A Study on An EMG Sensor with High Gain and Low Noise for Measuring Human Muscular Movement Patterns for Smart Healthcare
Abstract
:1. Introduction
2. System Overview
2.1. Block Diagram of the EMG Sensor
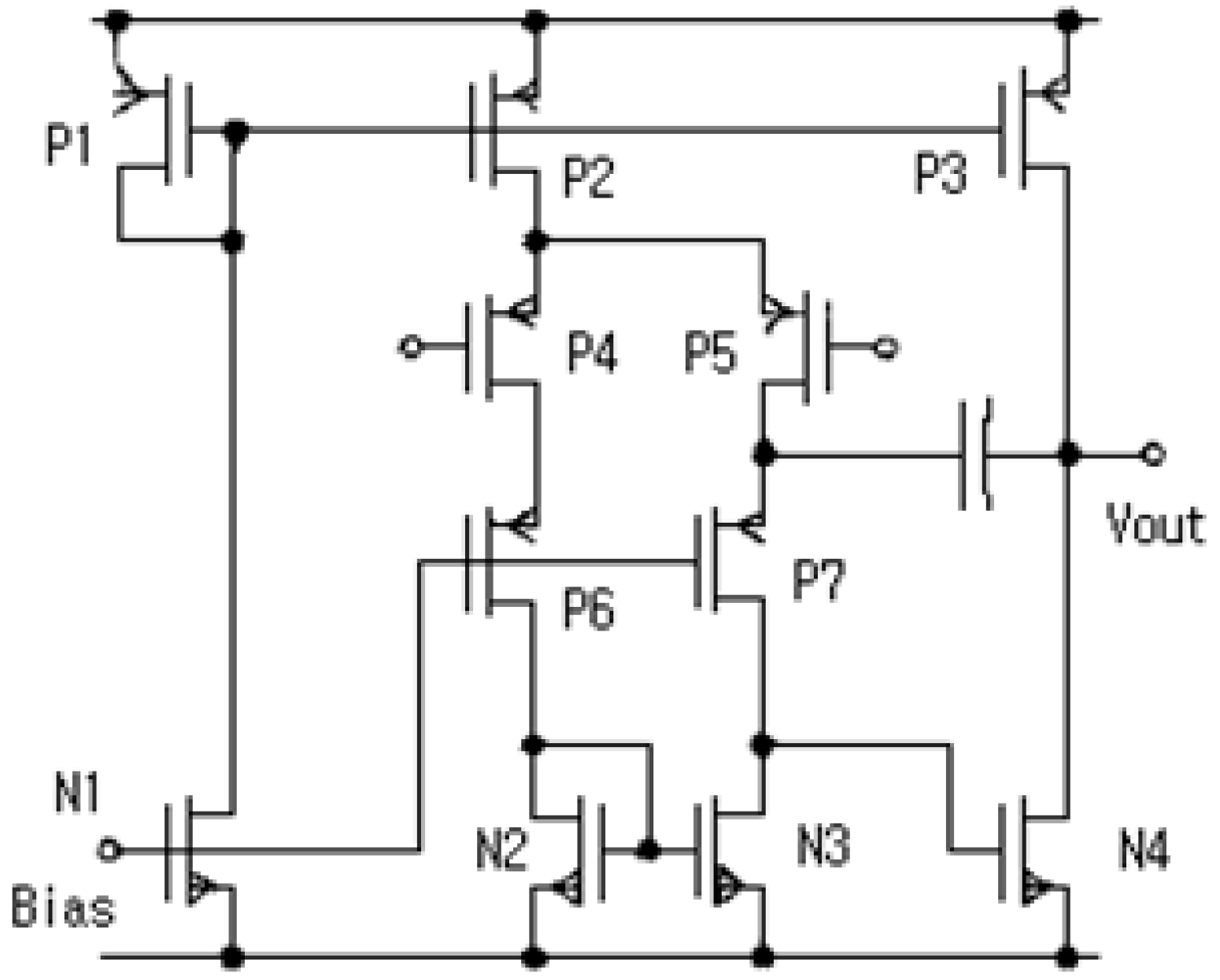
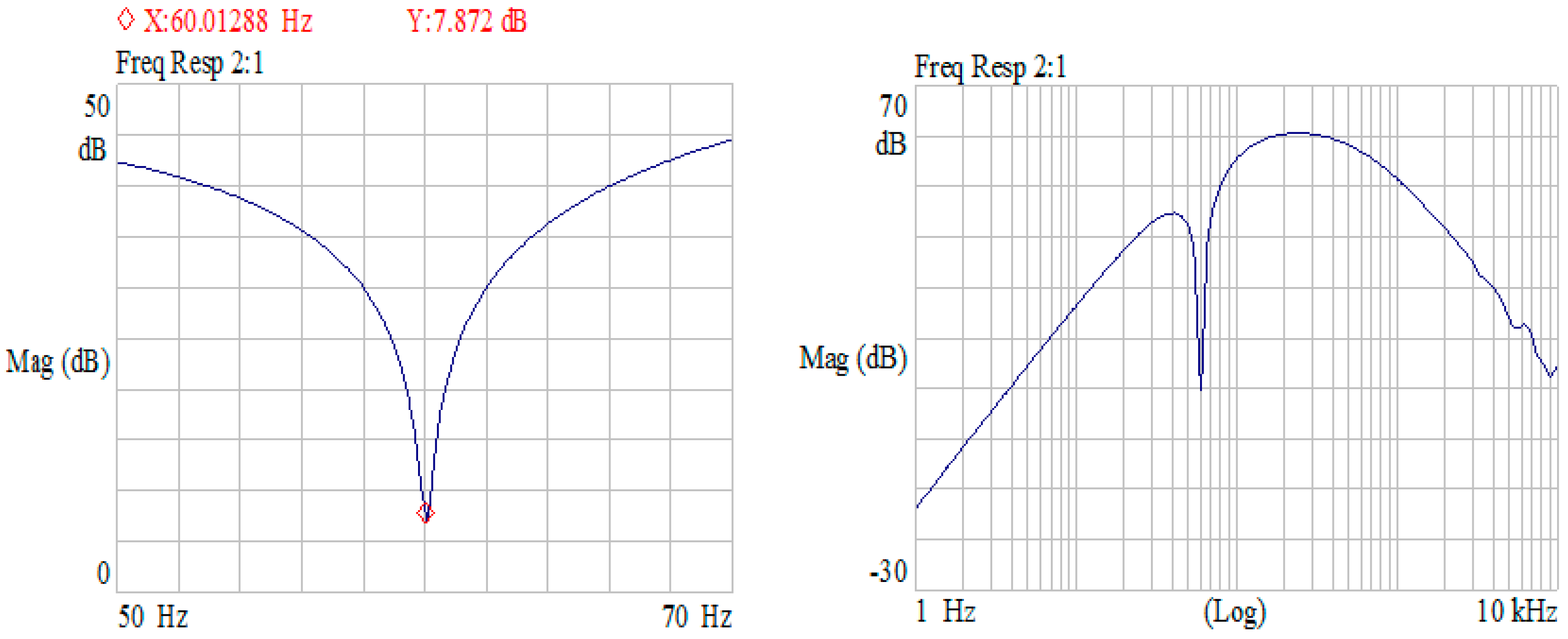
2.2. ASIC of Core-Amplifier
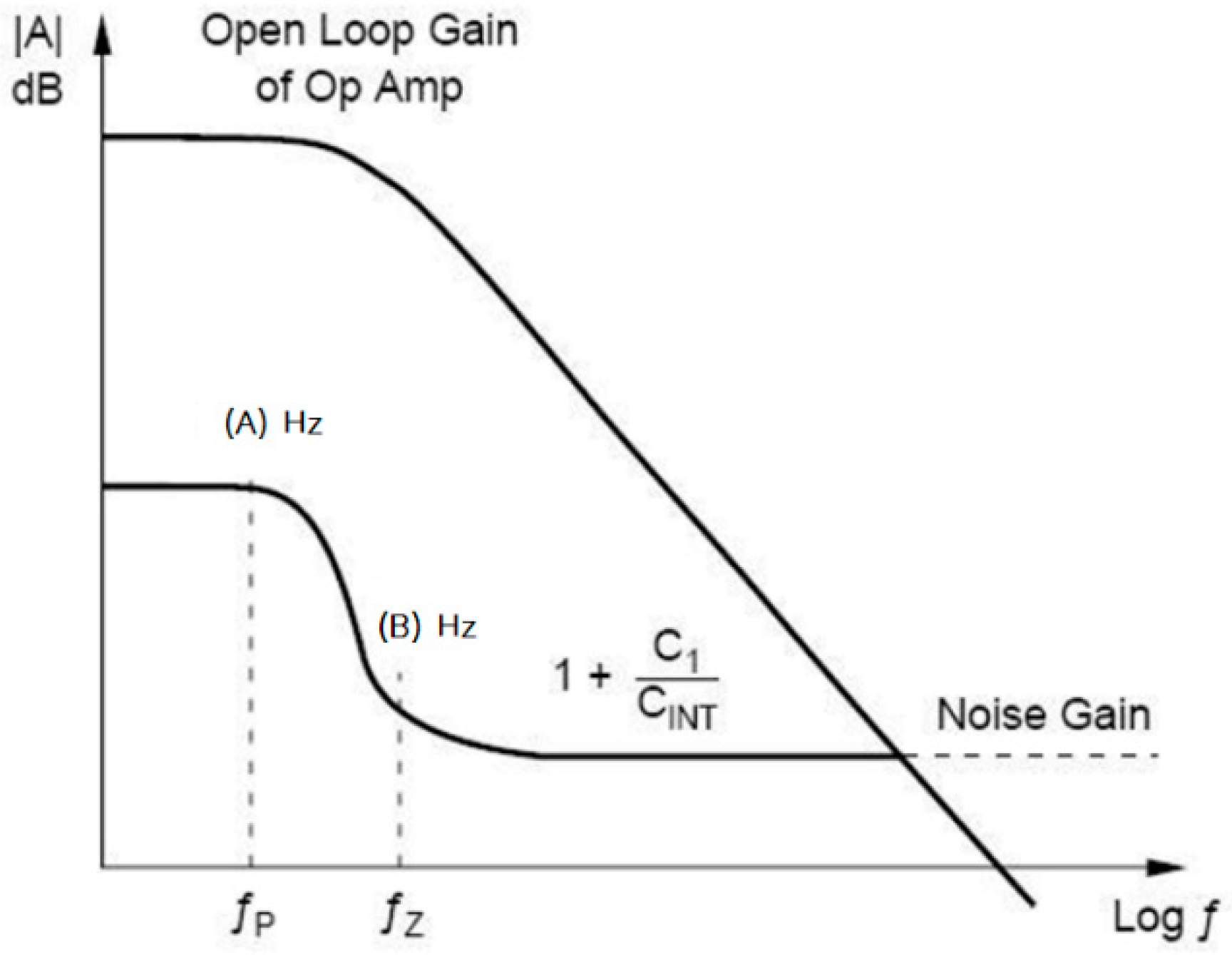
2.3. Noise Analysis of Core-Amplifier
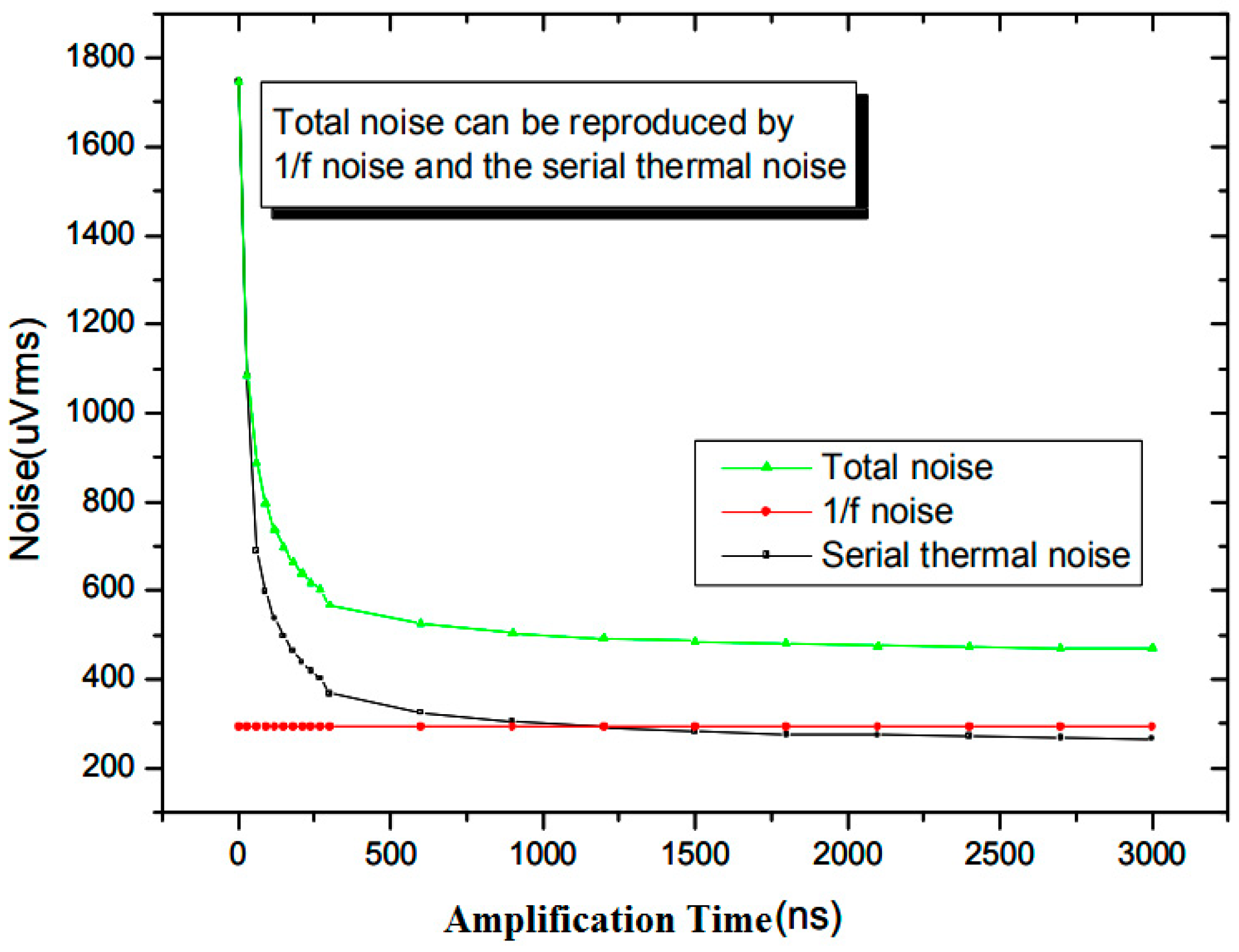
2.3.1. 1/f Noise
2.3.2. Serial Thermal Noise
2.3.3. Parallel Noise
2.3.4. Design of EMG Sensor
3. Result and Discussion
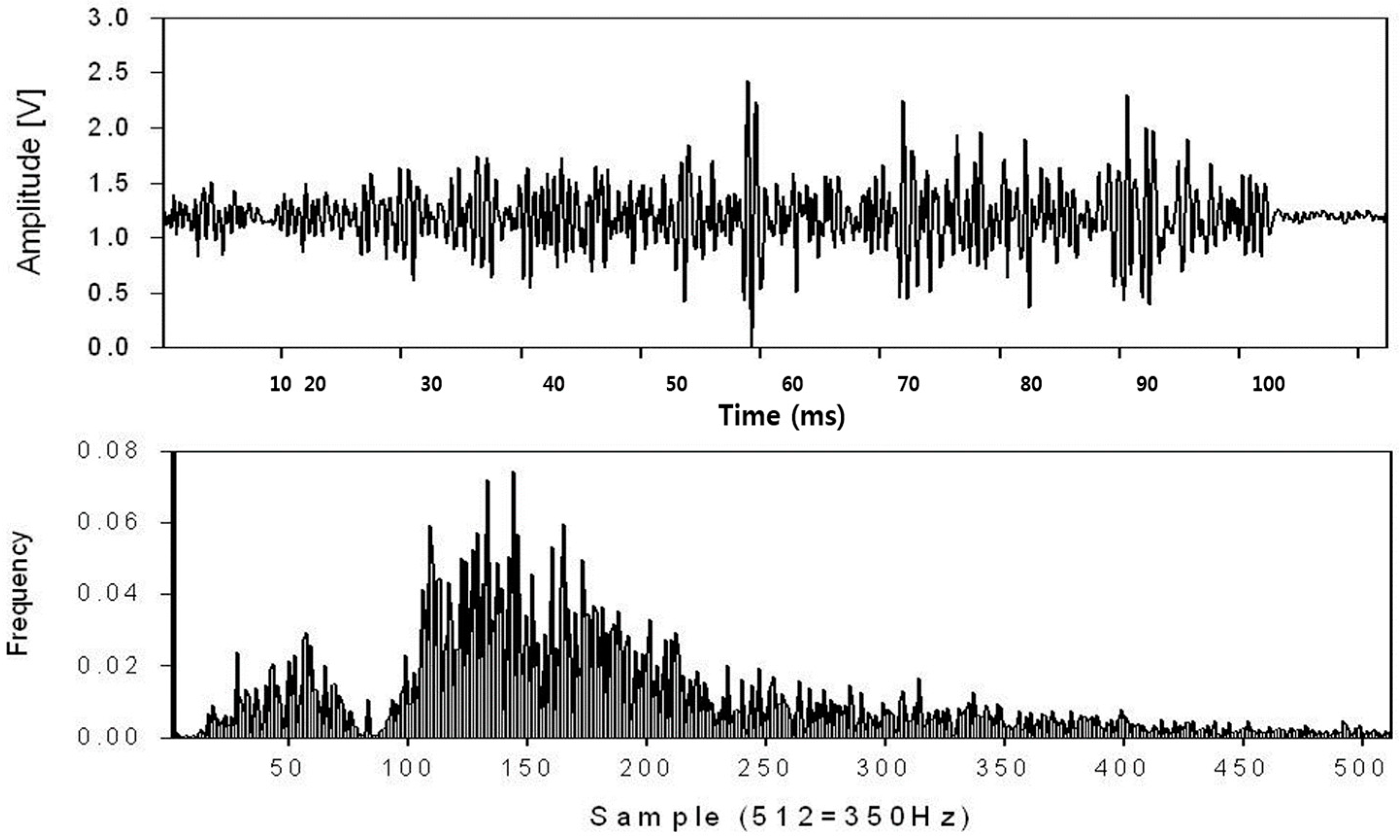
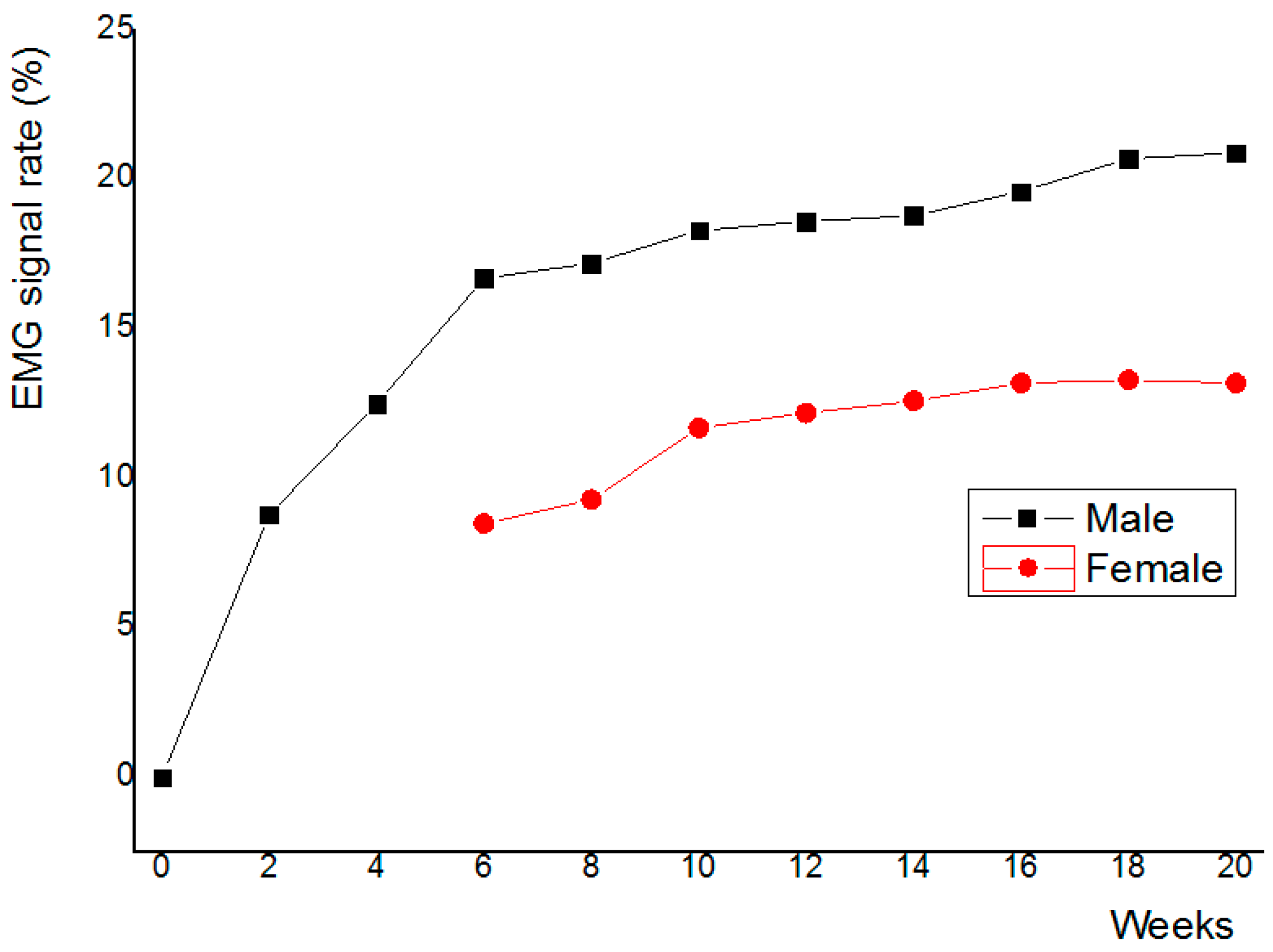
3.1. Myoelectric Signal Analysis Results
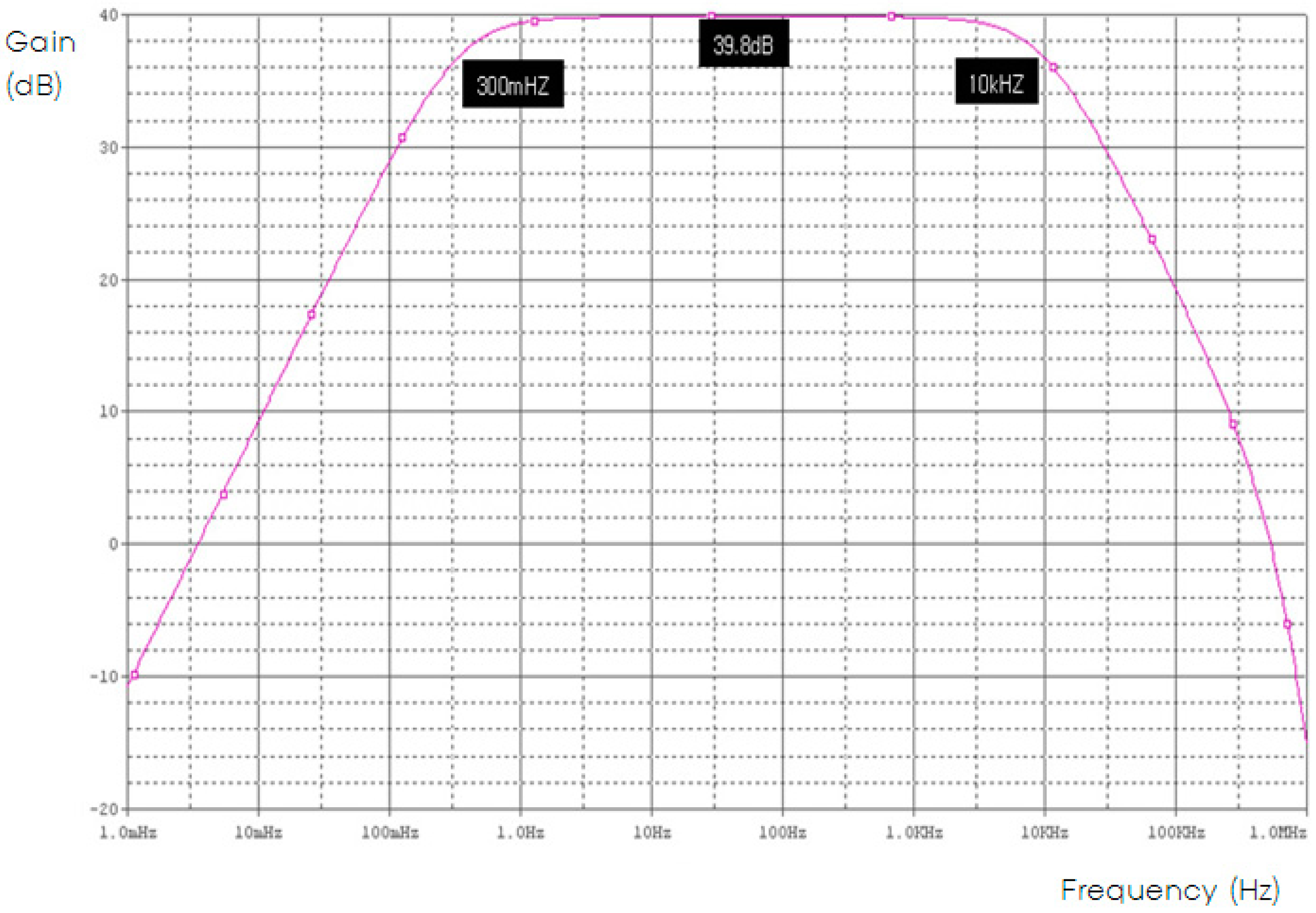
3.2. Noise Analysis Results
3.3. Verification of Results of Research
3.4. Clinical Trial Results
4. Conclusions
Author Contributions
Conflicts of Interest
Appendix A


References
- De Luca, C.J. The use of surface EMG electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Nicolai, H.; Teodorsscu, L.; Jain, L.C. Intelligent Systems and Technologies in Rehabilitation Engineering; CRC Press: Boca Raton, FL, USA, 2001; pp. 243–246. [Google Scholar]
- Farina, D.; Merletti, R. A novel approach for estimating muscle fiber conduction velocity by spatial and temporal filtering of surface EMG signals. IEEE Trans. Biomed. Eng. 2003, 50, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Melaku, A. Electrode Distance and magnitude of SEMG. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Houston, TX, USA, 23–26 October 2002; pp. 2477–2480. [Google Scholar]
- De Luca, C.J. Myoelectric manifestations of localized muscular fatigue in humans. CRC Crit. Rev. Biomed. Eng. 1985, 11, 251–279. [Google Scholar]
- Roy, S.H.; De Luca, C.J. Evolving characteristics of the median frequency of the EMG signal. In Computer Aided Electromyography and Expert Systems; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1989; pp. 205–221. [Google Scholar]
- Ismail, M.; Fiez, T. Analog VLSI; McGraw Hill: New York, NY, USA, 1994. [Google Scholar]
- Aspell, P. A fast, low power CMOS Amplifier on SOI for sensor applications in a radiation environment of up to 20 Mrad(Si). IEEE Trans. Nucl. Sci. 1995, 42, 1636–1640. [Google Scholar] [CrossRef]
- Graeme, J.G. Photodiode Amplifiers; McGraw Hill: New York, NY, USA, 1996. [Google Scholar]
- Fessler, P. An important step forward in continuous spectroscopic imaging of ionizing radiations using ASICs. Nucl. Instrum. Methods Phys. Res. A 1999, 421, 130–141. [Google Scholar] [CrossRef]
- Yuk, S.W. A Study on Design of PIN Photodiode Coupled with Pixellated Scintillator for Low Energy Detector. Ph.D. Thesis, Korea University, Seoul, Korea, 2006. [Google Scholar]
- Schaumann, R.; Van Valkenburg, M.E. Design of Analog Filters; Oxford University Press, Inc.: Oxford, UK, 2001. [Google Scholar]
- Franco, S. Design with Operational Amplifiers and Analog Integrated Circuits; McGrow Hill Companies, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Todorov, V.; Filzmoser, P. Robust statistic for the one-way MANOVA. Comput. Data Stat. Anal. 2010, 54, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.H.; De Luca, C.J.; Casavant, D.A. Lumbar muscle fatigue and chronic low back pain. Spine 1989, 14, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.H.; De Luca, C.J.; Snyder-Mackler, L.; Emley, M.S.; Crenshaw, R.L.; Lyons, J.P. Fatigue, recovery and low back pain in varsity rowers. Med. Sci. Sports Exerc. 1990, 22, 463–469. [Google Scholar] [CrossRef] [PubMed]
- St-Amant, Y.; Rancourt, D.; Clancy, E.A. Influence of smoothing window length on electromyogram amplitude estimates. IEEE Trans. Biomed. Eng. 1998, 45, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.; Mann, R.W. Myoelectric signal processing: Optimal estimation applied to electromyography—Part II: Experimental demonstration of optimal myoprocessor performance. IEEE Trans. Biomed. Eng. 1980, BME-27, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Hogan, N. Theoretic and experimental comparison of root-mean-square and mean-absolute-value electromyogram amplitude detectors. In Proceedings of the the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 30 October–2 November 1997; pp. 1267–1270. [Google Scholar]
- Clancy, E.A.; Hogan, N. Single site electromyograph amplitude estimation. IEEE Trans. Biomed. Eng. 1994, 41, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constable, C.R.; Thornbill, R.J. Using the discrete wavelet transform for time-frequency analysis of the surface EMG signal. Biomed. Sci. Instrum. 1993, 29, 121–127. [Google Scholar] [CrossRef]
- Etawil, H.A.Y.; Stashuk, D.W. Resolving superimposed motor unit action potentials. Med. Biol. Eng. Comput. 1996, 34, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Agarwal, G.C.; Shahani, B.T. Decomposition of multiunit electromyographic signals. IEEE Trans. Biomed. Eng. 1999, 46, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Colombo, R.; Merletti, R.; Baare Olsen, H. Evaluation of intra-muscular EMG signal decomposition algorithms. J. Electromyogr. Kinesiol. 2001, 11, 175–187. [Google Scholar] [CrossRef]
- Gemperline, J.J.; Allen, S.; Walk, D.; Rymer, W.Z. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve 1995, 18, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Gronlund, C.; Roeleveld, K.; Holtermann, A.; Karlsson, J.S. On-line signal quality estimation of multichannel surface electromyograms. Med. Biol. Eng. Comput. 2005, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Drost, G.; Stegeman, D.F.; van Engelen, B.G.; Zwarts, M.J. Clinical applications of high-density surface EMG: A systematic review. J. Electromyogr. Kinesiol. 2006, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Existing Product (Ottobock) | Value (Manufactured EMG Sensor) |
|---|---|---|
| Gain | By feedback resistance | 10 mV/fC |
| Noise | 477 uVrms | 500 uVrms |
| Output Swing | 5 V | 2.8 V |
| GBW (gain bandwidth) | 10 kHz | 200 MHz |
| Input Range | 10 fC | 4 fC |
| Linearity | 0.3% S.d | 0.3% S.d |
| Power Consumption | About 2 mW | 2 mW |
| CMOS Technology | N/A | 0.25 μm |
| Device | Z/L | Device | Z/L | Device | Z/L |
|---|---|---|---|---|---|
| P1 | 60/20 | P1 | 50/10 | N2 | 120/75 |
| P1 | 350/20 | P1 | 120/10 | N3 | 120/75 |
| P1 | 300/20 | P1 | 120/10 | N4 | 60/20 |
| P1 | 50/10 | N1 | 10/10 | - | - |
| Phase Margin | Phase of Signal | Noise | % Error Rate |
|---|---|---|---|
| 45° | 0.134 ms | 145 uVrms | 0.23% |
| 50° | 0.145 ms | 195 uVrms | 0.43% |
| 55° | 0.168 ms | 286 uVrms | 0.75% |
| 60° | 0.198 ms | 369 uVrms | 0.98% |
| 65° | 0.226 ms | 469 uVrms | 1.08% |
| Parameter | Gain | Noise | System Error |
|---|---|---|---|
| Number | 10 | 10 | 10 |
| Avg | 9.8 mV/fC | 487 uVrms | 1.11% |
| Median | 9.9 mV/fC | 502 uVrms | 1.03% |
| Z-score | 0.83 | 0.96 | 0.98 |
| 1. The EMG signal is measured for the upper limb cutter. |
| 2. Research participants should change to the same lab uniform. To measure the EMG of the upper extremity muscles, use the EMG sensor where the upper extremity moves most strongly. In addition, the other hand is grounded to obtain accurate experimental measurement data. Do not collect signals whose signal levels are shaken or whose thresholds are not exceeded [25,26]. |
| 3. All experimental data use only data values that meet 95% confidence with robust statistical processing techniques. |
| 4. Experimental method A. EMG measurement for 5 min in rest (sitting comfortably) B. Perform the same type of measurement every week at the right time C. Assessment of whether all data satisfy 95% confidence level in robust statistics D.Measure muscle movement (It is measured by the EMG signal level because it is impossible to measure with the naked eye.) E. Record the temperature rise of the system at all measurements. F. Record system error rate as temperature increases G. Record until after the system has an idle period until the error is less than 1%. H. The same type of measurement should be performed at intervals of 2 weeks for 20 weeks. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuk, S.-W.; Hwang, I.-H.; Cho, H.-R.; Park, S.-G. A Study on An EMG Sensor with High Gain and Low Noise for Measuring Human Muscular Movement Patterns for Smart Healthcare. Micromachines 2018, 9, 555. https://doi.org/10.3390/mi9110555
Yuk S-W, Hwang I-H, Cho H-R, Park S-G. A Study on An EMG Sensor with High Gain and Low Noise for Measuring Human Muscular Movement Patterns for Smart Healthcare. Micromachines. 2018; 9(11):555. https://doi.org/10.3390/mi9110555
Chicago/Turabian StyleYuk, Sun-Woo, In-Ho Hwang, Hyeon-Rae Cho, and Sang-Geon Park. 2018. "A Study on An EMG Sensor with High Gain and Low Noise for Measuring Human Muscular Movement Patterns for Smart Healthcare" Micromachines 9, no. 11: 555. https://doi.org/10.3390/mi9110555




