Light-Controlled Swarming and Assembly of Colloidal Particles
Abstract
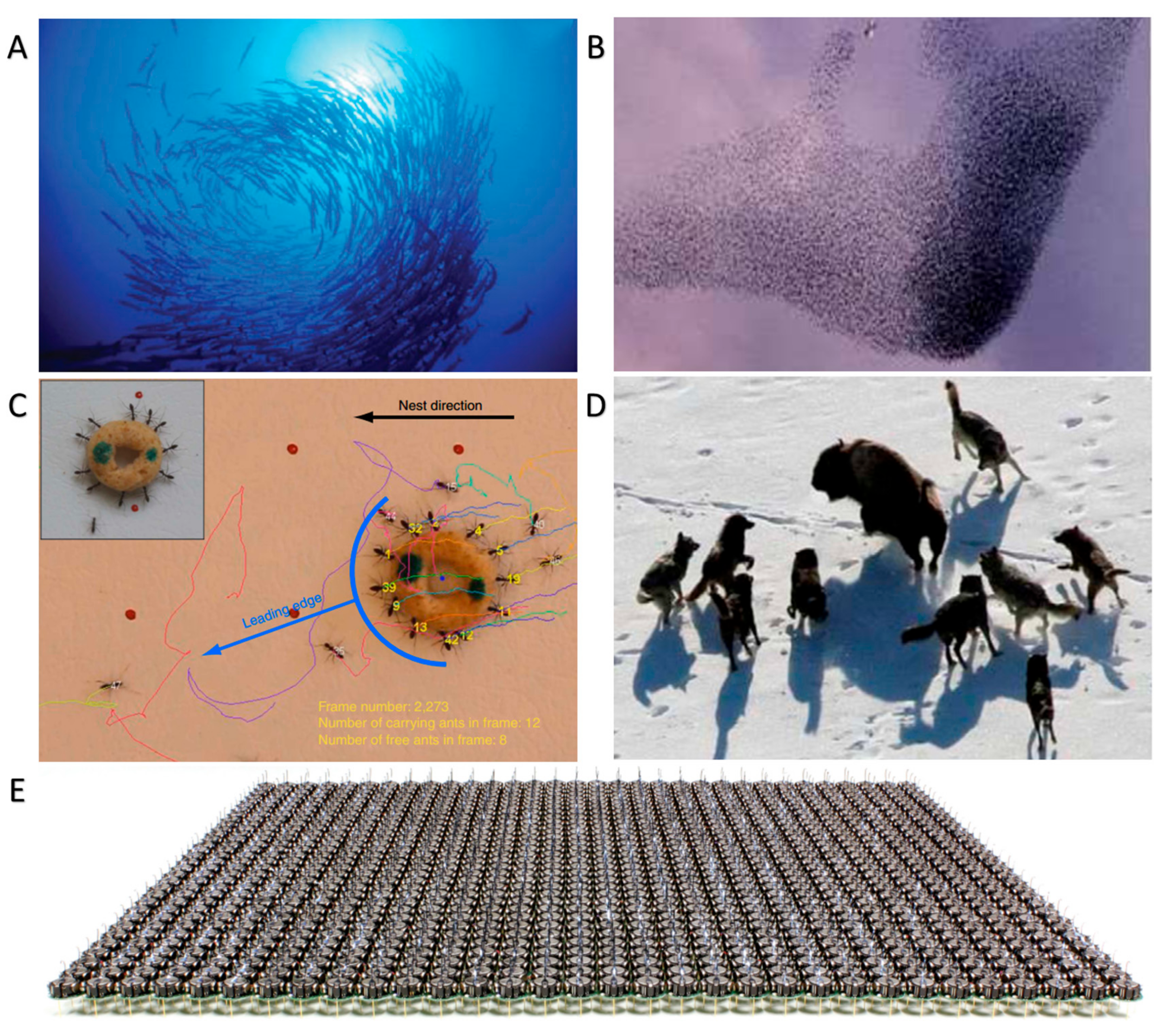
:1. Introduction
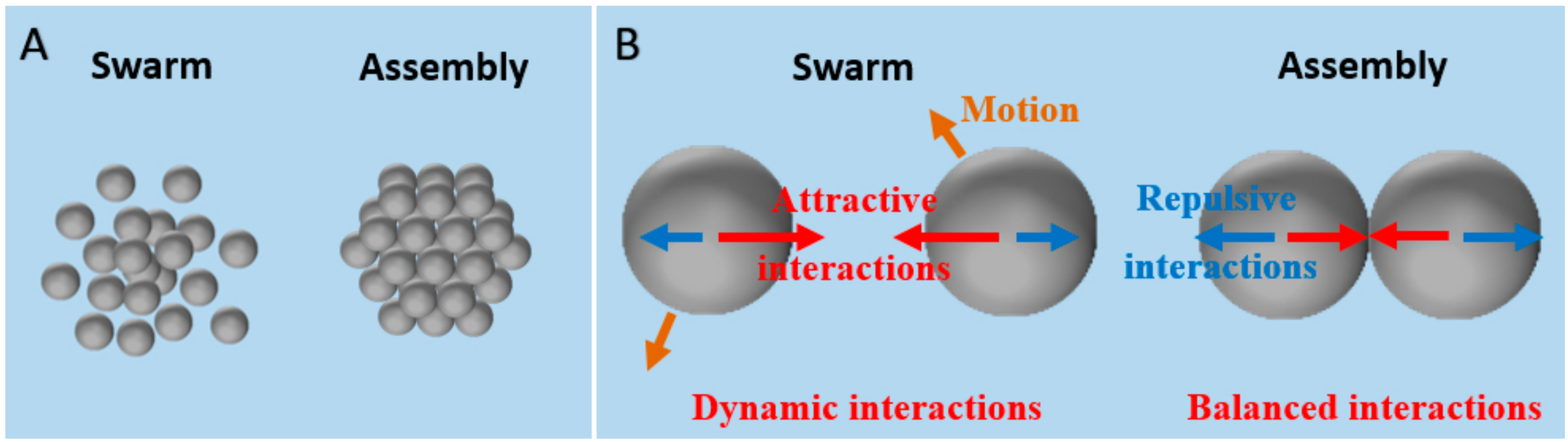
2. General Principles for Light-Controlled Swarming and Assembly of Colloidal Particles (CPs)
3. Light-Controlled Swarming and Assembly of CPs
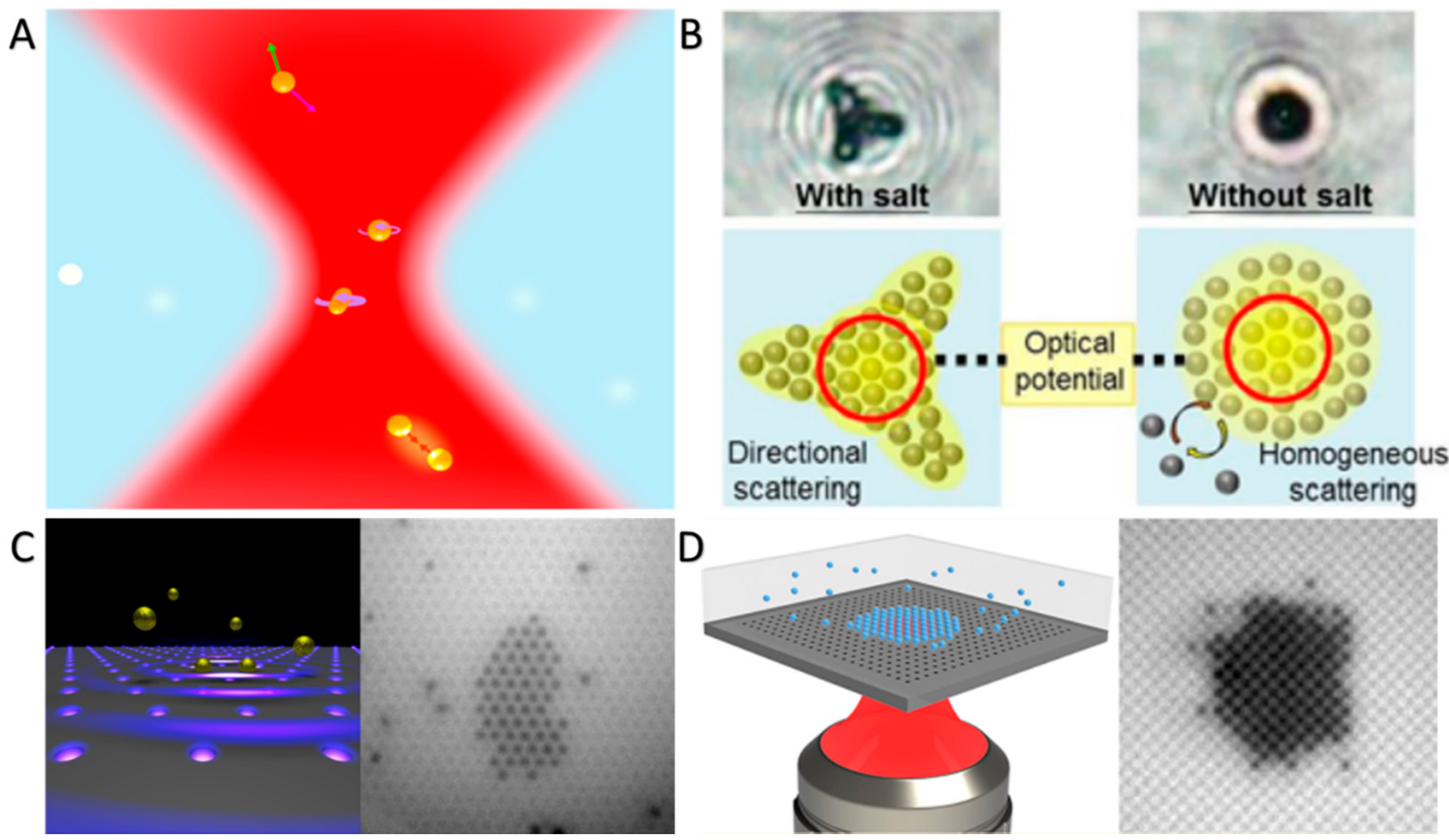
3.1. Optical Forces-Maneuvered Swarming and Assembly of CPs
3.2. Photochemical Reaction-Triggered Swarming and Assembly of CPs
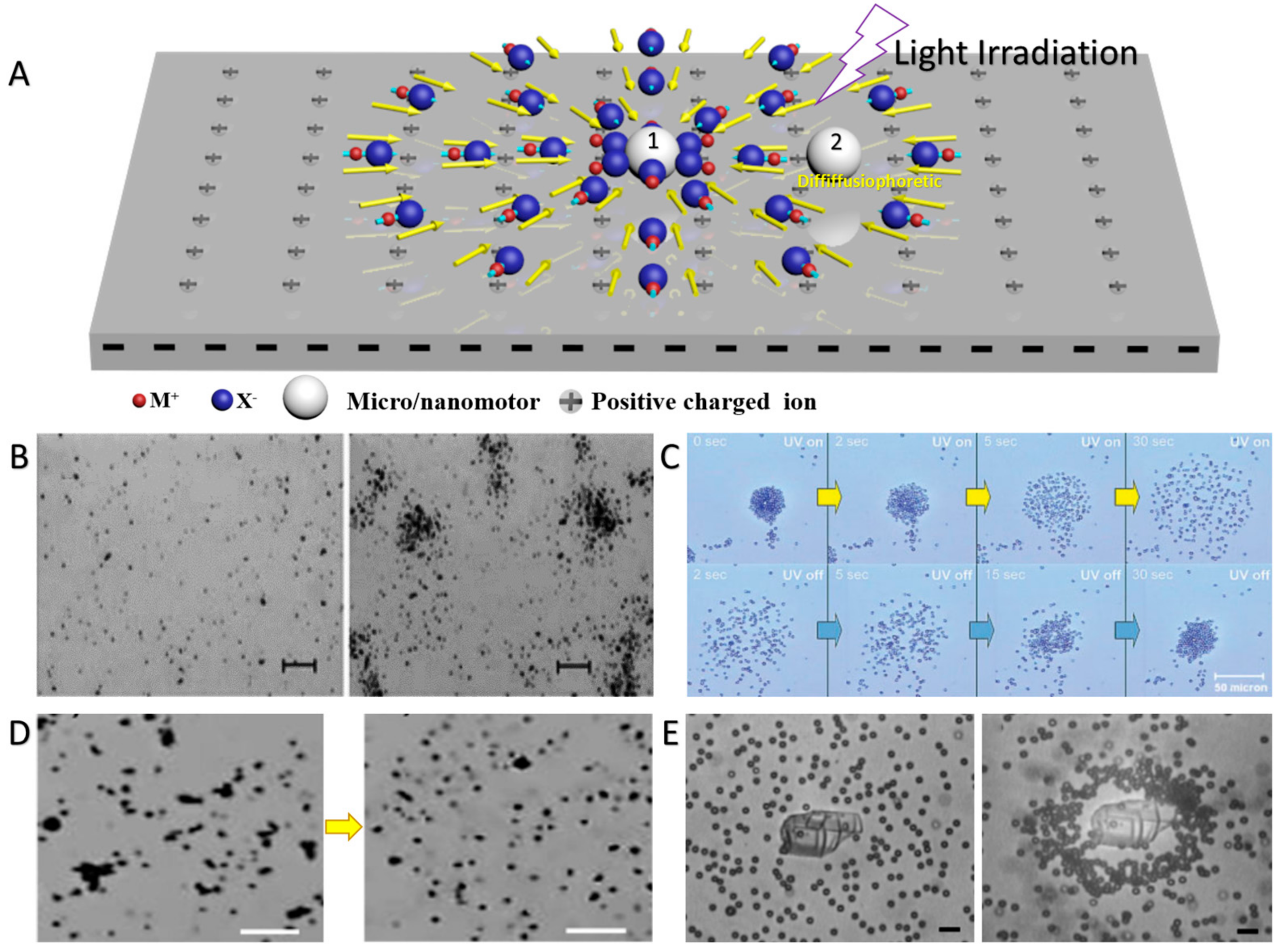
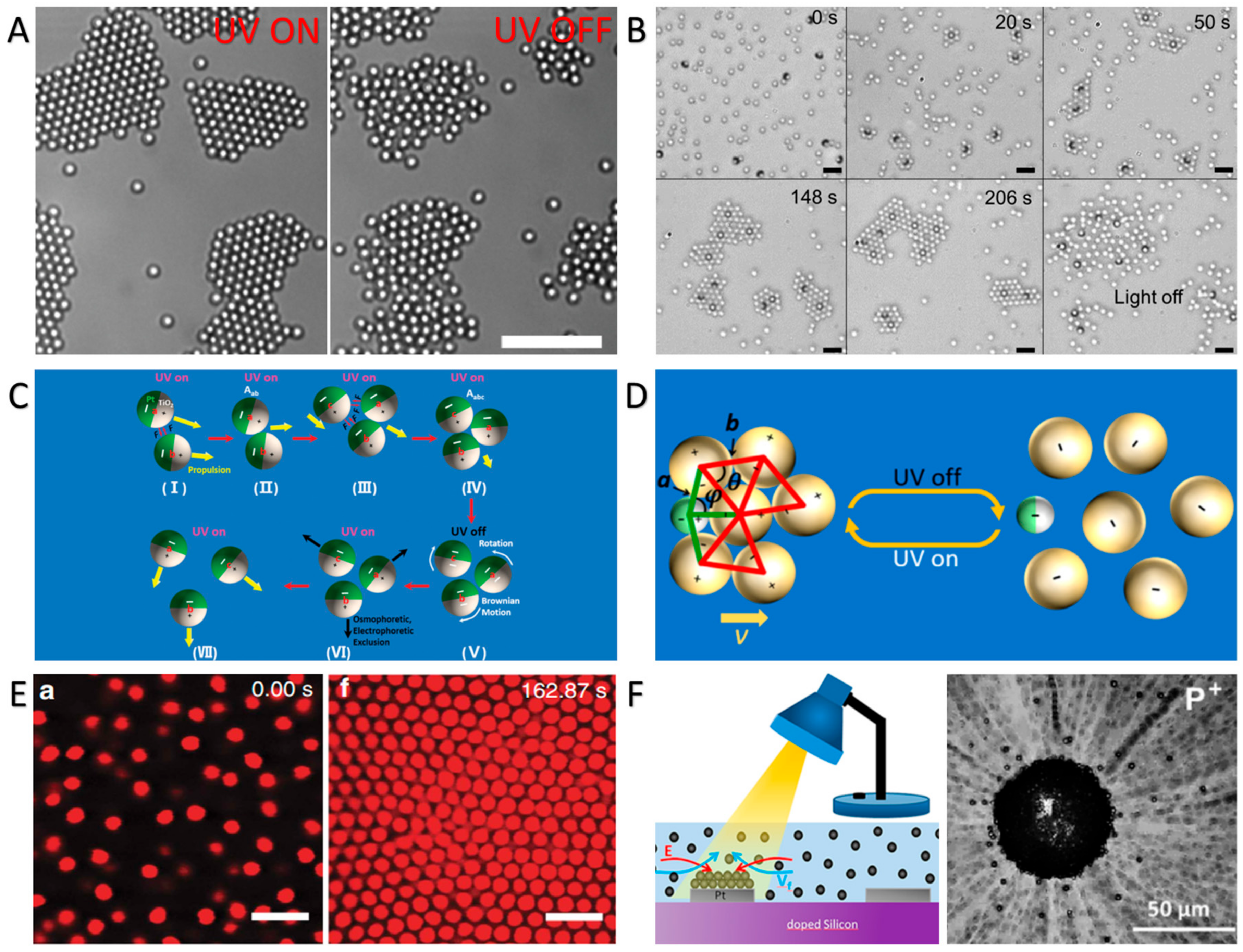
3.3. Photothermal Effect-Induced Swarming and Assembly of CPs
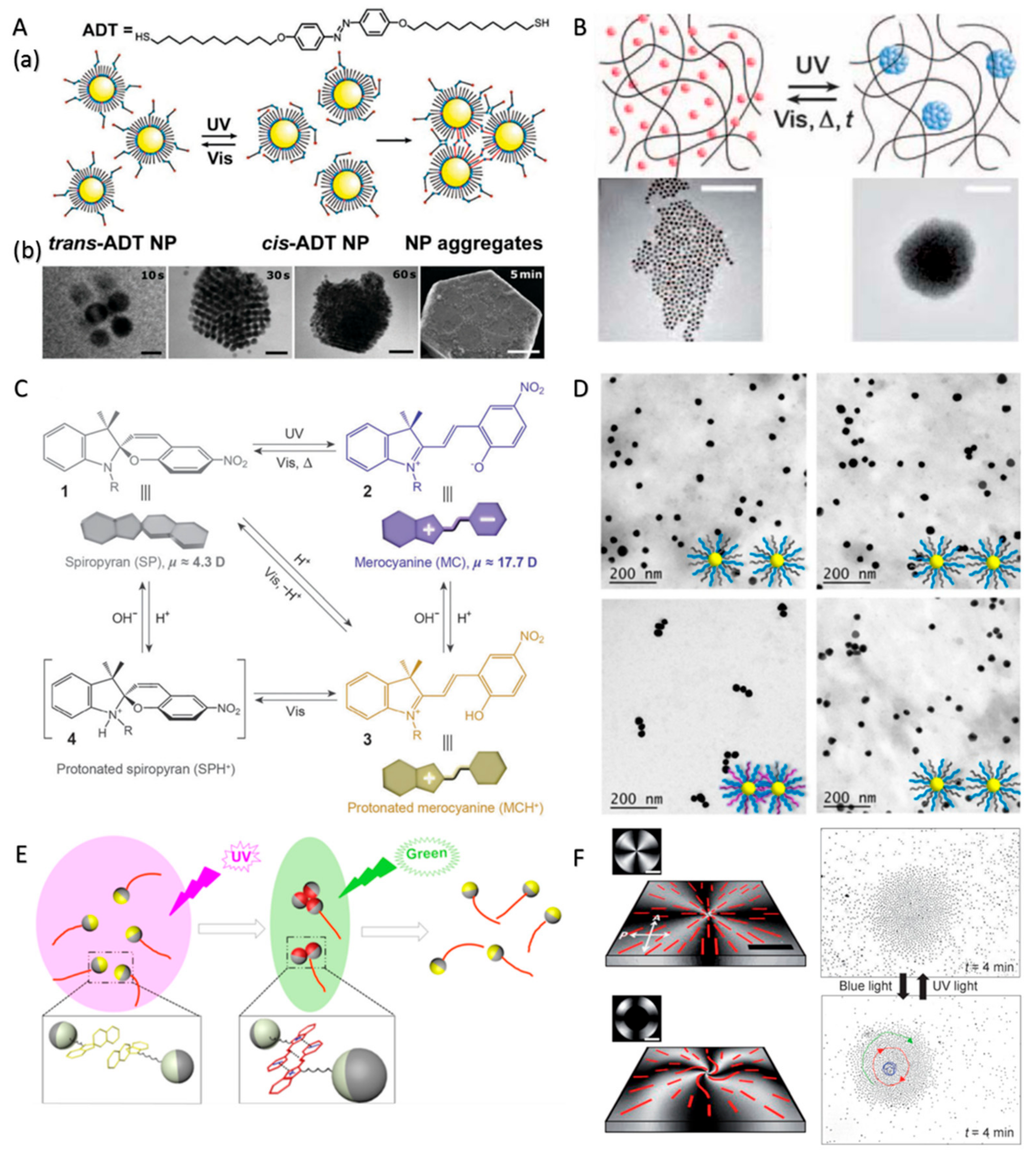
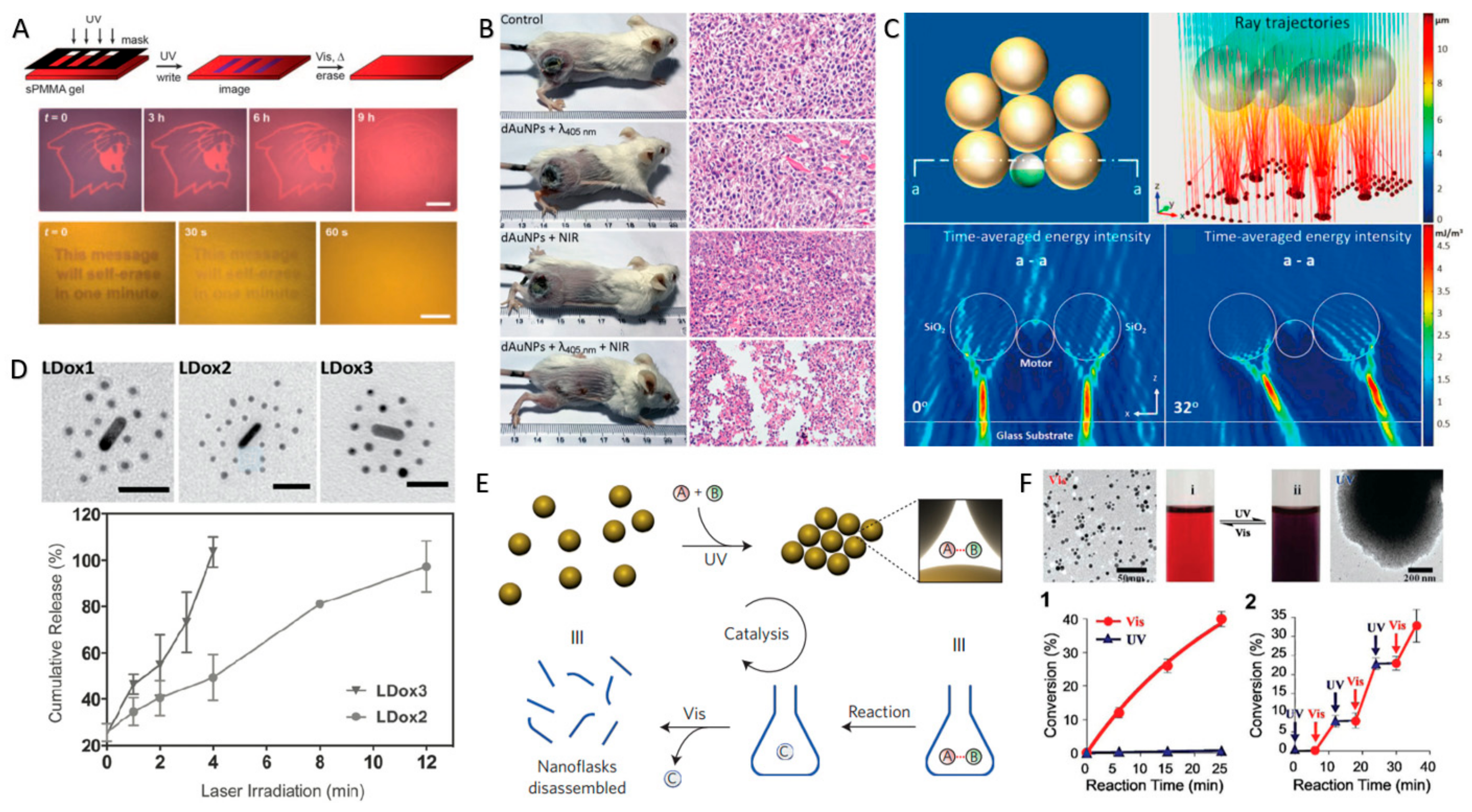
3.4. Photoisomerization-Controlled Swarming and Assembly of CPs
4. Applications of Light-Controlled CP Swarms and Assemblies
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solovev, A.A.; Sanchez, S.; Schmidt, O.G. Collective behaviour of self-propelled catalytic micromotors. Nanoscale 2013, 5, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Mirkin, C.A. Self-assembly gets new direction. Nature 2012, 491, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Fitzner, K.; Paczesny, J.; Granick, S. From dynamic self-assembly to networked chemical systems. Chem. Soc. Rev. 2017, 46, 5647–5678. [Google Scholar] [CrossRef] [PubMed]
- Bricard, A.; Caussin, J.B.; Desreumaux, N.; Dauchot, O.; Bartolo, D. Emergence of macroscopic directed motion in populations of motile colloids. Nature 2013, 503, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.K.; Edelsteinkeshet, L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 1999, 284, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, S.; Shi, X.-Q.; Chaté, H.; Wu, Y. Weak synchronization and large-scale collective oscillation in dense bacterial suspensions. Nature 2017, 542, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Academic Press: New York, NY, America, 1976; ISBN 978-0030839931. [Google Scholar]
- Lopez, U.; Gautrais, J.; Couzin, I.D.; Theraulaz, G. From behavioural analyses to models of collective motion in fish schools. Interface Focus 2012, 2, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef]
- Gelblum, A.; Pinkoviezky, I.; Fonio, E.; Ghosh, A.; Gov, N.; Feinerman, O. Ant groups optimally amplify the effect of transiently informed individuals. Nat. Commun. 2015, 6, 7729. [Google Scholar] [CrossRef] [PubMed]
- Muro, C.; Escobedo, R.; Spector, L.; Coppinger, R.P. Wolf-pack (Canis lupus) hunting strategies emerge from simple rules in computational simulations. Behav. Process. 2011, 88, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, M.; Cornejo, A.; Nagpal, R. Programmable self-assembly in a thousand-robot swarm. Science 2014, 345, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, X.; Pei, A.; Gu, Y.; Li, J.; Wang, J. Seawater-driven magnesium based Janus micromotors for environmental remediation. Nanoscale 2013, 5, 4696–4700. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Chen, C.; Zhong, Q.; Yin, Y.; Ma, H.; Guan, J. Autonomous motion and temperature-controlled drug delivery of Mg/Pt-poly(N-isopropylacrylamide) Janus micromotors driven by simulated body fluid and blood plasma. ACS Appl. Mater. Interfaces 2014, 6, 9897–9903. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Coutinho, E.; Rodrigues, A.S.; Baleizao, C.; Farinha, J.P.S. Hybrid mesoporous silica nanocarriers with thermovalve-regulated controlled release. Nanoscale 2017, 9, 13485–13494. [Google Scholar] [CrossRef] [PubMed]
- Huergo, M.A.; Maier, C.M.; Castez, M.F.; Vericat, C.; Nedev, S.; Salvarezza, R.C.; Urban, A.S.; Feldmann, J. Optical nanoparticle sorting elucidates synthesis of plasmonic nanotriangles. ACS Nano 2016, 10, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Shioi, A. Self-propelled nano/micromotors with a chemical reaction: Underlying physics and strategies of motion control. KONA Powder Part. J. 2015, 32, 2–22. [Google Scholar] [CrossRef]
- Chen, C.; Mou, F.; Xu, L.; Wang, S.; Guan, J.; Feng, Z.; Wang, Q.; Kong, L.; Li, W.; Wang, J.; et al. Light-steered isotropic semiconductor micromotors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Xu, L.; Ma, H.; Guan, J.; Chen, D.R.; Wang, S. Facile preparation of magnetic gamma-Fe2O3/TiO2 Janus hollow bowls with efficient visible-light photocatalytic activities by asymmetric shrinkage. Nanoscale 2012, 4, 4650–4657. [Google Scholar] [CrossRef] [PubMed]
- Vach, P.J.; Fratzl, P.; Klumpp, S.; Faivre, D. Fast magnetic micropropellers with random shapes. Nano Lett. 2015, 15, 7064–7070. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Yoshinaga, N.; Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 2010, 105, 268302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto-acoustic hybrid nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kim, K.; Lei, K.W.; Fan, D.L. Ultra-durable rotary micromotors assembled from nanoentities by electric fields. Nanoscale 2015, 7, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luijten, E.; Grzybowski, B.A.; Granick, S. Active colloids with collective mobility status and research opportunities. Chem. Soc. Rev. 2017, 46, 5551–5569. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Sen, A.; Mallouk, T.E. From one to many: Dynamic assembly and collective behavior of self-propelled colloidal motors. Acc. Chem. Res. 2015, 48, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Retsch, M.; Fustin, C.A.; Del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Diaz, M.; Córdova-Figueroa, U.M.; Sen, A. Light-driven titanium-dioxide-based reversible microfireworks and micromotor/micropump systems. Adv. Funct. Mater. 2010, 20, 1568–1576. [Google Scholar] [CrossRef]
- Duan, W.; Liu, R.; Sen, A. Transition between collective behaviors of micromotors in response to different stimuli. J. Am. Chem. Soc. 2013, 135, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Kagan, D.; Balasubramanian, S.; Wang, J. Chemically triggered swarming of gold microparticles. Angew. Chem. Int. Ed. 2011, 50, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Freedman, J.D.; Grinstaff, M.; Sen, A. Bone-crack detection, targeting, and repair. Angew. Chem. Int. Ed. 2013, 52, 10997–11001. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, S.; Wu, D.T.; Wu, N. Electric-field-induced assembly and propulsion of chiral colloidal clusters. Proc. Natl. Acad. Sci. USA 2015, 112, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, M.; Zhang, J.; Xu, C.; Luijten, E.; Granick, S. Reconfiguring active particles by electrostatic imbalance. Nat. Mater. 2016, 15, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Underhill, G.H.; Wassermann, T.B.; Sah, R.L.; Bhatia, S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods 2006, 3, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Baasch, T.; Blondel, N.; Laubli, N.; Dual, J.; Nelson, B.J. Neutrophil-inspired propulsion in a combined acoustic and magnetic field. Nat. Commun. 2017, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Gentekos, D.T.; Fink, C.A.; Mallouk, T.E. Self-assembly of nanorod motors into geometrically regular multimers and their propulsion by ultrasound. ACS Nano 2014, 8, 11053–11060. [Google Scholar] [CrossRef] [PubMed]
- Vanherberghen, B.; Manneberg, O.; Christakou, A.; Frisk, T.; Ohlin, M.; Hertz, H.M.; Onfelt, B.; Wiklund, M. Ultrasound-controlled cell aggregation in a multi-well chip. Lab Chip 2010, 10, 2727–2732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Zhang, Z.; Sun, M.; Sen, A.; Mallouk, T.E. A tale of two forces: Simultaneous chemical and acoustic propulsion of bimetallic micromotors. Chem. Commun. 2015, 51, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mou, F.; Feng, Y.; Che, S.; Li, W.; Xu, L.; Guan, J. Dynamic colloidal molecules maneuvered by light-controlled Janus micromotors. ACS Appl. Mater. Interfaces 2017, 9, 22704–22712. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Soto, F.; Gao, W.; Dong, R.; Garcia-Gradilla, V.; Magana, E.; Zhang, X.; Wang, J. Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. J. Am. Chem. Soc. 2015, 137, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Lidgi-Guigui, N.; Dablemont, C.; Veautier, D.; Viau, G.; Seneor, P.; Nguyen Van Dau, F.; Mangeney, C.; Vaurès, A.; Deranlot, C.; Friederich, A. Grafted 2D Assembly of colloidal metal nanoparticles for application as a variable capacitor. Adv. Mater. 2007, 19, 1729–1733. [Google Scholar] [CrossRef]
- Ling, D.; Park, W.; Park, S.-J.; Lu, Y.; Kim, K.S.; Hackett, M.J.; Kim, B.H.; Yim, H.; Jeon, Y.S.; Na, K.; et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J. Am. Chem. Soc. 2014, 136, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Cheng, D.; Gong, F.M.; Miao, X.M.; Shuai, X.T. Design of multifunctional micelle for tumor-targeted intracellular drug release and fluorescent imaging. Adv. Mater. 2012, 24, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Pavlick, R.A.; Meckler, S.M.; Sen, A. Triggered detection and deposition: Toward the repair of microcracks. Chem. Mater. 2014, 26, 4647–4652. [Google Scholar] [CrossRef]
- Gallo, J.; Kamaly, N.; Lavdas, I.; Stevens, E.; Quang-De, N.; Wylezinska-Arridge, M.; Aboagye, E.O.; Long, N.J. CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced magnetic resonance imaging. Angew. Chem. Int. Ed. 2014, 53, 9550–9554. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Pauzauskie, P.J.; Radenovic, A.; Trepagnier, E.; Shroff, H.; Yang, P.D.; Liphardt, J. Optical trapping and integration of semiconductor nanowire assemblies in water. Nat. Mater. 2006, 5, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Ikin, L.; Carberry, D.M.; Gibson, G.M.; Padgett, M.J.; Miles, M.J. Assembly and force measurement with SPM-like probes in holographic optical tweezers. New J. Phys. 2009, 11, 023012. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly efficient light-driven TiO2-Au Janus micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-controlled propulsion, aggregation and separation of water-fuelled TiO2/Pt Janus submicromotors and their “on-the-fly” photocatalytic activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Li, Y.; Chen, C.; Li, W.; Yin, Y.; Ma, H.; Guan, J. Single-component TiO2 tubular microengines with motion controlled by light-induced bubbles. Small 2015, 11, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Navarro, S.; Tierno, P.; Farrera, J.A.; Ignes-Mullol, J.; Sagues, F. Reconfigurable swarms of nematic colloids controlled by photoactivated surface patterns. Angew. Chem. Int. Ed. 2014, 53, 10696–10700. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic nanoparticle assemblies for biomedical applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S. The mechanics and statistics of active matter. Ann. Rev. Condens. Matter Phys. 2010, 1, 323–345. [Google Scholar] [CrossRef]
- Marchetti, M.C.; Joanny, J.F.; Ramaswamy, S.; Liverpool, T.B.; Prost, J.; Rao, M.; Simha, R.A. Hydrodynamics of soft active matter. Rev. Mod. Phys. 2013, 85, 1143–1189. [Google Scholar] [CrossRef]
- Reynolds, C.W. Flocks, herds and schools: A distributed behavioral model. In ACM SIGGRAPH Computer Graphics; Association for Computing Machinery (ACM): New York, NY, USA, 1987; Volume 21, pp. 25–34. [Google Scholar]
- Juanico, D.E.O. Self-organized pattern formation in a diverse attractive-repulsive swarm. Europhys. Lett. 2009, 86, 48004. [Google Scholar] [CrossRef]
- Li, F.; Josephson, D.P.; Stein, A. Colloidal assembly: The road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Bishop, K.J.M.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale forces and their uses in self-assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef] [PubMed]
- Illien, P.; Golestanian, R.; Sen, A. ‘Fuelled’ motion: Phoretic motility and collective behaviour of active colloids. Chem. Soc. Rev. 2017, 46, 5508–5518. [Google Scholar] [CrossRef] [PubMed]
- Palacci, J.; Sacanna, S.; Kim, S.H.; Yi, G.R.; Pine, D.J.; Chaikin, P.M. Light-activated self-propelled colloids. Philos. Trans. R. Soc. A 2014, 372, 20130372. [Google Scholar] [CrossRef] [PubMed]
- Weinert, F.M.; Braun, D. Observation of slip flow in thermophoresis. Phys. Rev. Lett. 2008, 101, 168301. [Google Scholar] [CrossRef] [PubMed]
- Lehmuskero, A.; Johansson, P.; Rubinsztein-Dunlop, H.; Tong, L.M.; Kall, M. Laser trapping of colloidal metal nanoparticles. ACS Nano 2015, 9, 3453–3469. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Kudo, T.; Yuyama, K.I.; Sugiyama, T.; Masuhara, H. Optically evolved assembly formation in laser trapping of polystyrene nanoparticles at solution surface. Langmuir 2016, 32, 12488–12496. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Martinez, L.J.; Jaquay, E.; Nakano, A.; Povinelli, M.L. Optical epitaxial growth of gold nanoparticle arrays. Nano Lett. 2015, 15, 5841–5845. [Google Scholar] [CrossRef] [PubMed]
- Jaquay, E.; Martinez, L.J.; Mejia, C.A.; Povinelli, M.L. Light-assisted, templated self-assembly using a photonic-crystal slab. Nano Lett. 2013, 13, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.R.; Iwata, J.; Lammert, P.E.; Mallouk, T.E.; Sen, A.; Velegol, D. Catalytically driven colloidal patterning and transport. J. Phys. Chem. B 2006, 110, 24513–24521. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Yadav, V.; Zhang, H.; Pavlick, R.; Sen, A. Triggered “on/off” micropumps and colloidal photodiode. J. Am. Chem. Soc. 2012, 134, 15688–15691. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Ramaswamy, S.; Menon, N. Long-lived giant number fluctuations in a swarming granular nematic. Science 2007, 317, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Gruler, H.; Dewald, U.; Eberhardt, M. Nematic liquid crystals formed by living amoeboid cells. Eur. Phys. J. B Condens. Matter Complex Syst. 1999, 11, 187–192. [Google Scholar] [CrossRef]
- Palacci, J.; Sacanna, S.; Steinberg, A.P.; Pine, D.J.; Chaikin, P.M. Living crystals of light-activated colloidal surfers. Science 2013, 339, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Choudhury, U.; Fischer, P.; Mark, A.G. Non-equilibrium assembly of light-activated colloidal mixtures. Adv. Mater. 2017, 29, 1701328. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shah, A.A.; Solomon, M.J. Spatially and temporally reconfigurable assembly of colloidal crystals. Nat. Commun. 2014, 5, 3676. [Google Scholar] [CrossRef] [PubMed]
- Esplandiu, M.J.; Afshar Farniya, A.; Bachtold, A. Silicon-based chemical motors: An efficient pump for triggering and guiding fluid motion using visible light. ACS Nano 2015, 9, 11234–11240. [Google Scholar] [CrossRef] [PubMed]
- Golestanian, R. Collective behavior of thermally active colloids. Phys. Rev. Lett. 2012, 108, 038303. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Golestanian, R. Emergent cometlike swarming of optically driven thermally active colloids. Phys. Rev. Lett. 2014, 112, 068302. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, X.; Wang, M.; Scarabelli, L.; Mao, Z.; Liz-Marzan, L.M.; Becker, M.F.; Zheng, Y. Light-directed reversible assembly of plasmonic nanoparticles using plasmon-enhanced thermophoresis. ACS Nano 2016, 9659–9668. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Zhang, J.L.; Peng, X.L.; Wu, Z.L.; Coughlan, A.C.H.; Mao, Z.M.; Bevan, M.A.; Zheng, Y.B. Opto-thermophoretic assembly of colloidal matter. Sci. Adv. 2017, 3, e1700458. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R.; Stoddart, J.F.; Grzybowski, B.A. Nanoparticles functionalised with reversible molecular and supramolecular switches. Chem. Soc. Rev. 2010, 39, 2203–2237. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R.; Bishop, K.J.; Grzybowski, B.A. Light-controlled self-assembly of reversible and irreversible nanoparticle suprastructures. Proc. Natl. Acad. Sci. USA 2007, 104, 10305–10309. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R.; Wesson, P.J.; Bishop, K.J.M.; Grzybowski, B.A. Writing self-erasing images using metastable nanoparticle “Inks”. Angew. Chem. Int. Ed. 2009, 48, 7035–7039. [Google Scholar] [CrossRef] [PubMed]
- Fissi, A.; Pieroni, O.; Angelini, N.; Lenci, F. Photoresponsive polypeptides. Photochromic and conformational behavior of spiropyran-containing Poly(l-glutamate)s under acid conditions. Macromolecules 1999, 32, 7116–7121. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, L.; Rong, Y.; Liu, Z.; Tong, D.; Huang, Y.; Chen, T. Light-triggered reversible self-assembly of gold nanoparticle oligomers for tunable SERS. Langmuir 2015, 31, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, R.; Chang, X.; Ren, B.; Tong, Z. Spiropyran-decorated SiO2-Pt Janus micromotor: preparation and light-Induced dynamic self-assembly and disassembly. ACS Appl. Mater. Interfaces 2015, 7, 24585–24591. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.K.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Borner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-controlled self-assembly of non-photoresponsive nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef] [PubMed]
- Raeesi, V.; Chou, L.Y.; Chan, W.C. Tuning the drug loading and release of DNA-assembled gold-nanorod superstructures. Adv. Mater. 2016, 28, 8511–8518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sen, S.; Udayabhaskararao, T.; Sawczyk, M.; Kucanda, K.; Manna, D.; Kundu, P.K.; Lee, J.W.; Kral, P.; Klajn, R. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotechnol. 2016, 11, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, S.; Kim, J.; Soh, S.; Grzybowski, B.A. Photoswitchable catalysis mediated by dynamic aggregation of nanoparticles. J. Am. Chem. Soc. 2010, 132, 11018–11020. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, J.K.; Deak, A.; Brougham, D.F. Nanoparticle clusters: Assembly and control over internal order, current capabilities, and future potential. Adv. Mater. 2016, 28, 5400–5424. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.-H.; Zhang, H.; Zhao, L. UV irradiation induced transformation of TiO2 nanoparticles in water: aggregation and photoreactivity. Environ. Sci. Technol. 2014, 48, 11962–11968. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Optical trapping and manipulation of neutral particles using lasers. Proc. Natl. Acad. Sci. USA 1997, 94, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, J.; Granick, S. Directed self-assembly pathways of active colloidal clusters. Angew. Chem. Int. Ed. 2016, 55, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Furuhashi, S. Group-size distribution of skeins of wild geese. Phys. Rev. E 2012, 86, 031924. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Guo, J.; Mou, F.; Guan, J. Light-Controlled Swarming and Assembly of Colloidal Particles. Micromachines 2018, 9, 88. https://doi.org/10.3390/mi9020088
Zhang J, Guo J, Mou F, Guan J. Light-Controlled Swarming and Assembly of Colloidal Particles. Micromachines. 2018; 9(2):88. https://doi.org/10.3390/mi9020088
Chicago/Turabian StyleZhang, Jianhua, Jingjing Guo, Fangzhi Mou, and Jianguo Guan. 2018. "Light-Controlled Swarming and Assembly of Colloidal Particles" Micromachines 9, no. 2: 88. https://doi.org/10.3390/mi9020088






