Manipulating Microrobots Using Balanced Magnetic and Buoyancy Forces
Abstract
:1. Introduction
2. Materials and Methods
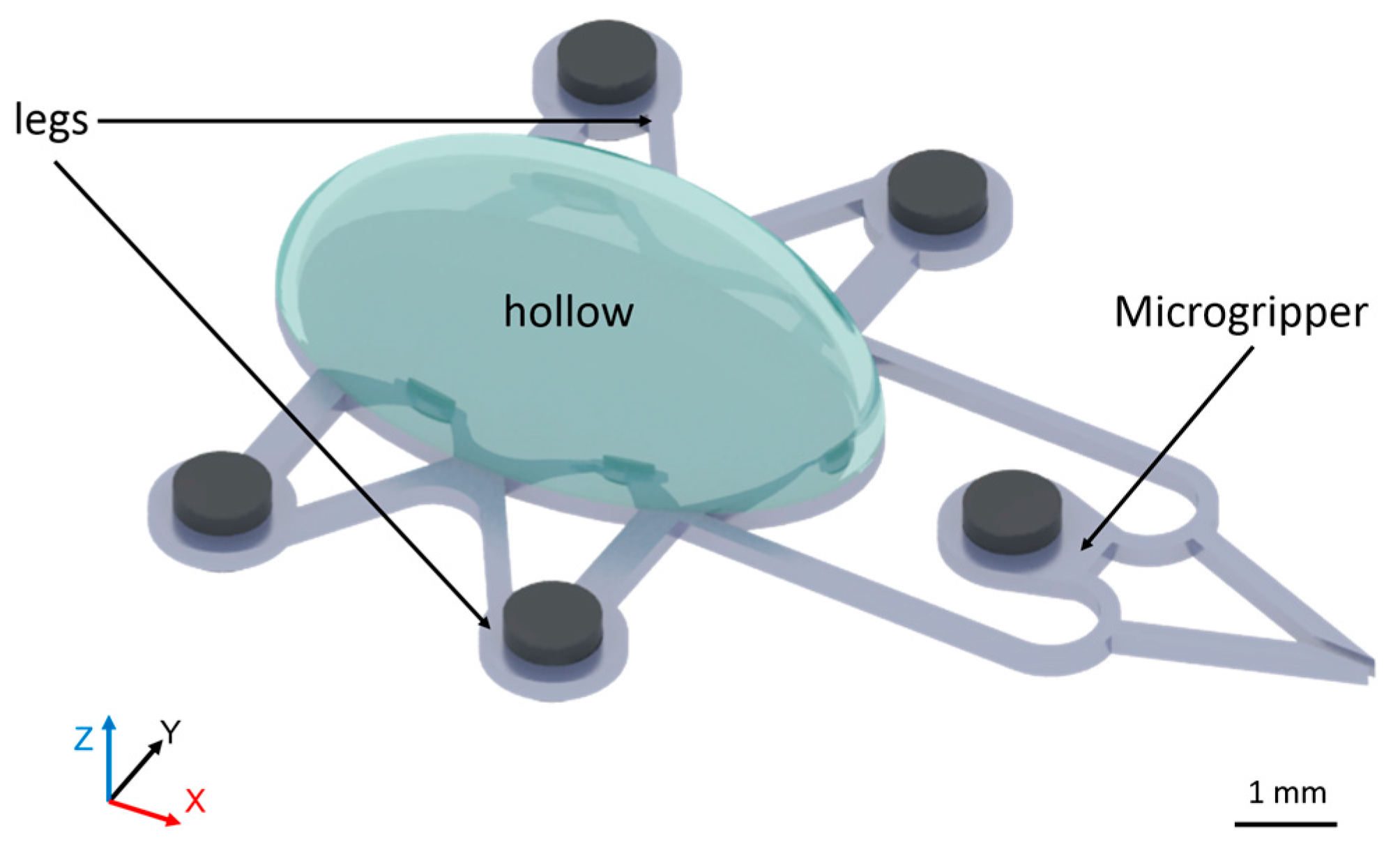
2.1. Microrobot Design
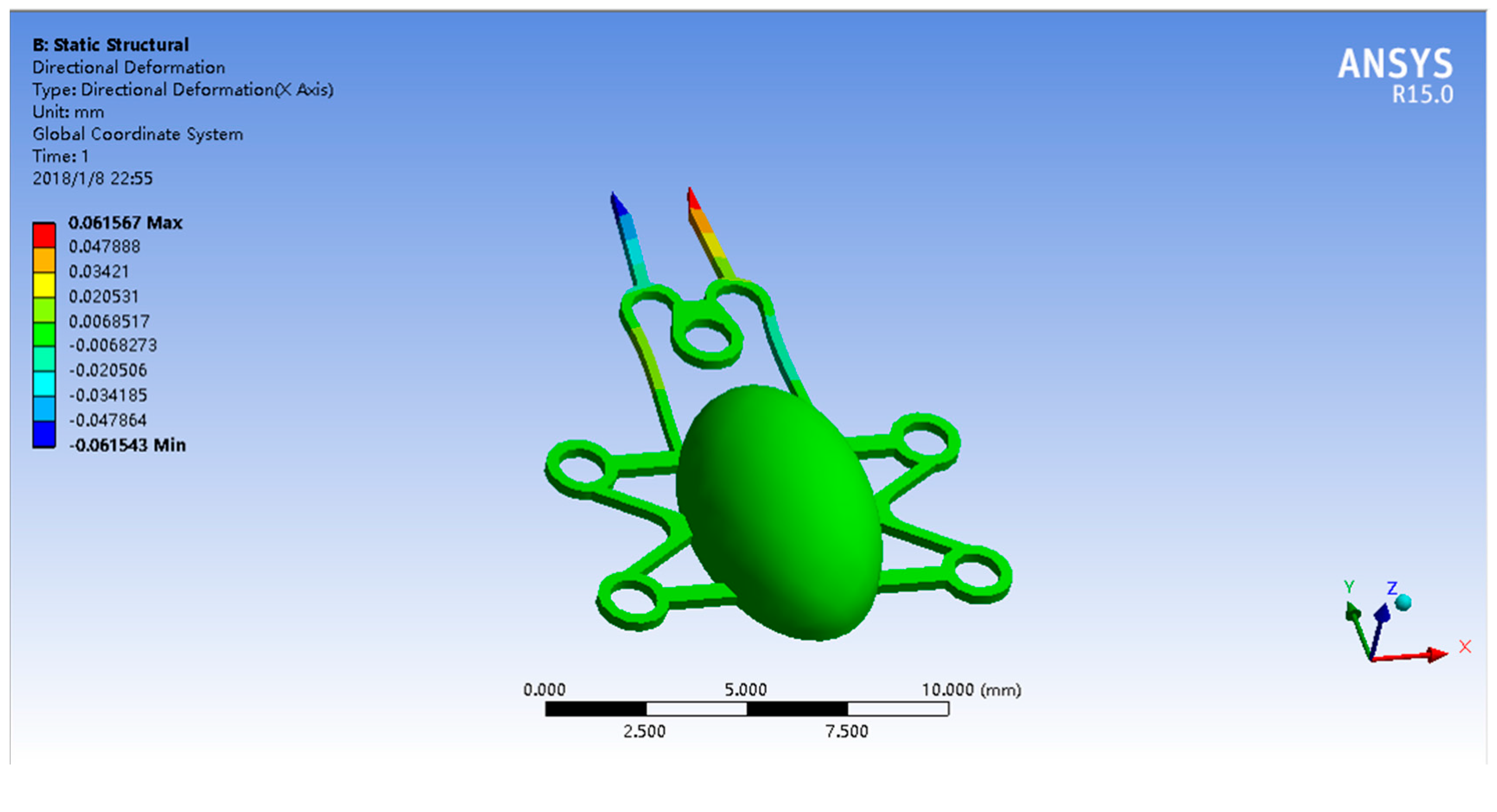
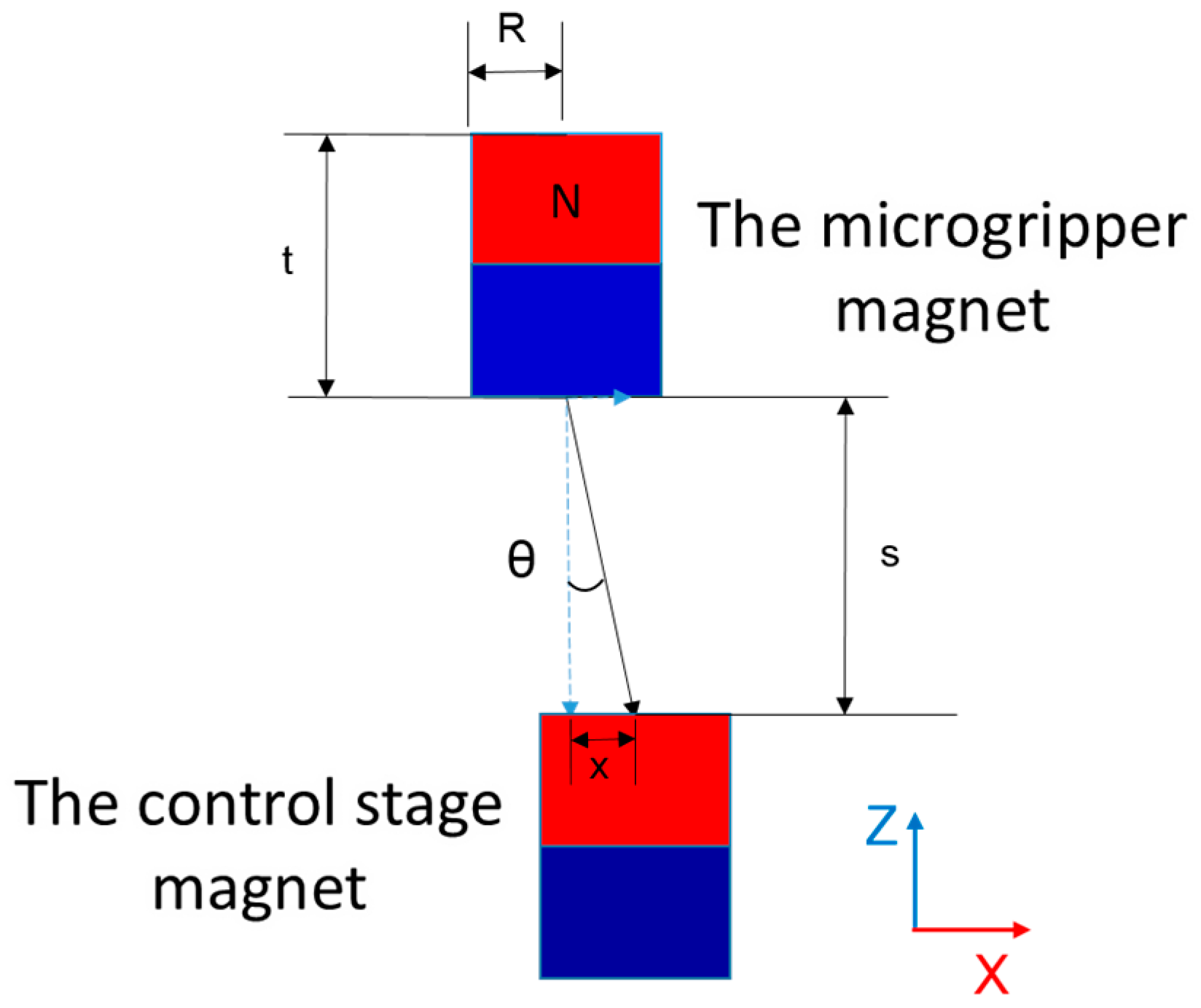
2.2. Microgripper Simulation
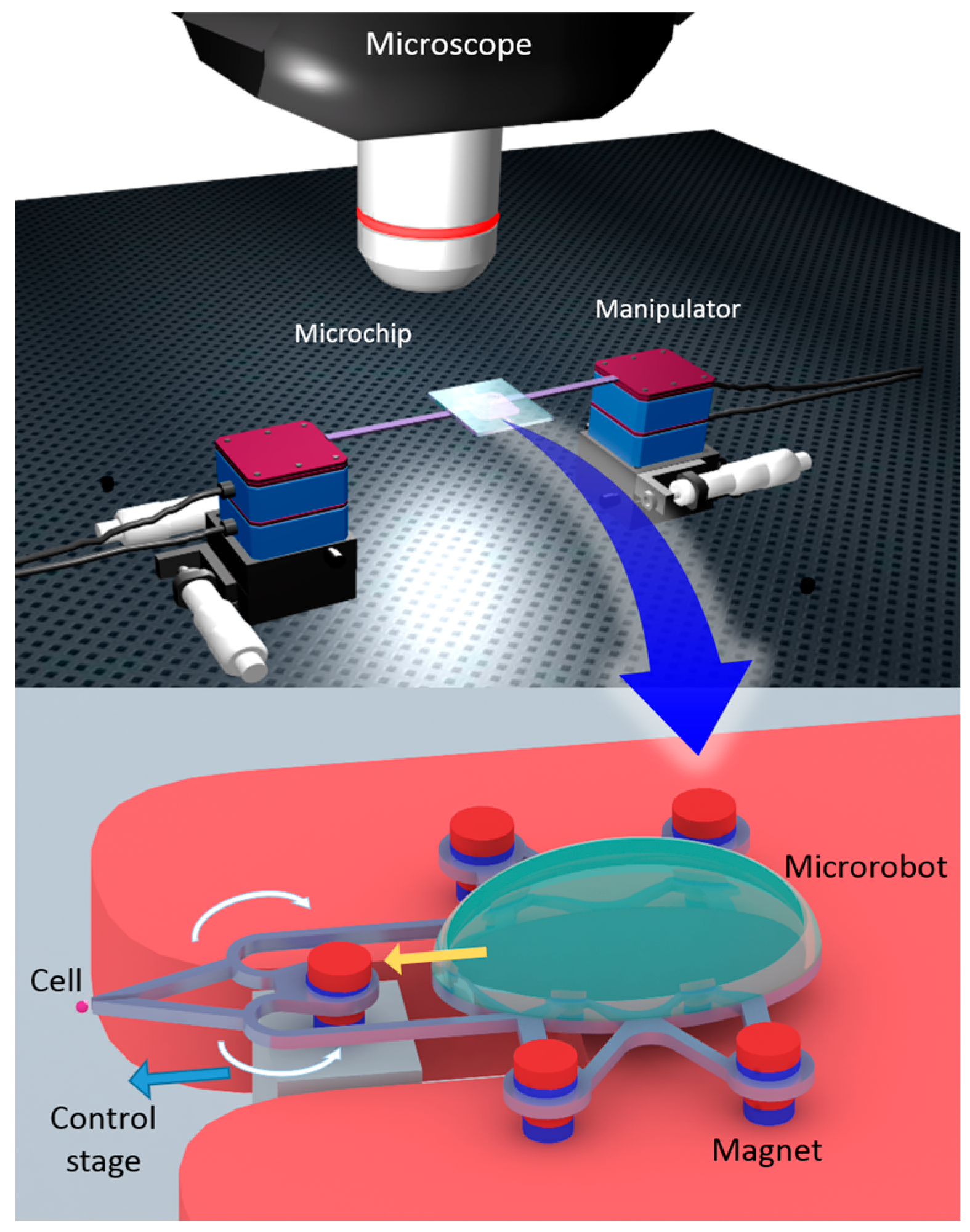
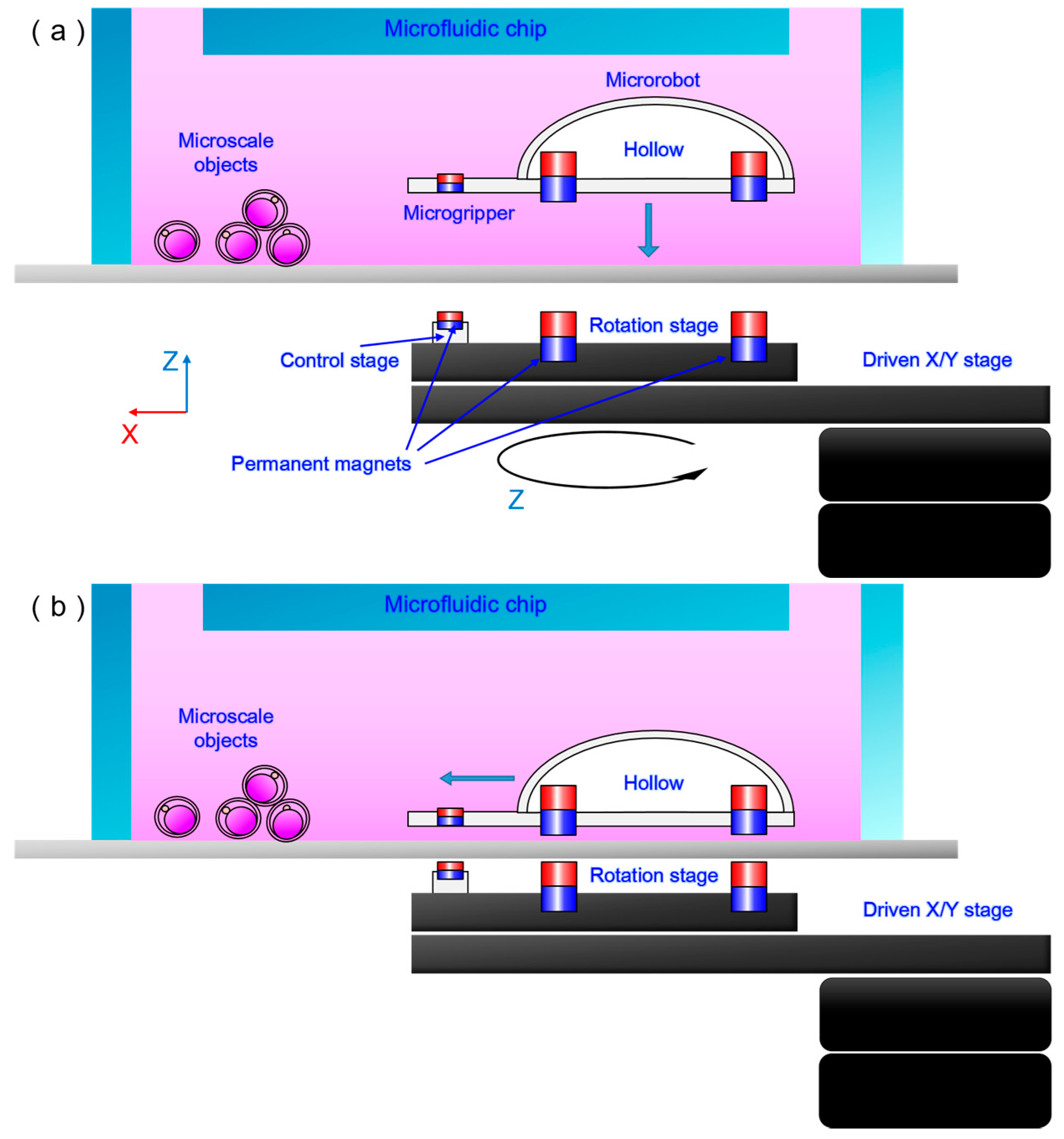
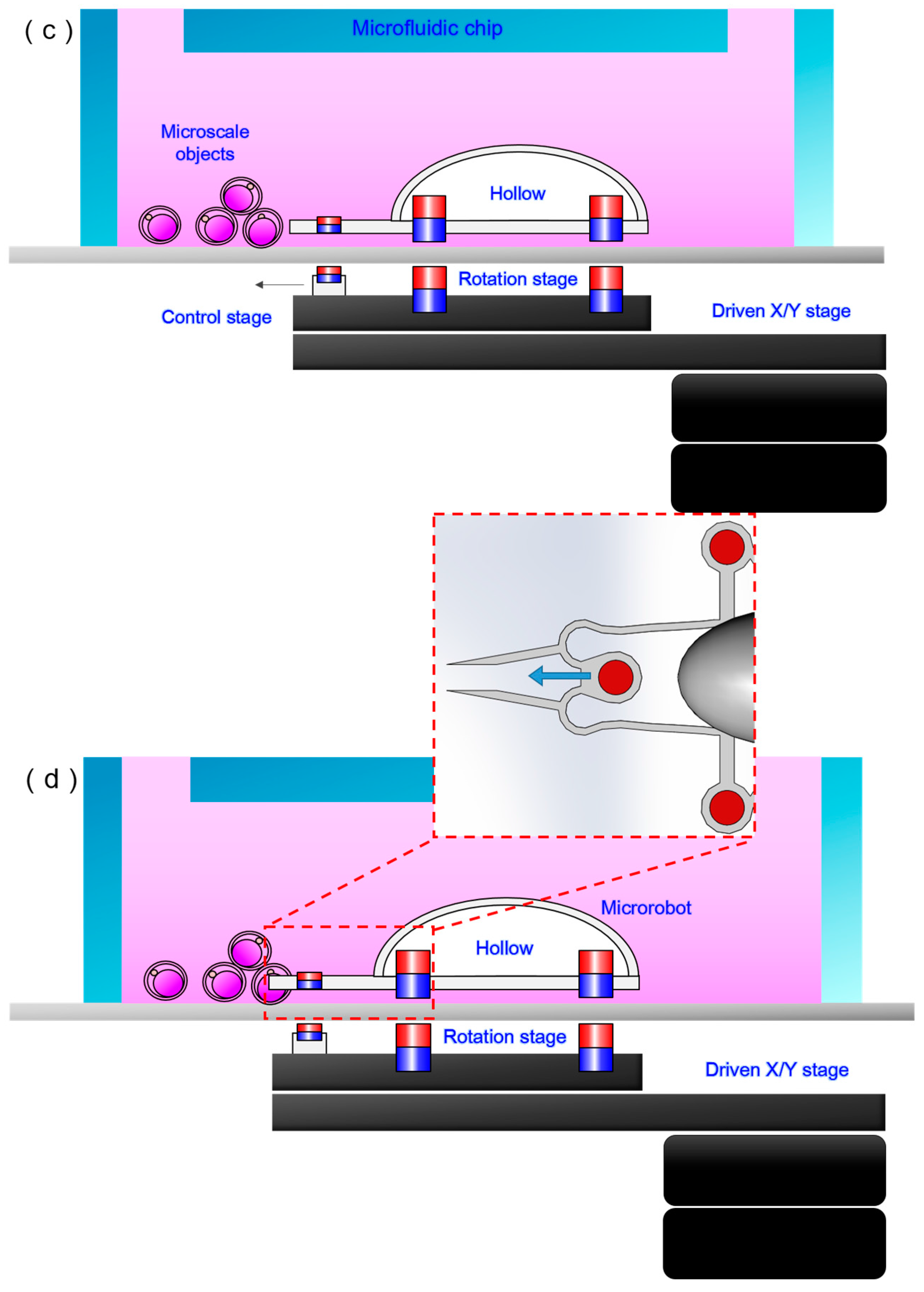
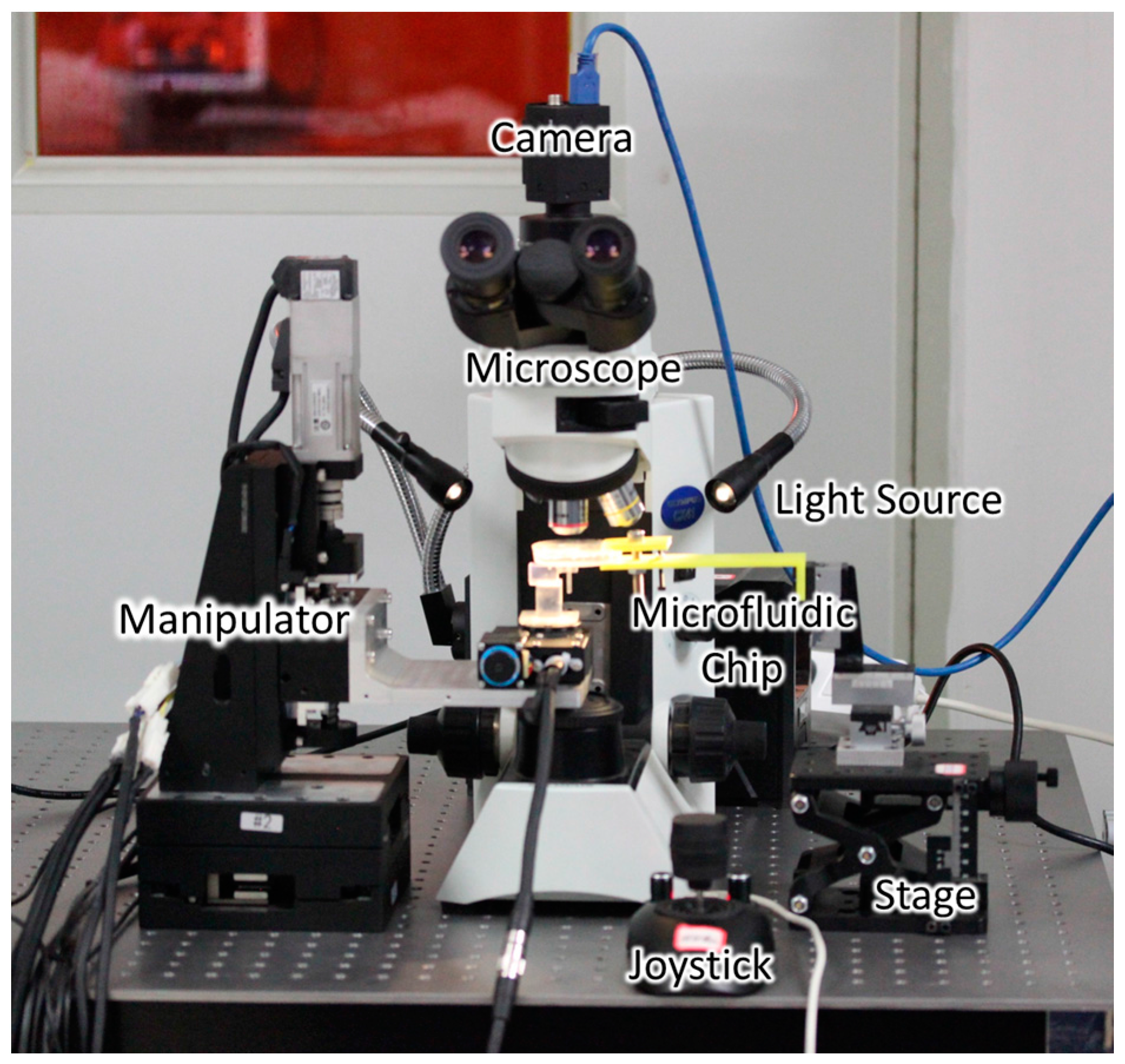
2.3. Experimental Setup
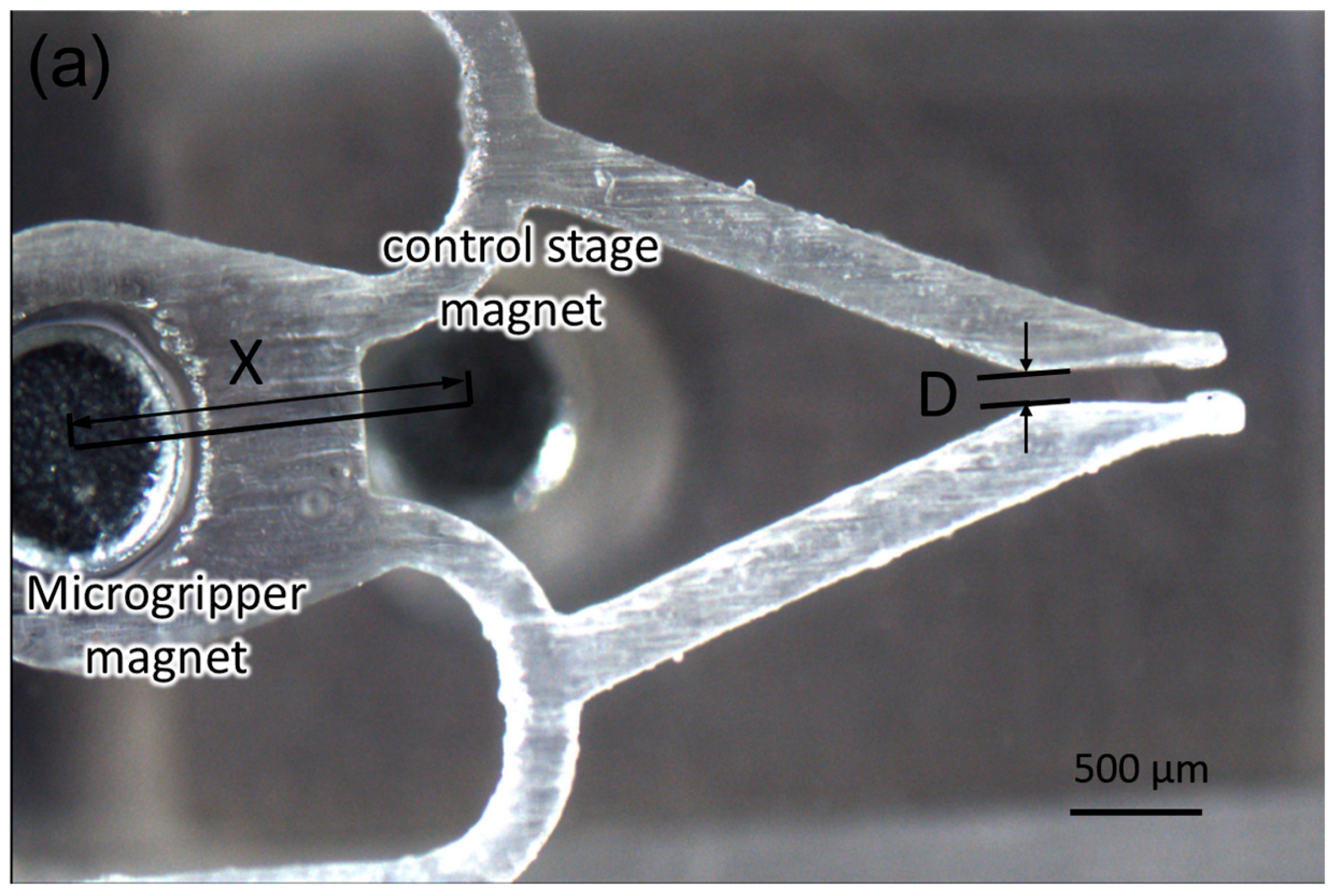
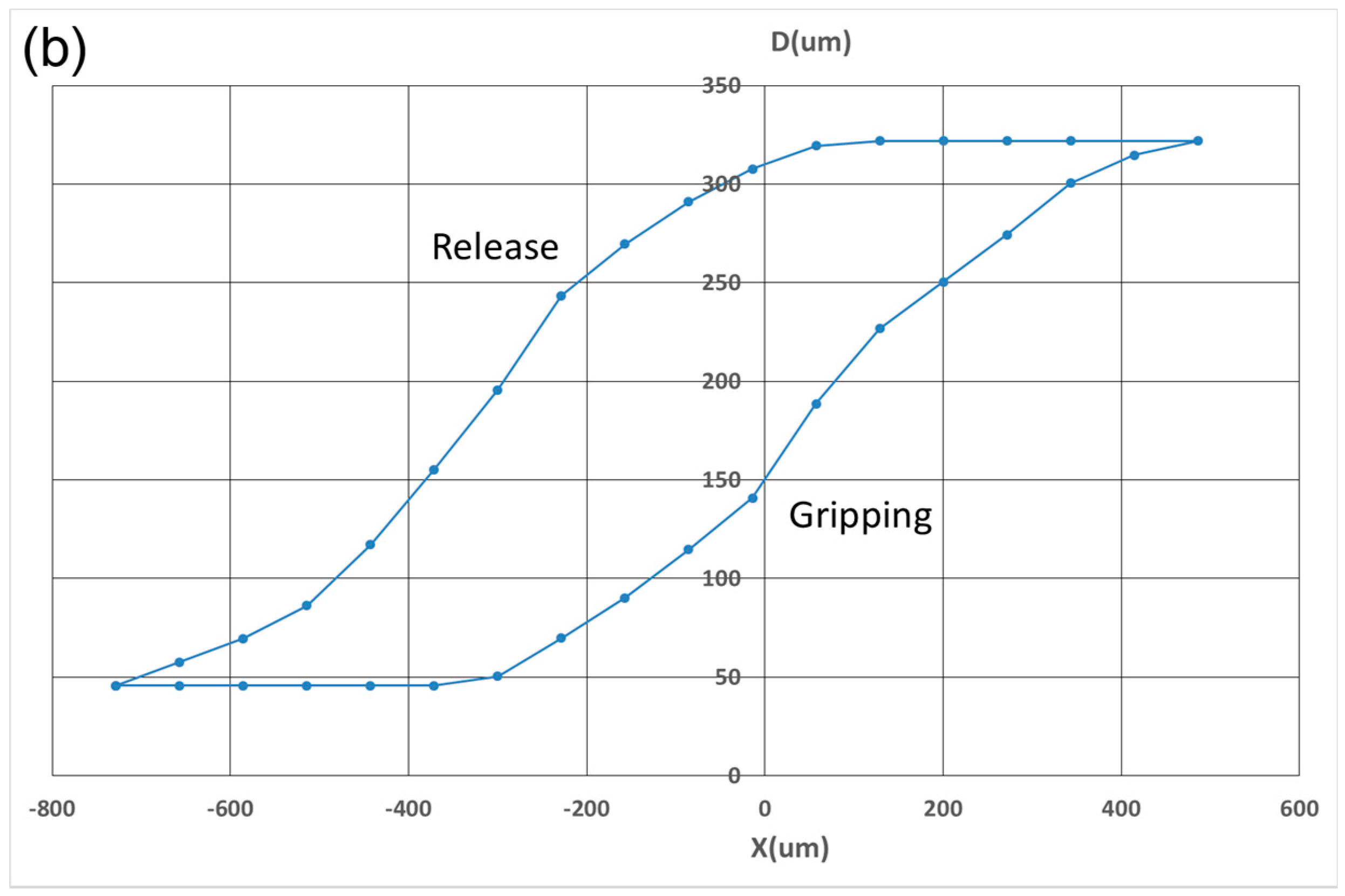
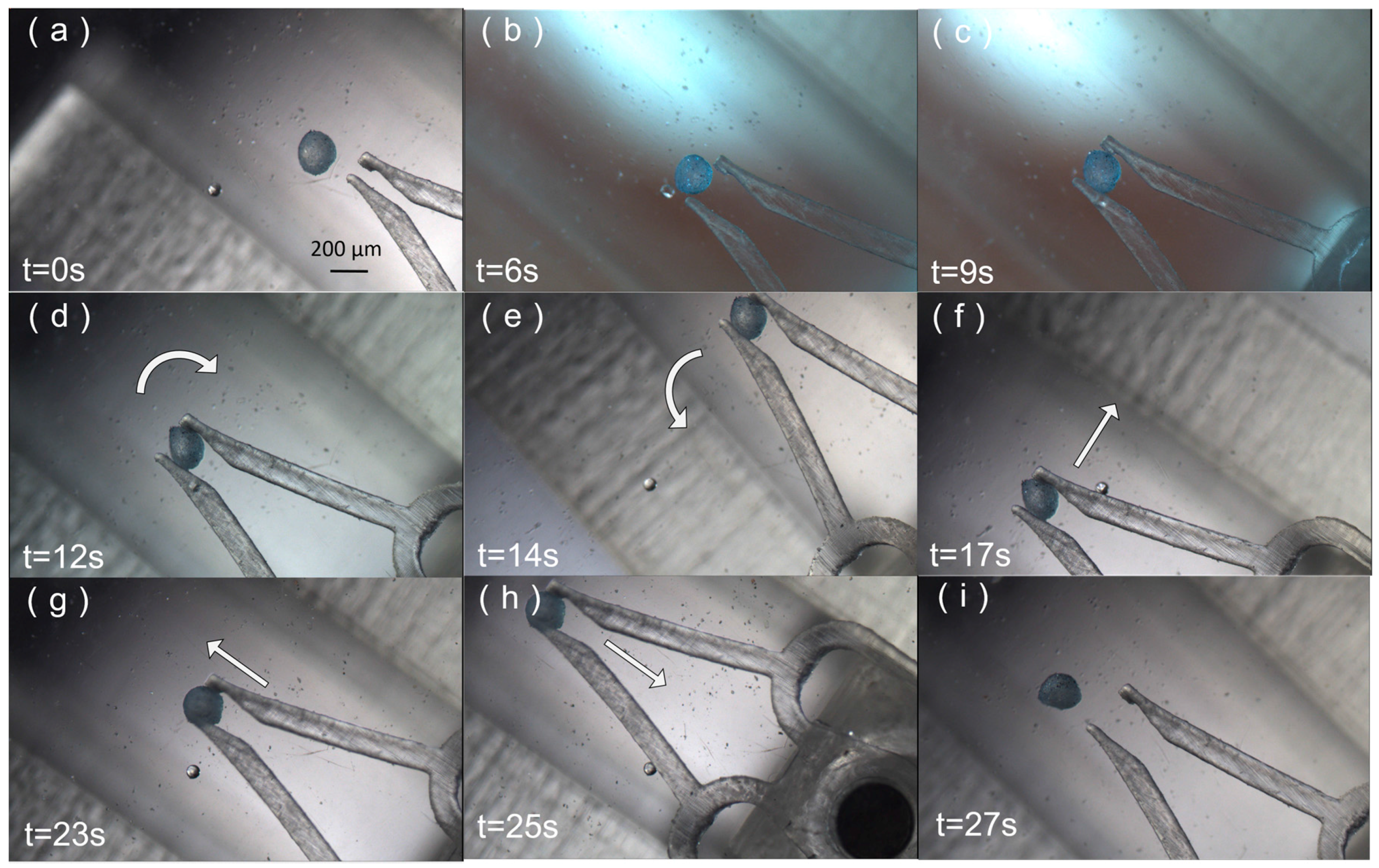
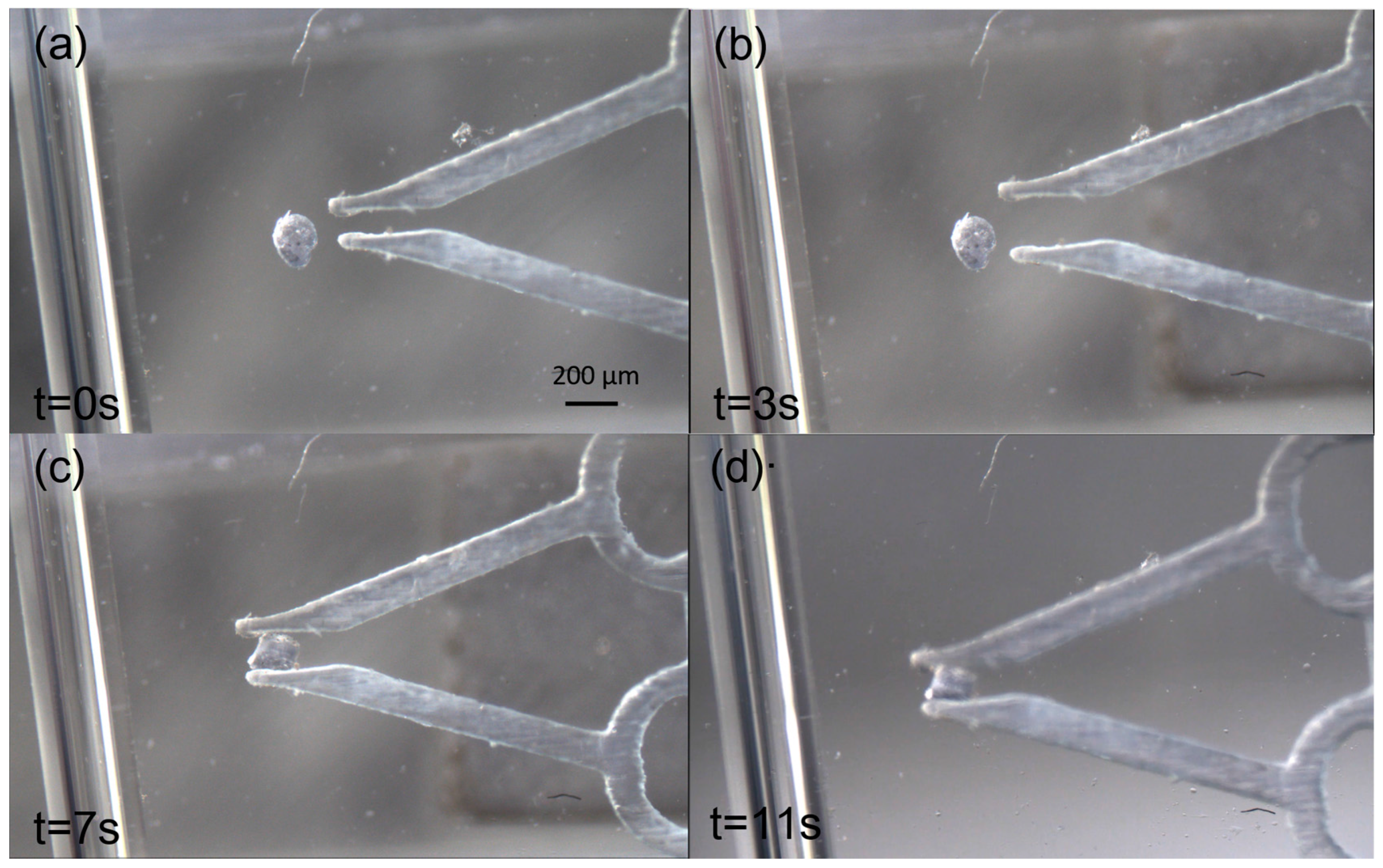
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujita, H. Recent Progress in Micromachining and Applications to Microactuators. Jpn. J. Appl. Phys. 1994, 33, 7163–7166. [Google Scholar] [CrossRef]
- Bayoudh, S.; Nieminen, T.A.; Heckenberg, N.R. Orientation of biological cells using plane-polarized Gaussian beam optical tweezers 1 Introduction. J. Mod. Opt. 2003, 50, 1581–1590. [Google Scholar] [CrossRef]
- Ichikawa, M.; Kubo, K.; Yoshikawa, K.; Kimura, Y. Tilt control in optical tweezers. J. Biomed. Opt. 2008, 13, 010503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, M. Investigation of inclined dual-fiber optical tweezers for 3D manipulation and force sensing. Opt. Express 2009, 17, 13624–13638. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-L.; Huang, Y.-P.; Lu, Y.-S.; Hou, M.T.; Yeh, J.A. Cell rotation using optoelectronic tweezers. Biomicrofluidics 2010, 4, 043003. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; Arai, F. Multi-beam bilateral teleoperation of holographic optical tweezers. Opt. Express 2012, 20, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.A. Some effects of nonuniform fields on dielectrics. J. Appl. Phys. 1958, 29, 1182–1188. [Google Scholar] [CrossRef]
- Nishioka, M.; Katsura, S.; Hirano, K.; Mizuno, A. Evaluation of cell characteristics by step-wise orientational/nrotation using optoelectrostatic micromanipulation. IEEE Trans. Ind. Appl. 1997, 33, 1353–1357. [Google Scholar] [CrossRef]
- Park, J.; Jung, S.-H.; Kim, Y.H.; Kim, B.; Lee, S.-K.; Ju, B.; Lee, K. An integrated bio cell processor for single embryo cell manipulation. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Sendai, Japan, 28 September–2 October 2004; pp. 242–247. [Google Scholar] [CrossRef]
- Kunikata, R.; Takahashi, Y.; Koide, M.; Itayama, T.; Yasukawa, T.; Shiku, H.; Matsue, T. Three dimensional microelectrode array device integrating multi-channel microfluidics to realize manipulation and characterization of enzyme-immobilized polystyrene beads. Sens. Actuators B Chem. 2009, 141, 256–262. [Google Scholar] [CrossRef]
- Gray, D.S.; Tan, J.L.; Voldman, J.; Chen, C.S. Dielectrophoretic registration of living cells to a microelectrode array. Biosens. Bioelectron. 2004, 19, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Beyeler, F.; Neild, A.; Oberti, S.; Bell, D.J.; Sun, Y.; Dual, J.; Nelson, B.J. Monolithically fabricated microgripper with integrated force sensor for manipulating microobjects and biological cells aligned in an ultrasonic field. J. Microelectromech. Syst. 2007, 16, 7–15. [Google Scholar] [CrossRef]
- Colinjivadi, K.S.; Lee, J.-B.; Draper, R. Viable cell handling with high aspect ratio polymer chopstick gripper mounted on a nano precision manipulator. Microsyst. Technol. 2008, 14, 1627–1633. [Google Scholar] [CrossRef]
- Jager, E.W.H. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Wester, B.A.; Rajaraman, S.; Ross, J.D.; Laplaca, M.C.; Allen, M.G. Development and characterization of a packaged mechanically actuated microtweezer system. Sens. Actuators A Phys. 2011, 167, 502–511. [Google Scholar] [CrossRef]
- Kawahara, T.; Sugita, M.; Hagiwara, M.; Arai, F.; Kawano, H.; Shihira-Ishikawa, I.; Miyawaki, A. On-chip microrobot for investigating the response of aquatic microorganisms to mechanical stimulation. Lab Chip 2013, 13, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Sakuma, S.; Sugita, M.; Shoda, T.; Tamakoshi, T.; Akagi, S.; Arai, F. On-chip enucleation of an oocyte by untethered microrobots. J. Micromech. Microeng. 2014, 24, 095004. [Google Scholar] [CrossRef]
- Ceylan, H.; Giltinan, J.; Kozielski, K.; Sitti, M.; Münster, R.; Mierka, O.; Turek, S.; Leshansky, A.M.; Fischer, P.; Zhang, L.; et al. Mobile microrobots for bioengineering applications. Lab Chip 2017, 17, 1705–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peyer, K.E.; Nelson, B.J. Artificial bacterial flagella for micromanipulation. Lab Chip 2010, 10, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Ger, T.-R.; Huang, H.-T.; Chen, W.-Y.; Lai, M.-F. Magnetically-controllable zigzag structures as cell microgripper. Lab Chip 2013, 13, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Dong, X.; Sitti, M. Three-dimensional heterogeneous assembly of coded microgels using an untethered mobile microgripper. Lab Chip 2015, 15, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Dimaki, M.; Svendsen, W.E. Manipulation of biological samples using micro and nano techniques. Integr. Biol. 2009, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Cugat, O.; Delamare, J.; Reyne, G. Magnetic Micro-Actuators and Systems (MAGMAS). IEEE Trans. Magn. 2003, 39, 3607–3612. [Google Scholar] [CrossRef]
- Inomata, N.; Mizunuma, T.; Yamanishi, Y.; Arai, F. Omnidirectional actuation of magnetically driven microtool for cutting of oocyte in a chip. J. Microelectromech. Syst. 2011, 20, 383–388. [Google Scholar] [CrossRef]
- Feng, L.; Hagiwara, M.; Ichikawa, A.; Arai, F. On-Chip enucleation of bovine oocytes using microrobot-assisted flow-speed control. Micromachines 2013, 4, 272–285. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Y.; Ohsumi, C.; Arai, F. Accurate dispensing system for single oocytes using air ejection. Biomicrofluidics 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Di, P.; Arai, F. High-precision motion of magnetic microrobot with ultrasonic levitation for 3-D rotation of single oocyte. Int. J. Robot. Res. 2016, 35, 1445–1458. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Yamanishi, Y.; Masuda, T.; Feng, L.; Arai, F. On-chip magnetically actuated robot with ultrasonic vibration for single cell manipulations. Lab Chip 2011, 11, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Feng, L.; Arai, F. On-demand and Size-controlled Production of emulsion droplets by magnetically driven microtool. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; Volume 1, pp. 4094–4099. [Google Scholar]
- Vokoun, D.; Beleggia, M.; Heller, L.; Šittner, P. Magnetostatic interactions and forces between cylindrical permanent magnets. J. Magn. Magn. Mater. 2009, 321, 3758–3763. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Yamanishi, Y.; Arai, F. Driving method of microtool by horizontally arranged permanent magnets for single cell manipulation. Appl. Phys. Lett. 2010, 97, 1–4. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Wu, X.; Jiang, Y.; Zhang, D.; Arai, F. Manipulating Microrobots Using Balanced Magnetic and Buoyancy Forces. Micromachines 2018, 9, 50. https://doi.org/10.3390/mi9020050
Feng L, Wu X, Jiang Y, Zhang D, Arai F. Manipulating Microrobots Using Balanced Magnetic and Buoyancy Forces. Micromachines. 2018; 9(2):50. https://doi.org/10.3390/mi9020050
Chicago/Turabian StyleFeng, Lin, Xiaocong Wu, Yonggang Jiang, Deyuan Zhang, and Fumihito Arai. 2018. "Manipulating Microrobots Using Balanced Magnetic and Buoyancy Forces" Micromachines 9, no. 2: 50. https://doi.org/10.3390/mi9020050
APA StyleFeng, L., Wu, X., Jiang, Y., Zhang, D., & Arai, F. (2018). Manipulating Microrobots Using Balanced Magnetic and Buoyancy Forces. Micromachines, 9(2), 50. https://doi.org/10.3390/mi9020050





