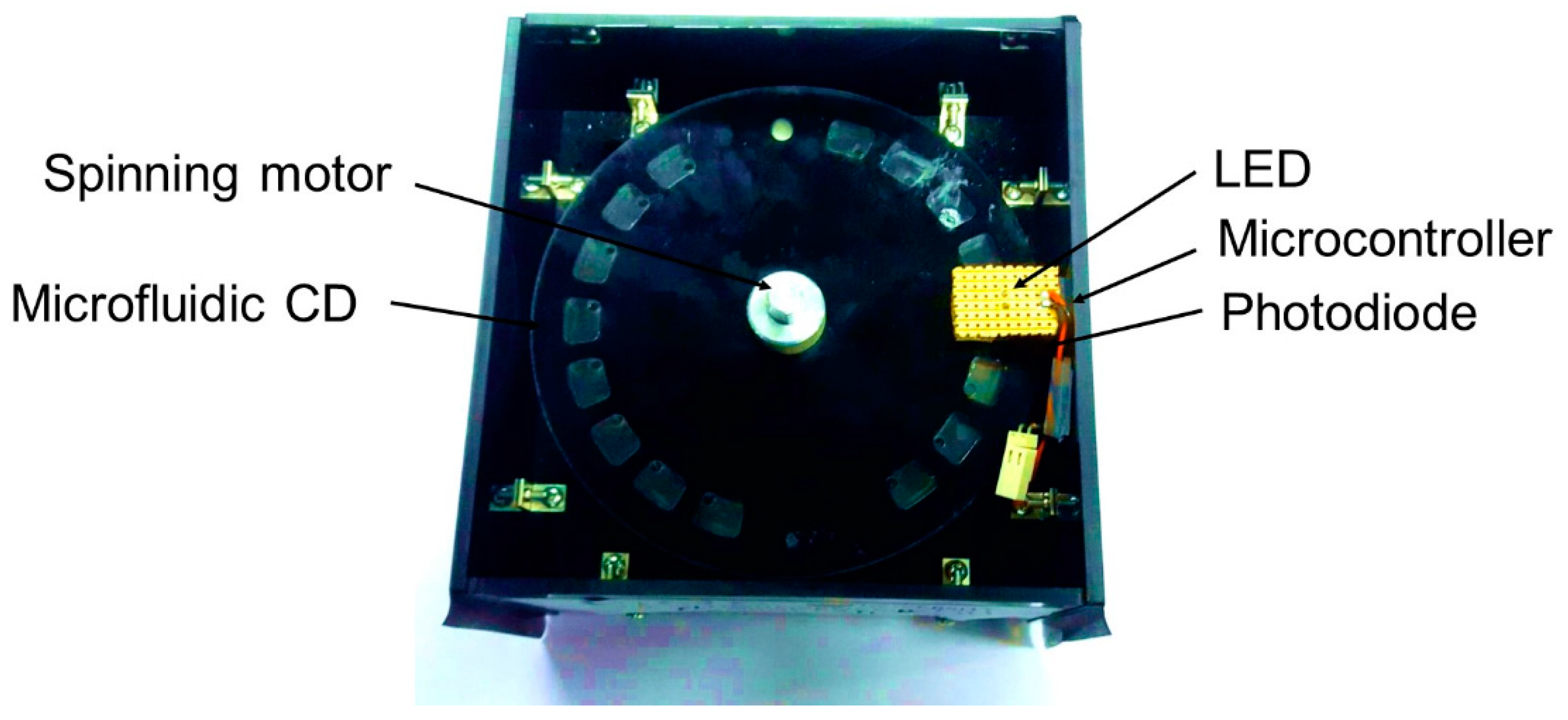
2.5. Absorbance Reading
3. Results and Discussion
3.1. Microfluidic CD Operations
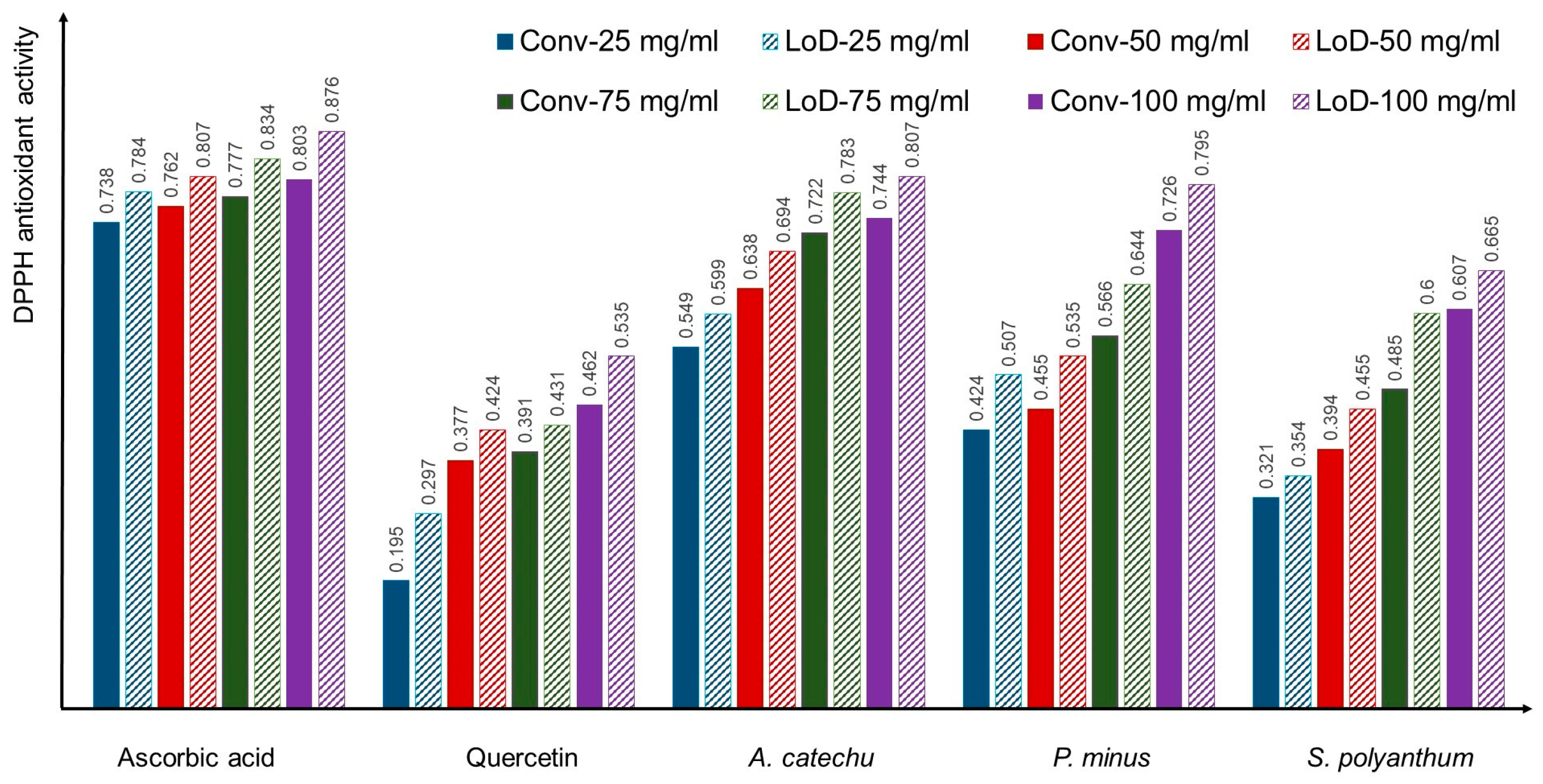
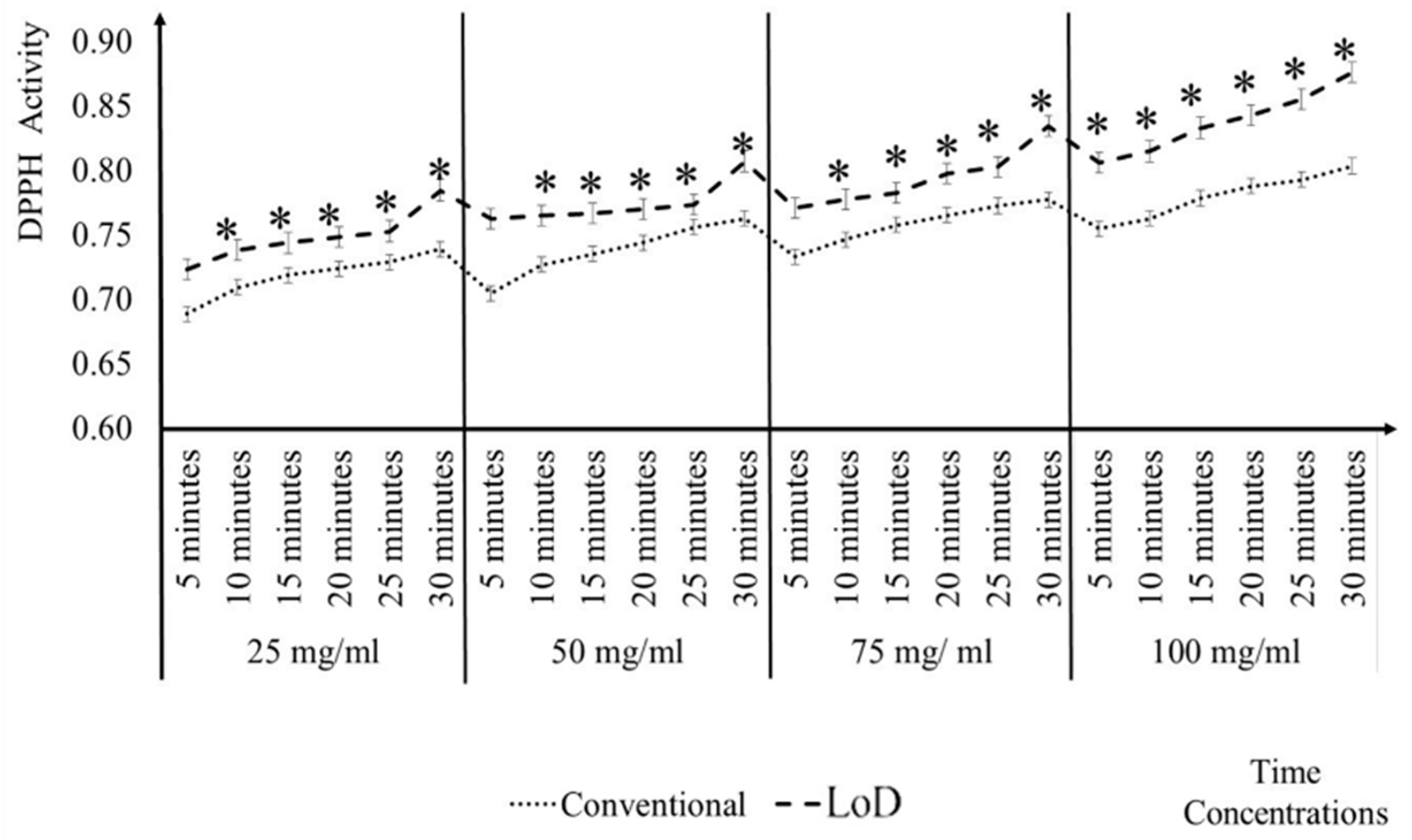
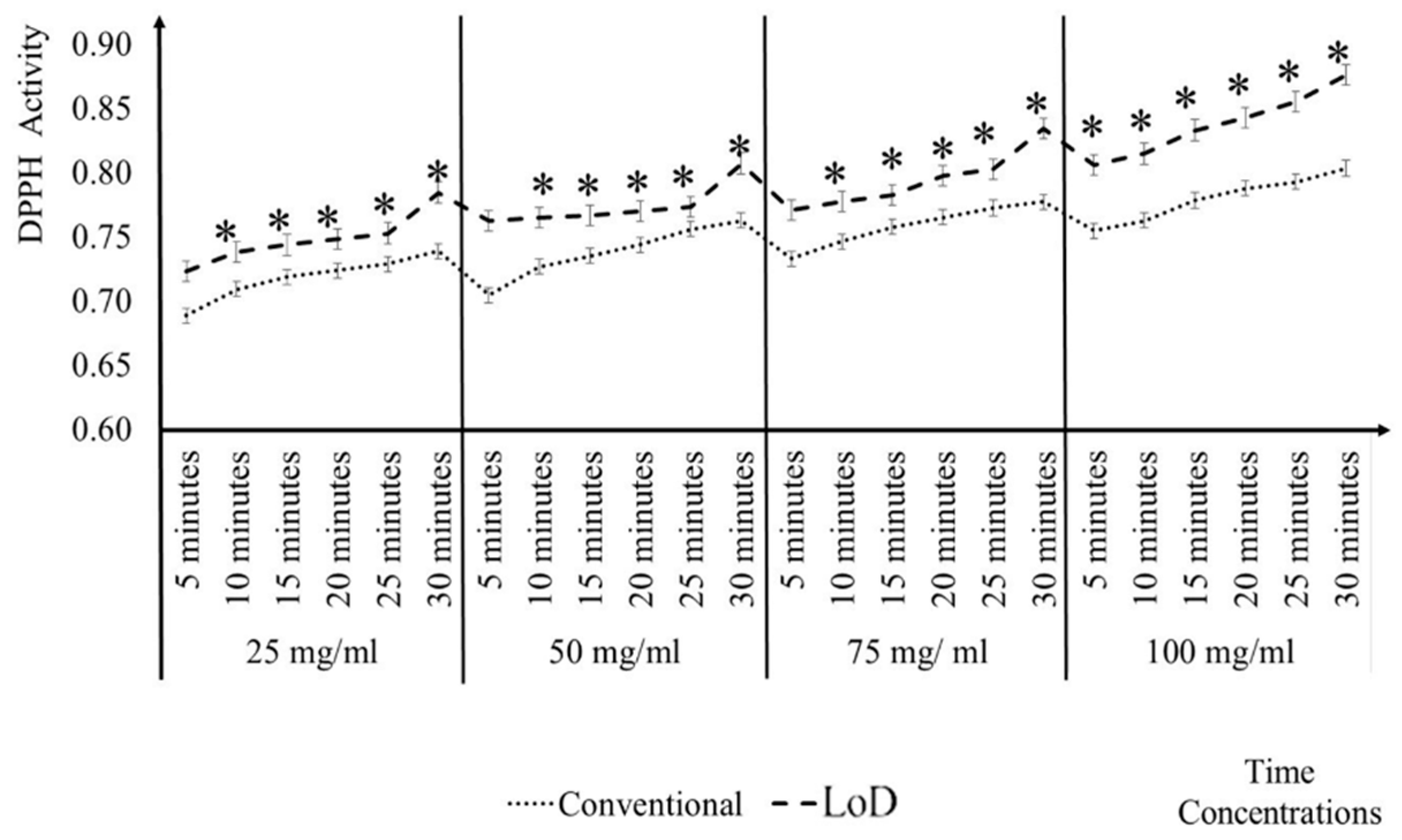
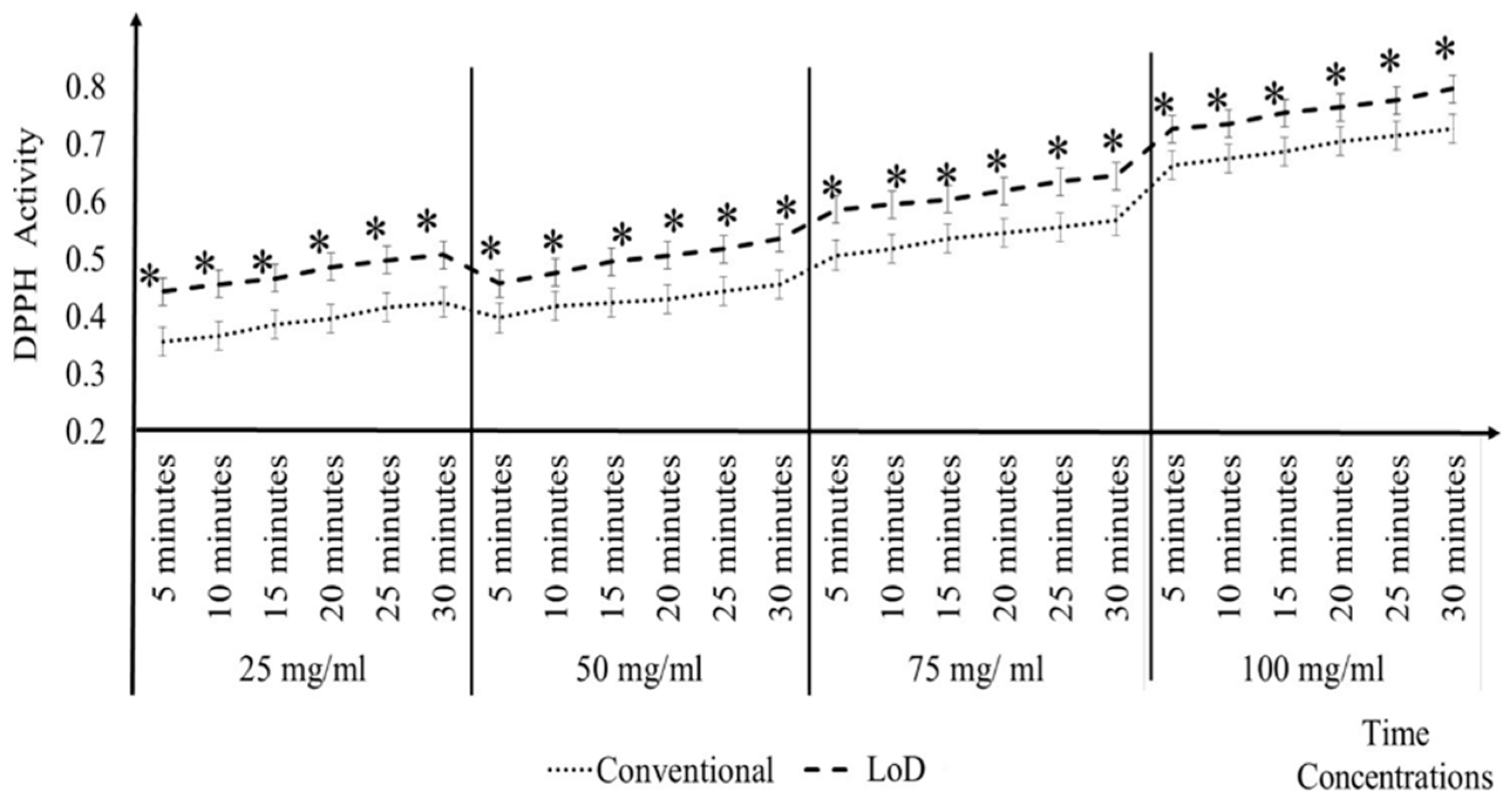
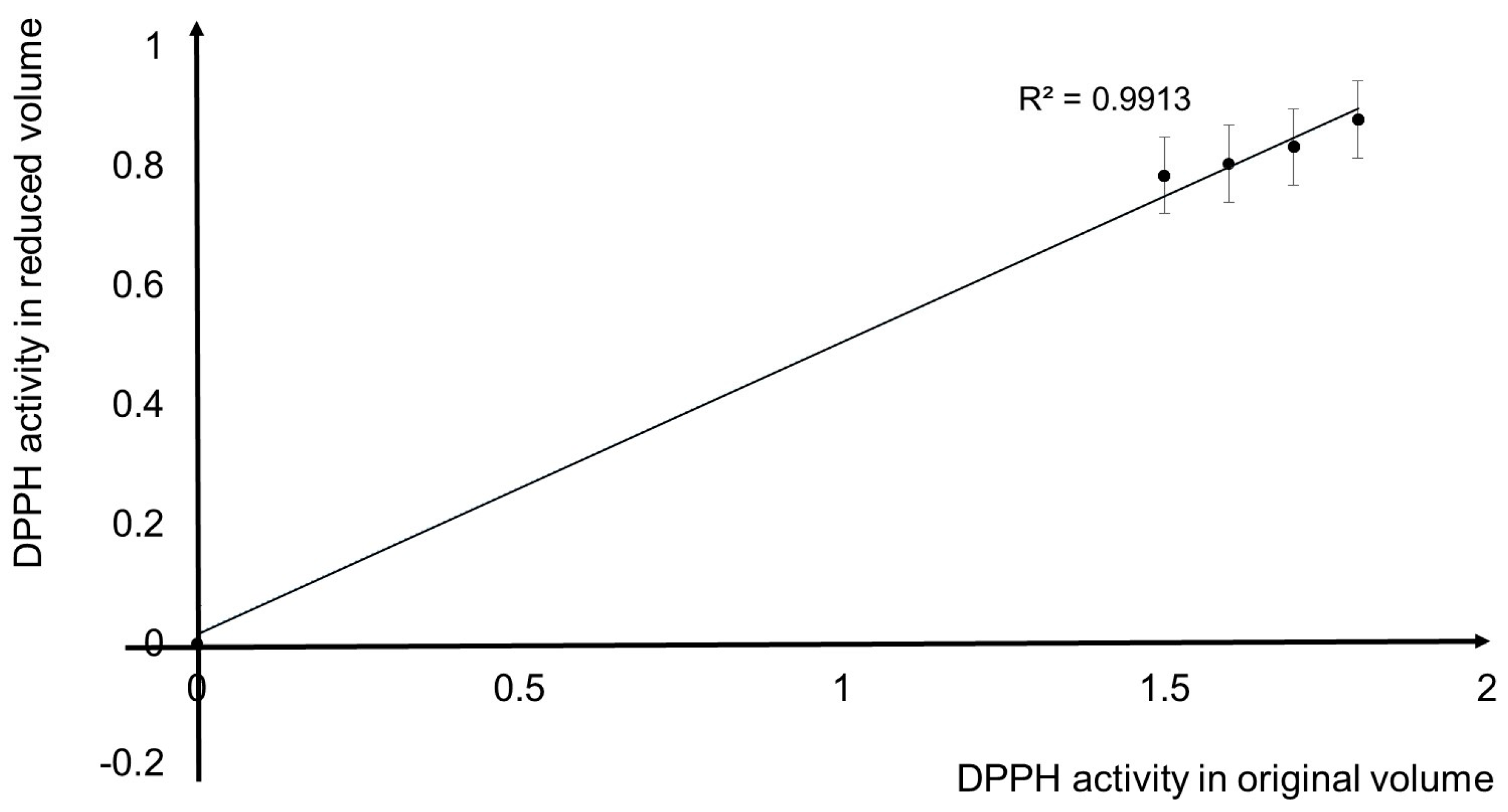
3.2. Comparisons of Conventional and LoD DPPH Antioxidant Activity Method
3.2.1. Ascorbic Acid
3.2.2. Quercetin
3.2.3. A. catechu
3.2.4. P. minus
3.2.5. S. polyanthum
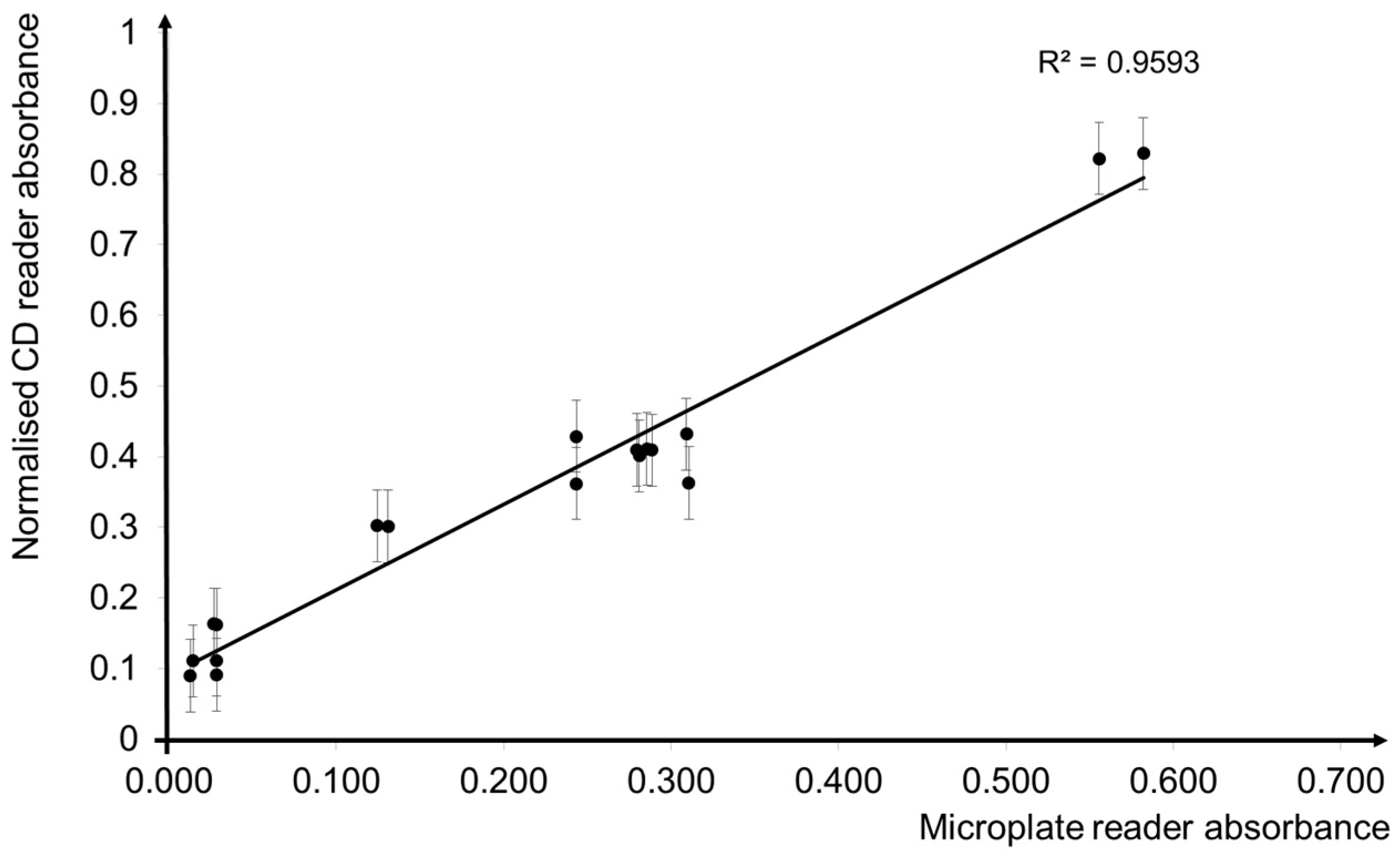
3.3. Comparisons of CD Reader and the Conventional Microplate Reader
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Ungvari, Z. Role of mitochondrial oxidative stress in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1417–H1427. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in parkinson’s disease. J. Parkinson Dis. 2013, 3, 461–491. [Google Scholar]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Vanessa Fiorentino, T.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Ashok, B.T.; Ali, R. The aging paradox: Free radical theory of aging. Exp. Gerontol. 1999, 34, 293–303. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.R.; Ha, S.K.; Choi, I.; Lee, S.H.; Kim, D.; Choi, N.; Sung, J.H. A microfluidic device for evaluating the dynamics of the metabolism-dependent antioxidant activity of nutrients. Lab Chip 2014, 14, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.J. Prospects for the use of antioxidant therapies. Drugs 1995, 49, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Radiance 2006, 5, 326–334. [Google Scholar]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Pekal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Deng, J.; Cheng, W.; Yang, G. A novel antioxidant activity index (aau) for natural products using the DPPH assay. Food Chem. 2011, 125, 1430–1435. [Google Scholar] [CrossRef]
- Rana, S.V. No Preanalytical Errors in Laboratory Testing: A Beneficial Aspect for Patients; Springer: Berlin, Germany, 2012. [Google Scholar]
- Hosseini, S.; Azari, P.; Aeinehvand, M.M.; Rothan, H.A.; Djordjevic, I.; Martinez-Chapa, S.O.; Madou, M.J. Intrant elisa: A novel approach to fabrication of electrospun fiber mat-assisted biosensor platforms and their integration within standard analytical well plates. Appl. Sci. 2016, 6, 336. [Google Scholar] [CrossRef]
- Sayad, A.A.; Ibrahim, F.; Uddin, S.M.; Pei, K.X.; Mohktar, M.S.; Madou, M.; Thong, K.L. A microfluidic lab-on-a-disc integrated loop mediated isothermal amplification for foodborne pathogen detection. Sens. Actuators B Chem. 2016, 227, 600–609. [Google Scholar] [CrossRef]
- Wang, X.; Amatatongchai, M.; Nacapricha, D.; Hofmann, O.; de Mello, J.C.; Bradley, D.D.; de Mello, A.J. Thin-film organic photodiodes for integrated on-chip chemiluminescence detection–application to antioxidant capacity screening. Sens. Actuators B Chem. 2009, 140, 643–648. [Google Scholar] [CrossRef]
- George, A.; Ng, C.P.; O’Callaghan, M.; Jensen, G.S.; Wong, H.J. In vitro and ex vivo cellular antioxidant protection and cognitive enhancing effects of an extract of polygonum minus huds (lineminus™) demonstrated in a barnes maze animal model for memory and learning. BMC Complement. Altern. Med. 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Antioxidant, anti-inflammatory, and chemoprotective properties of acacia catechu heartwood extracts. Phytother. Res. 2015, 29, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, K.; Krishnakumar, G.; Sooryaprakash, S. Phytochemical composition, antioxidant, and antibacterial activities of two Syzygium spp. J. Herbs Spices Med. Plants 2014, 20, 45–54. [Google Scholar] [CrossRef]
- Saha, M.R.; Dey, P.; Begum, S.; De, B.; Chaudhuri, T.K.; De Sarker, D.; Das, A.P.; Sen, A. Effect of acacia catechu (lf) willd. On oxidative stress with possible implications in alleviating selected cognitive disorders. PLoS ONE 2016, 11, e0150574. [Google Scholar] [CrossRef] [PubMed]
- Thio, T.H.G.; Soroori, S.; Ibrahim, F.; Al-Faqheri, W.; Soin, N.; Kulinsky, L.; Madou, M. Theoretical development and critical analysis of burst frequency equations for passive valves on centrifugal microfluidic platforms. Med. Biol. Eng. Comput. 2013, 51, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, A.; Ganesan, P.; Ibrahim, F.; He, S.; Madou, M.J. The effect of contact angles and capillary dimensions on the burst frequency of super hydrophilic and hydrophilic centrifugal microfluidic platforms, a CFD study. PLoS ONE 2013, 8, e73002. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tang, M.; Wang, G.; Kong, S.-K.; Ho, H.-P. A review of biomedical centrifugal microfluidic platforms. Micromachines 2016, 7, 26. [Google Scholar] [CrossRef]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. In Microfluidics Based Microsystems; Springer: Berlin, Germany, 2010; pp. 305–376. [Google Scholar]
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. BMJ 2005, 331, 903. [Google Scholar] [CrossRef] [PubMed]
- Phonchai, A.; Kim, Y.; Chantiwas, R.; Cho, Y.-K. Lab-on-a-disc for simultaneous determination of total phenolic content and antioxidant activity of beverage samples. Lab Chip 2016, 16, 3268–3275. [Google Scholar] [CrossRef] [PubMed]
- Papariello, G.; Janish, M. Diphenylpicrylhydrazyl as an organic analytical reagent in the spectrophotometric analysis of phenols. Anal. Chem. 1966, 38, 211–214. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Schaich, K.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.; Ibrahim, F.; Sayad, A.A.; Thiha, A.; Pei, K.X.; Mohktar, M.S.; Hashim, U.; Cho, J.; Thong, K.L. A portable automatic endpoint detection system for amplicons of loop mediated isothermal amplification on microfluidic compact disk platform. Sensors 2015, 15, 5376–5389. [Google Scholar] [CrossRef] [PubMed]
- Thiha, A.; Ibrahim, F. A colorimetric enzyme-linked immunosorbent assay (elisa) detection platform for a point-of-care dengue detection system on a lab-on-compact-disc. Sensors 2015, 15, 11431–11441. [Google Scholar] [CrossRef] [PubMed]
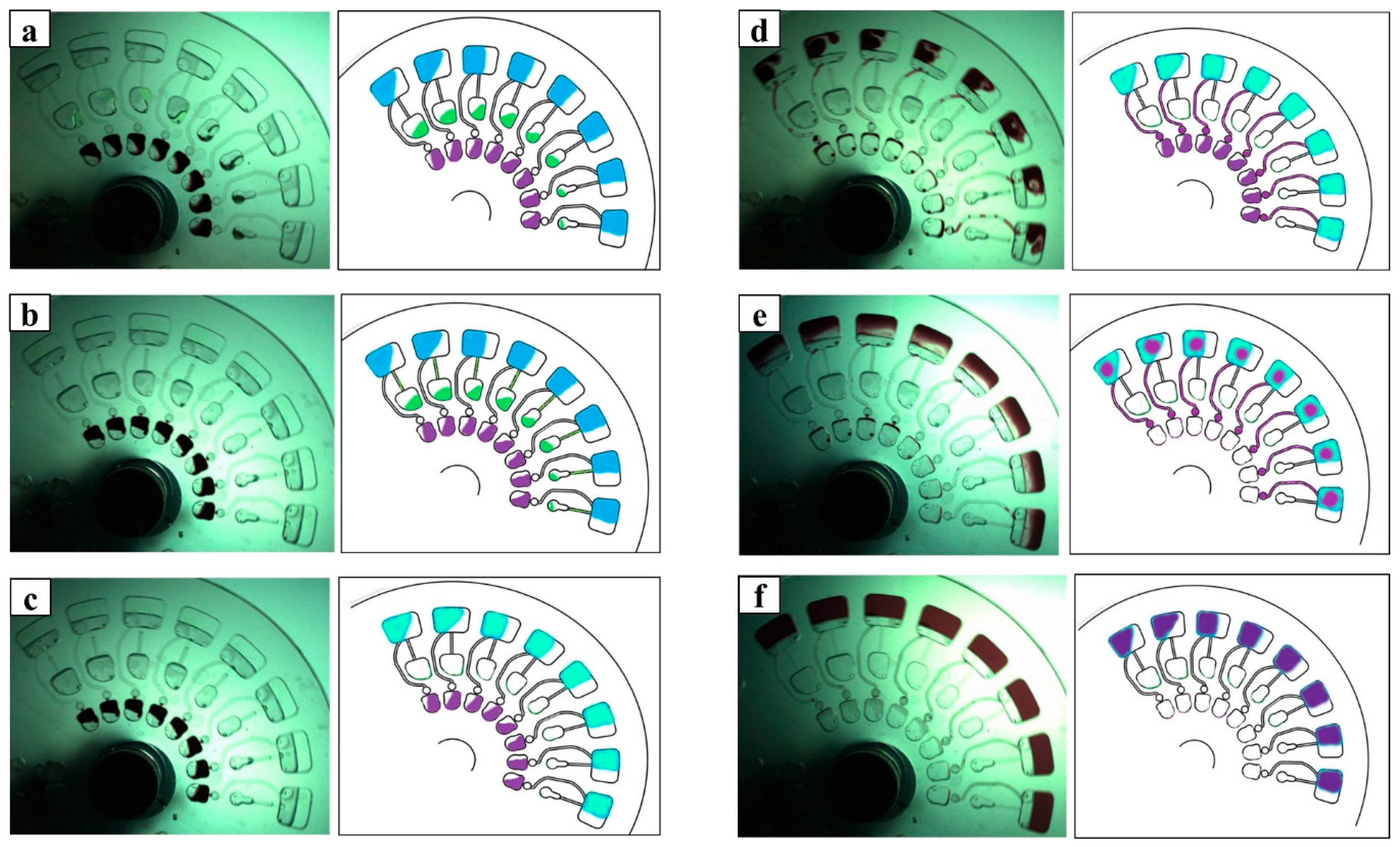


| Step | Spinning Speed (rpm) | Time * | Spinning Direction | Process |
|---|---|---|---|---|
| 1 | 0 | 1 min | nil | Sample preloading |
| 2 | 300 | 30 s | Clockwise | Plant extracts flowing to the reaction chamber and emptied |
| 3 | 800 | 15 s | Anticlockwise | 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution flow out from the DPPH chamber to the capillary valve and reached reaction chamber |
| 4 | 1400 | 5 min interval to 30 min | Clockwise and anticlockwise | The disc is rotated for mixing purposes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rahman, N.; Ibrahim, F.; Aeinehvand, M.M.; Yusof, R.; Madou, M. A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts. Micromachines 2018, 9, 140. https://doi.org/10.3390/mi9040140
Abd Rahman N, Ibrahim F, Aeinehvand MM, Yusof R, Madou M. A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts. Micromachines. 2018; 9(4):140. https://doi.org/10.3390/mi9040140
Chicago/Turabian StyleAbd Rahman, Nurhaslina, Fatimah Ibrahim, Mohammad M. Aeinehvand, Rohana Yusof, and Marc Madou. 2018. "A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts" Micromachines 9, no. 4: 140. https://doi.org/10.3390/mi9040140
APA StyleAbd Rahman, N., Ibrahim, F., Aeinehvand, M. M., Yusof, R., & Madou, M. (2018). A Microfluidic Lab-on-a-Disc (LOD) for Antioxidant Activities of Plant Extracts. Micromachines, 9(4), 140. https://doi.org/10.3390/mi9040140