An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Human Serum Samples
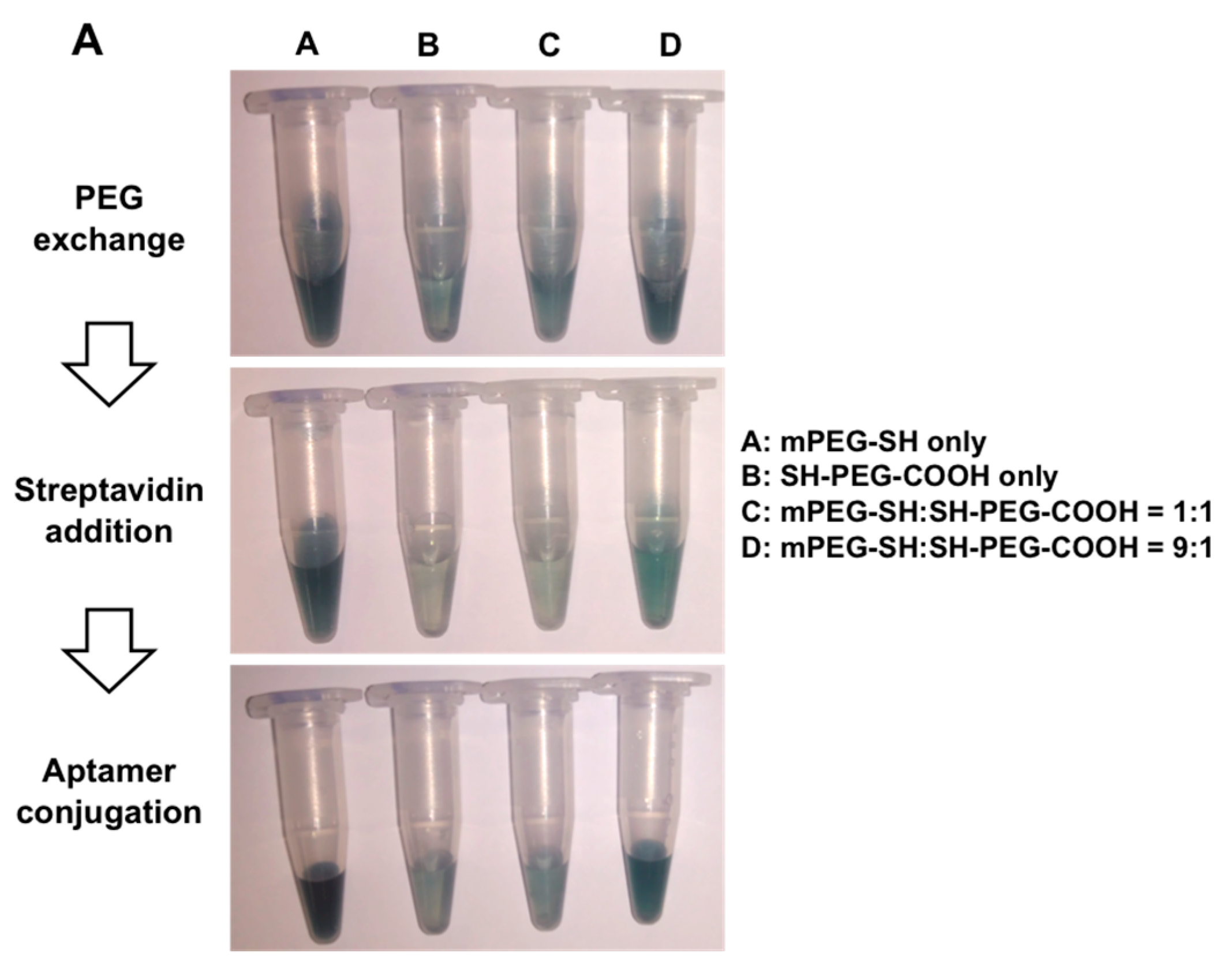
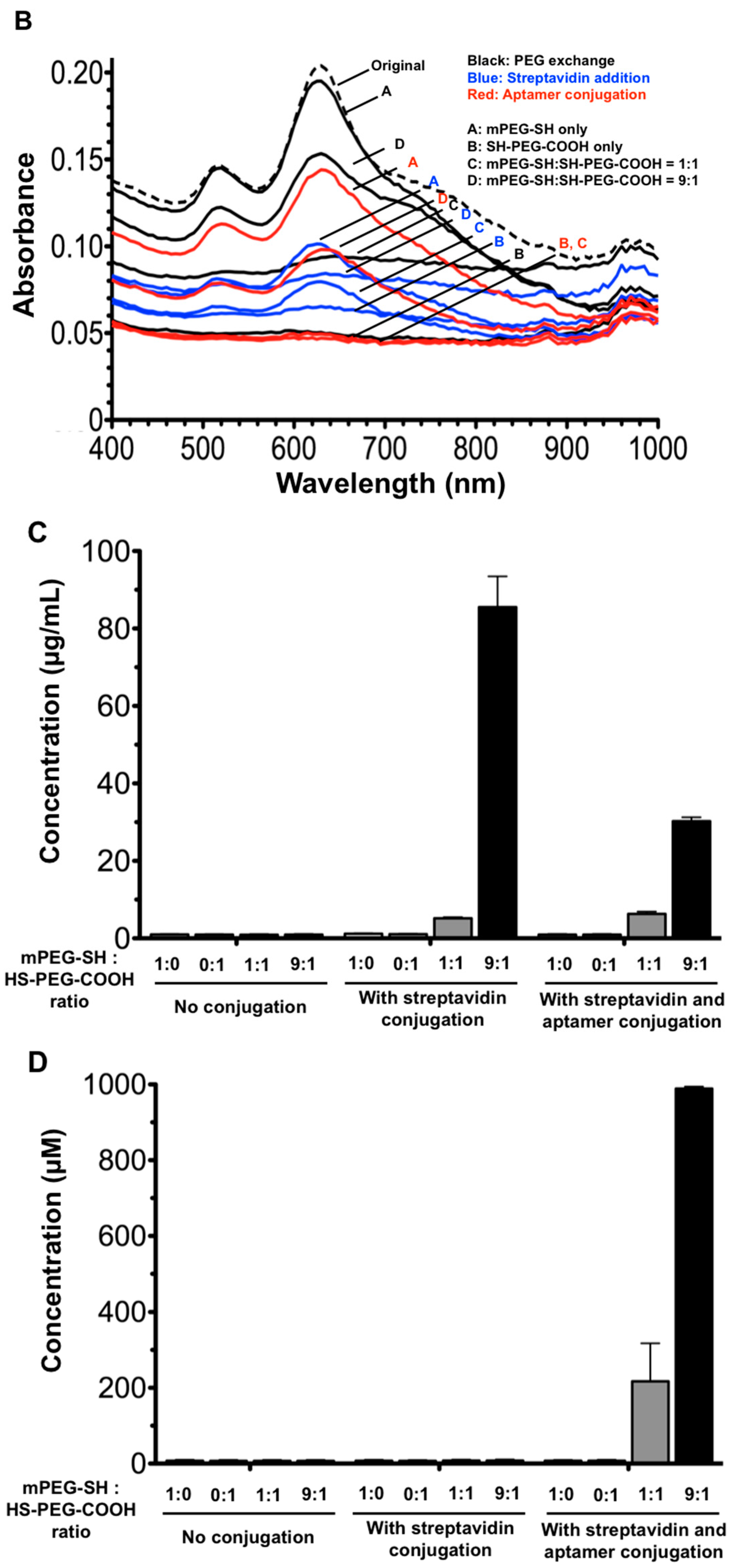
2.2. AuNR Preparation and Functionalization
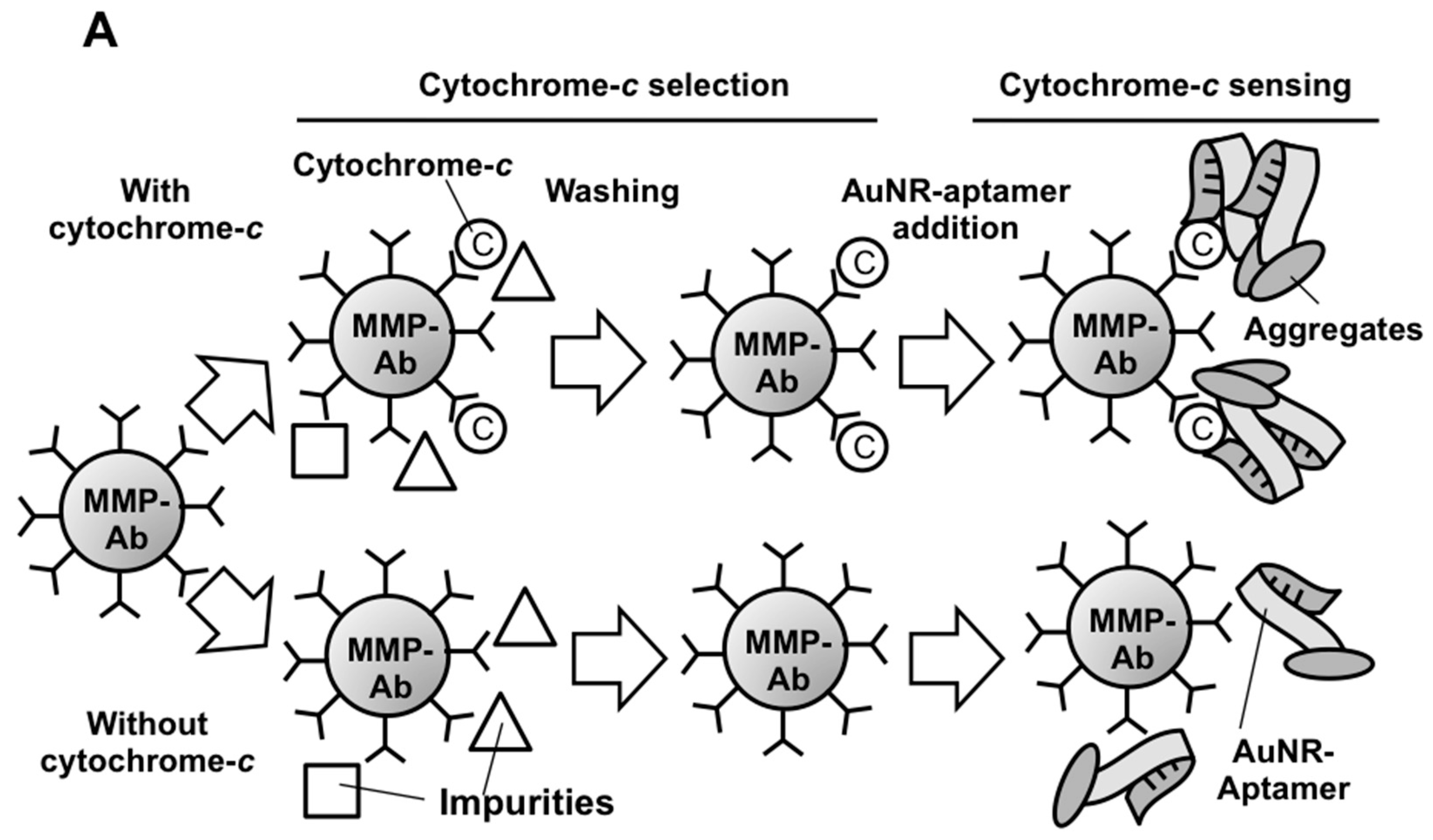
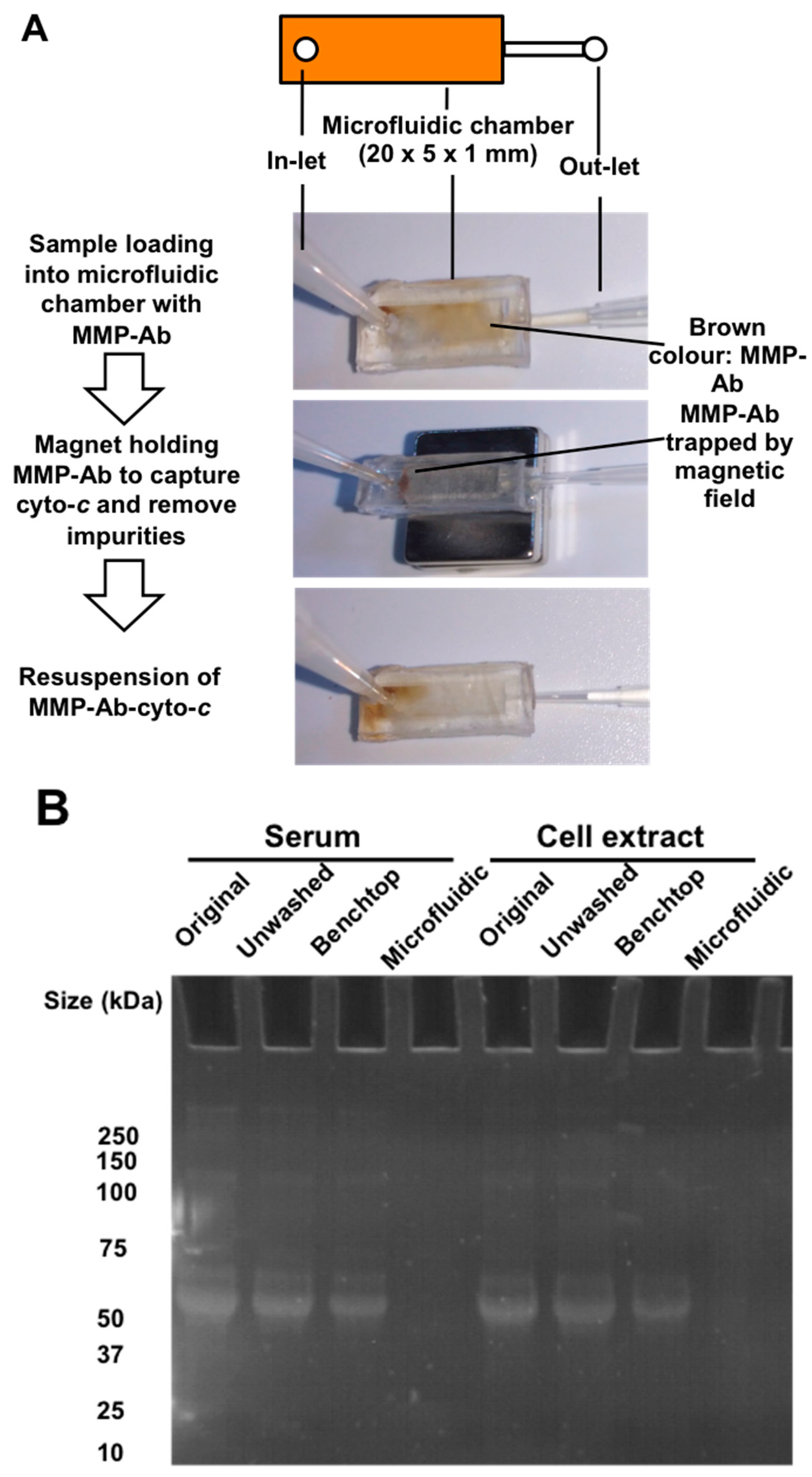
2.3. Microfluidics Design and Fabrication
2.4. AuNR-LSPR Assay
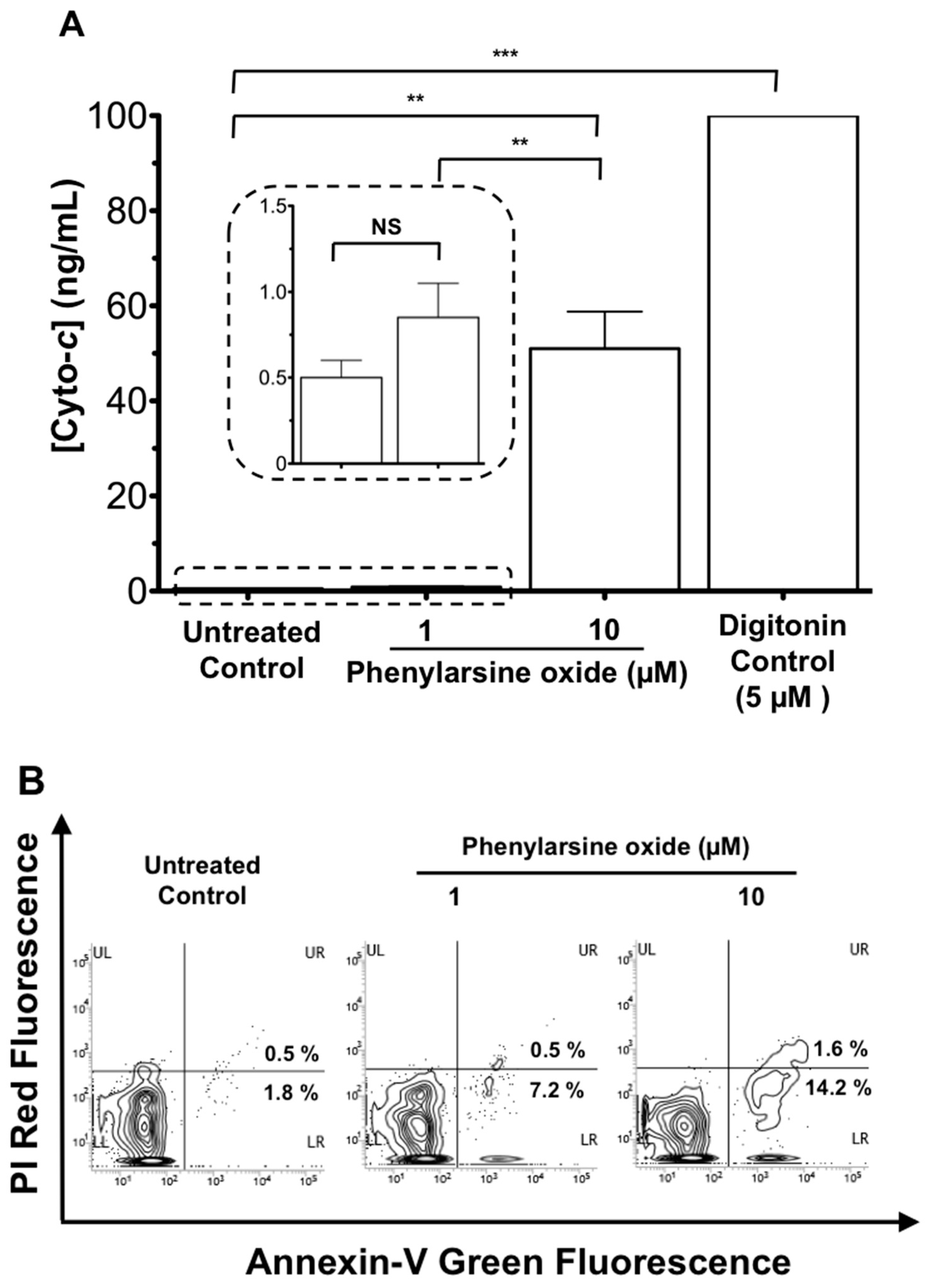
2.5. Cancer Cells and Anti-Cancer Drug Treatment
2.6. Data Analysis
3. Results and Discussion
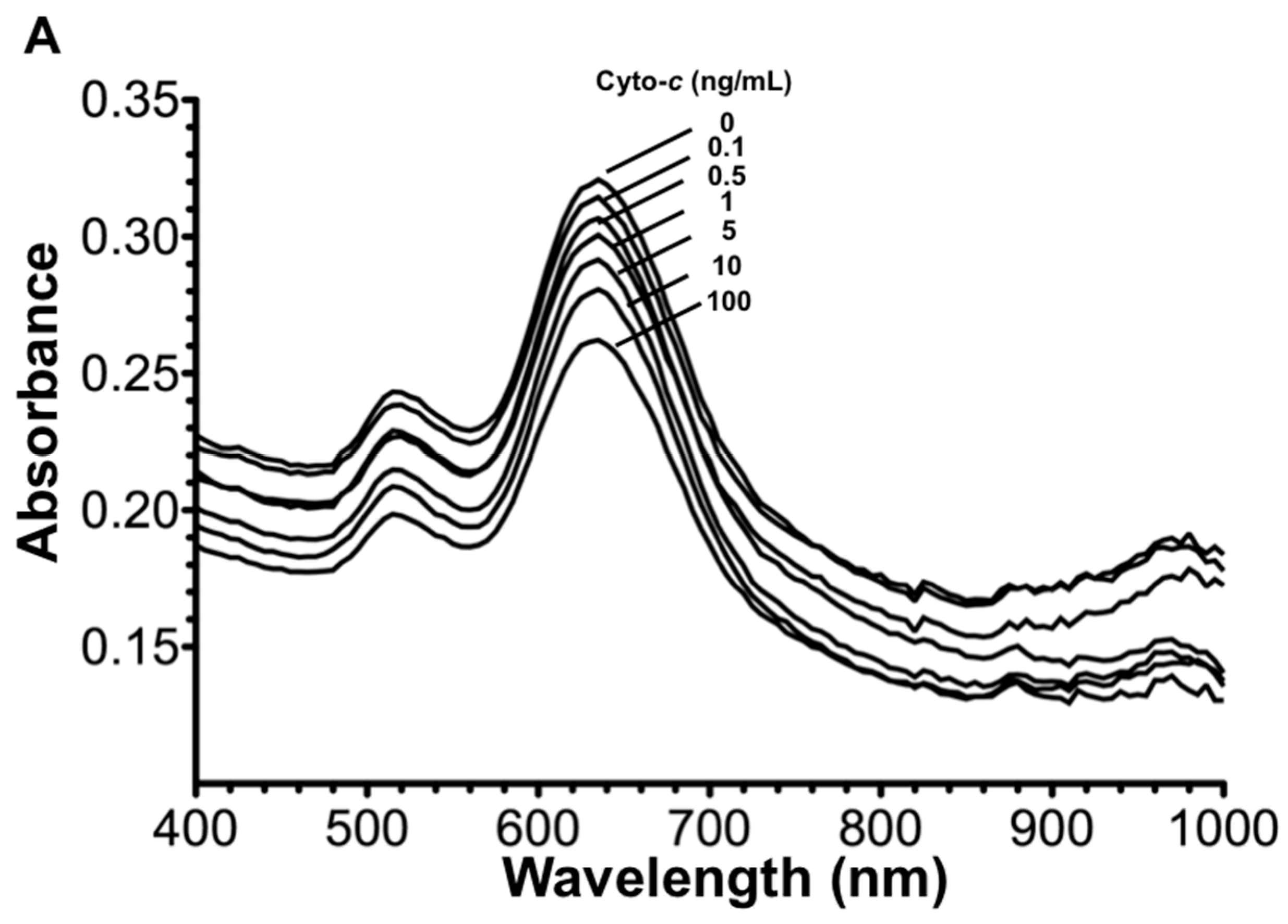
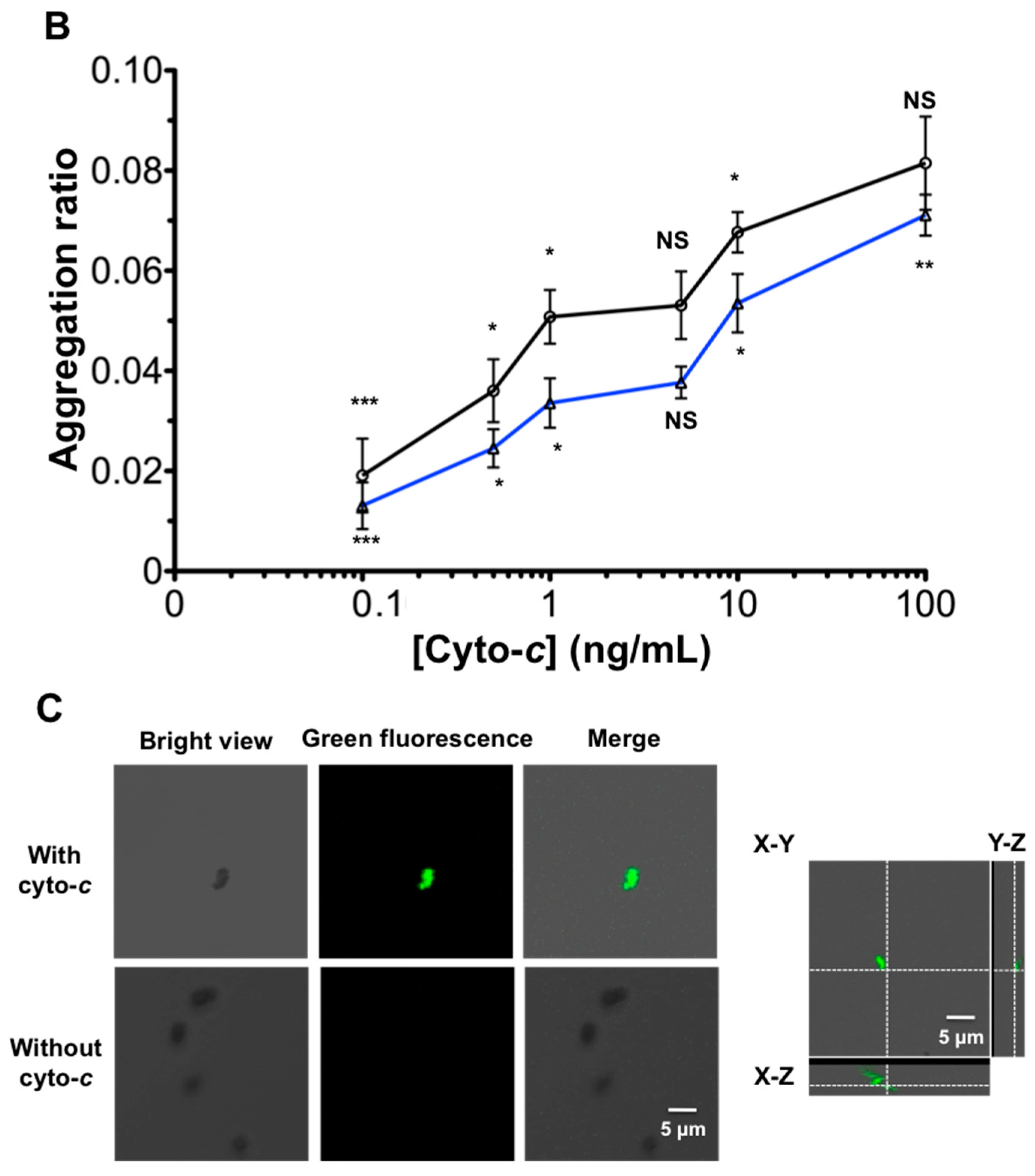
3.1. Optimization of AuNR-Functionalized Aptamer
3.2. Signal Enhancement and Sensitivity Using the AuNR-Aptamer on AuNR-LSPR Assay
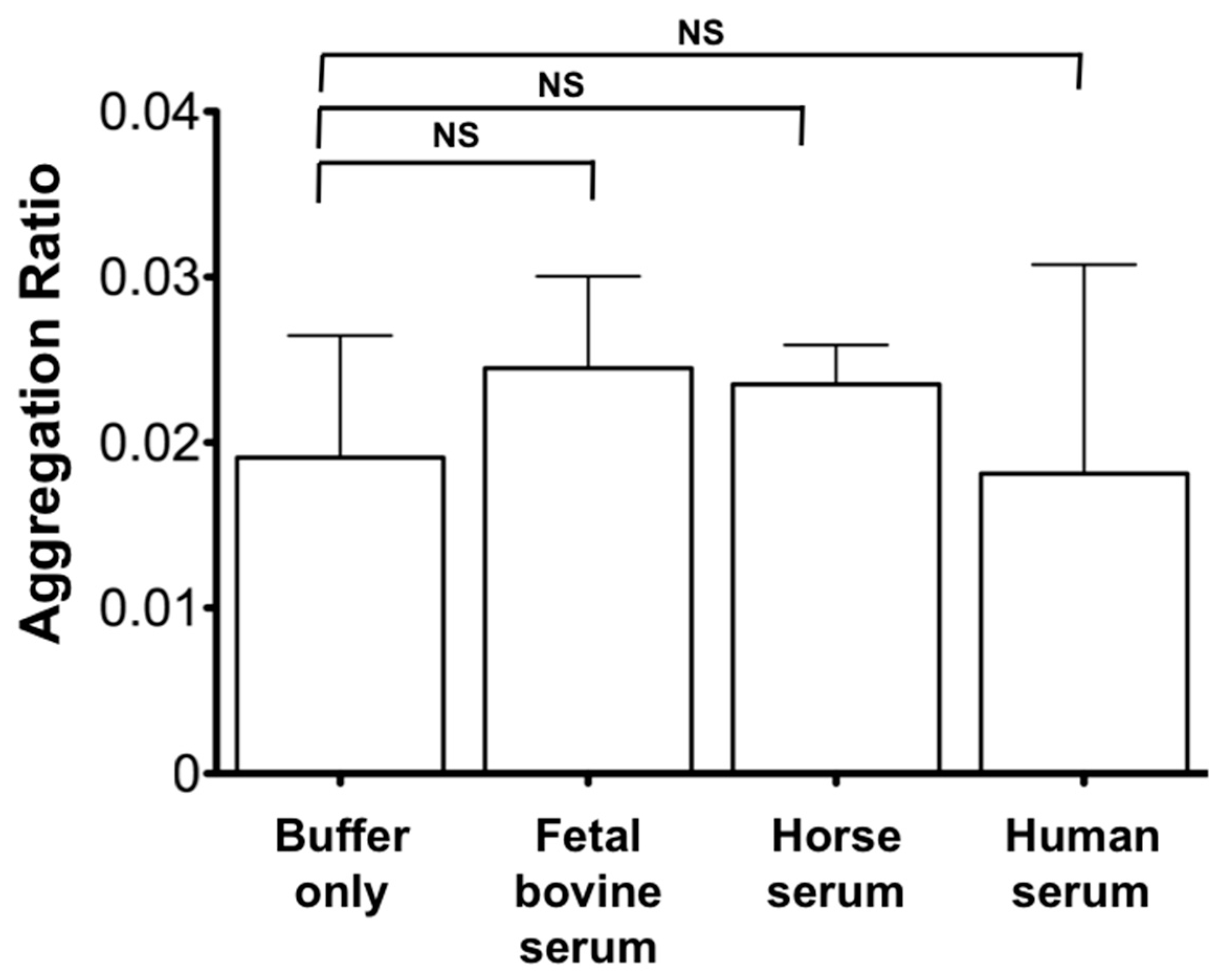
3.3 Determination of Cyto-c Concentration in Serum Using AuNR-LSPR Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alleyne, T.; Joseph, J.; Sampson, V. Cytochrome-c detection: A diagnostic marker for myocardial infarction. Appl. Biochem. Biotechnol. 2001, 90, 97–105. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a potentially clinical useful marker of mitochondrial and cellular damage. Front. Immunol. 2016, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Jemmerson, R.; LaPlante, B.; Treeful, A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death Differ. 2002, 9, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, K.; Kreuter, M.; Pryjma, J.; Booy, E.P.; Maddika, S.; Ghavami, S.; Berdel, W.E.; Roth, J.; Los, M. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int. J. Cancer 2005, 116, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Wang, S.; Ayoub, I.M.; Kolarova, J.D.; Levine, R.F.; Gazmuri, R.J. Circulating levels of cytochrome c after resuscitation from cardiac arrest: A marker of mitochondrial injury and predictor of survival. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H767–H775. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Yang, S.; Chen, D.; Chaudry, I.; Raju, R. Influence of aging and hemorrhage injury on Sirt1 expression: Possible role of myc-Sirt1 regulation in mitochondrial function. Biochim. Biophys. Acta 2011, 1812, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Phaneuf, S.; Leeuwenburgh, C. Cytochrome c release from mitochondria in the aging heart: A possible mechanism for apoptosis with age. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R423–R430. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Hirota, M.; Hamaguchi, M.; Okamoto, K.; Watanabe, K.; Endo, F. Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin. Chim. Acta 2004, 342, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Renz, A.; Berdel, W.E.; Kreuter, M.; Belka, C.; Schulze-Osthoff, K.; Los, M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood 2001, 98, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Loo, F.C.; Ng, S.P.; Wu, C.M.L.; Kong, S.K. An aptasensor using DNA aptamer and white light common-path SPR spectral interferometry to detect cytochrome-c for anti-cancer drug screening. Actuators B Chem. 2014, 198, 416–423. [Google Scholar] [CrossRef]
- Loo, J.F.; Lau, P.M.; Ho, H.P.; Kong, S.K. An aptamer-based bio-barcode assay with isothermal recombinase polymerase amplification for cytochrome-c detection and anti-cancer drug screening. Talanta 2013, 115, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.P.; Ngan, E.K.; Loo, J.F.; Suen, Y.K.; Ho, H.P.; Kong, S.K. Aptamer-based bio-barcode assay for the detection of cytochrome-c released from apoptotic cells. Biochem. Biophys. Res. Commun. 2010, 395, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.F.; Yang, C.; Tsang, H.L.; Lau, P.M.; Yong, K.T.; Ho, H.P.; Kong, S.K. An Aptamer Bio-barCode (ABC) assay using SPR, RNase H, and probes with RNA and gold-nanorods for anti-cancer drug screening. Analyst 2017, 142, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Syahir, A.; Usui, K.; Tomizaki, K.Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosterd, L.; Buclin, T.; Guiducci, C. Label-free detection of tobramycin in serum by transmission-localized surface plasmon resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kerman, K.; Saito, M.; Sathuluri, R.R.; Endo, T.; Yamamura, S.; Kwon, Y.S.; Tamiya, E. Label-free DNA biosensor based on localized surface plasmon resonance coupled with interferometry. Anal. Chem. 2007, 79, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kerman, K.; Nagatani, N.; Takamura, Y.; Tamiya, E. Label-free detection of peptide nucleic acid—DNA hybridization using localized surface plasmon resonance based optical biosensor. Anal. Chem. 2005, 77, 6976–6984. [Google Scholar] [CrossRef] [PubMed]
- Delong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Tian, L.; Wang, T.; Wang, C. Optical DNA detection based on gold nanorods aggregation. Anal. Chim. Acta 2010, 673, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Chemical sensing and imaging with metallic nanorods. Chem. Commun. 2008, 7, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A light-driven therapy of pancreatic adenocarcinoma using Gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, D.; Crummett, W.B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
- Osaka, A.; Hasegawa, H.; Yamada, Y.; Yanagihara, K.; Hayashi, T.; Mine, M.; Aoyama, M.; Sawada, T.; Kamihira, S. A novel role of serum cytochrome c as a tumor marker in patients with operable cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 371–377. [Google Scholar] [CrossRef] [PubMed]
| Technique | Hardware Requirement | Signal Amplification | Detection Limit (ng/mL) | Reference |
|---|---|---|---|---|
| Aptamer-based LSPR | Absorbance reader | – | 0.1 | This study |
| Aptamer-based SPR | SPR sensor | – | 1 (80 pM) | [12] |
| ABC-Rash assay-RNase H digestion on RNA probes | SPR sensor | Enzyme-based | 1 (80 pM) | [15] |
| ABC-PCR assay | Real-time fluorescence thermocycler | Enzyme-based | 10 | [14] |
| ABC-RPA (Recombinase polymerase amplification) assay | Real-time fluorescence detector with temperature regulation | Enzyme-based | 10 | [13] |
| Aptamer-base ELISA | Absorbance reader | Enzyme-based | 100 | [12] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loo, J.F.-C.; Lau, P.-M.; Kong, S.-K.; Ho, H.-P. An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination. Micromachines 2017, 8, 338. https://doi.org/10.3390/mi8110338
Loo JF-C, Lau P-M, Kong S-K, Ho H-P. An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination. Micromachines. 2017; 8(11):338. https://doi.org/10.3390/mi8110338
Chicago/Turabian StyleLoo, Jacky Fong-Chuen, Pui-Man Lau, Siu-Kai Kong, and Ho-Pui Ho. 2017. "An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination" Micromachines 8, no. 11: 338. https://doi.org/10.3390/mi8110338





