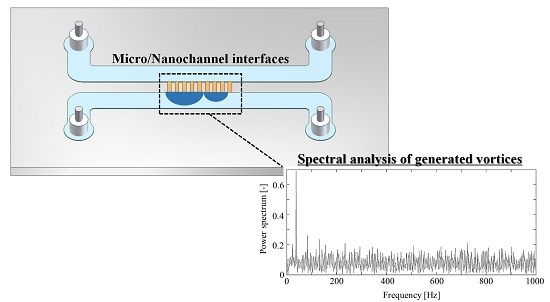
Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Spectral Analysis
Abstract
:1. Introduction
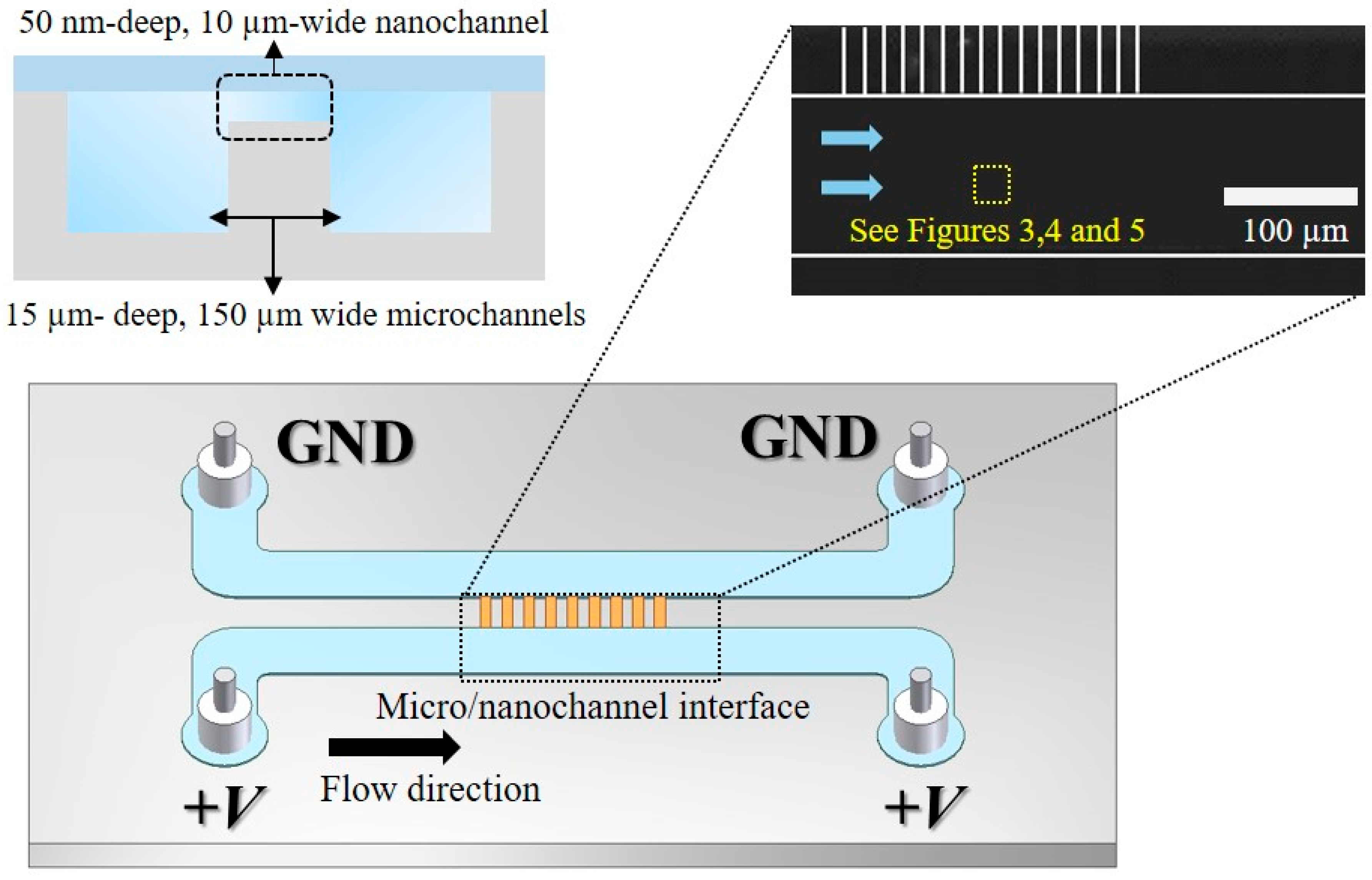
2. Experimental
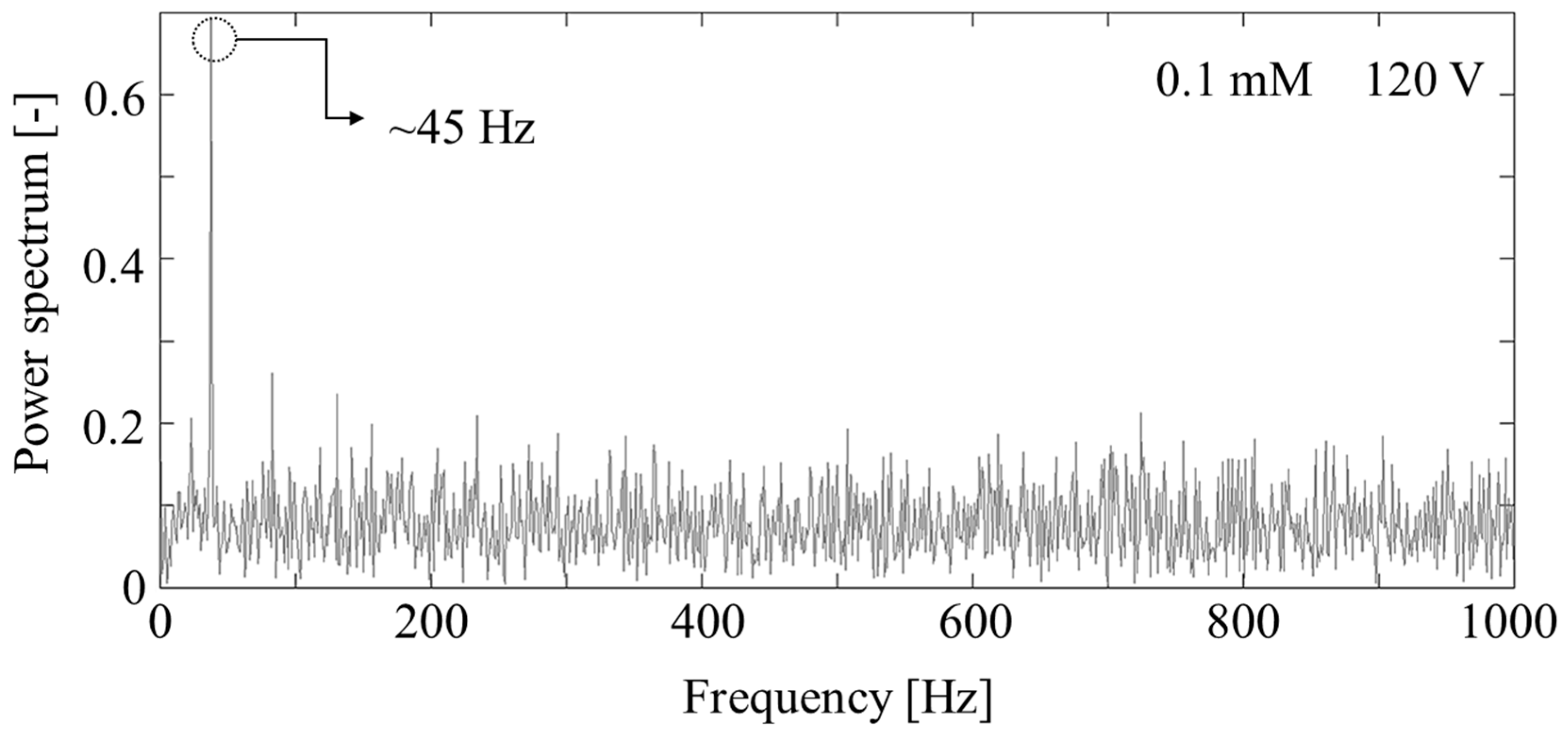
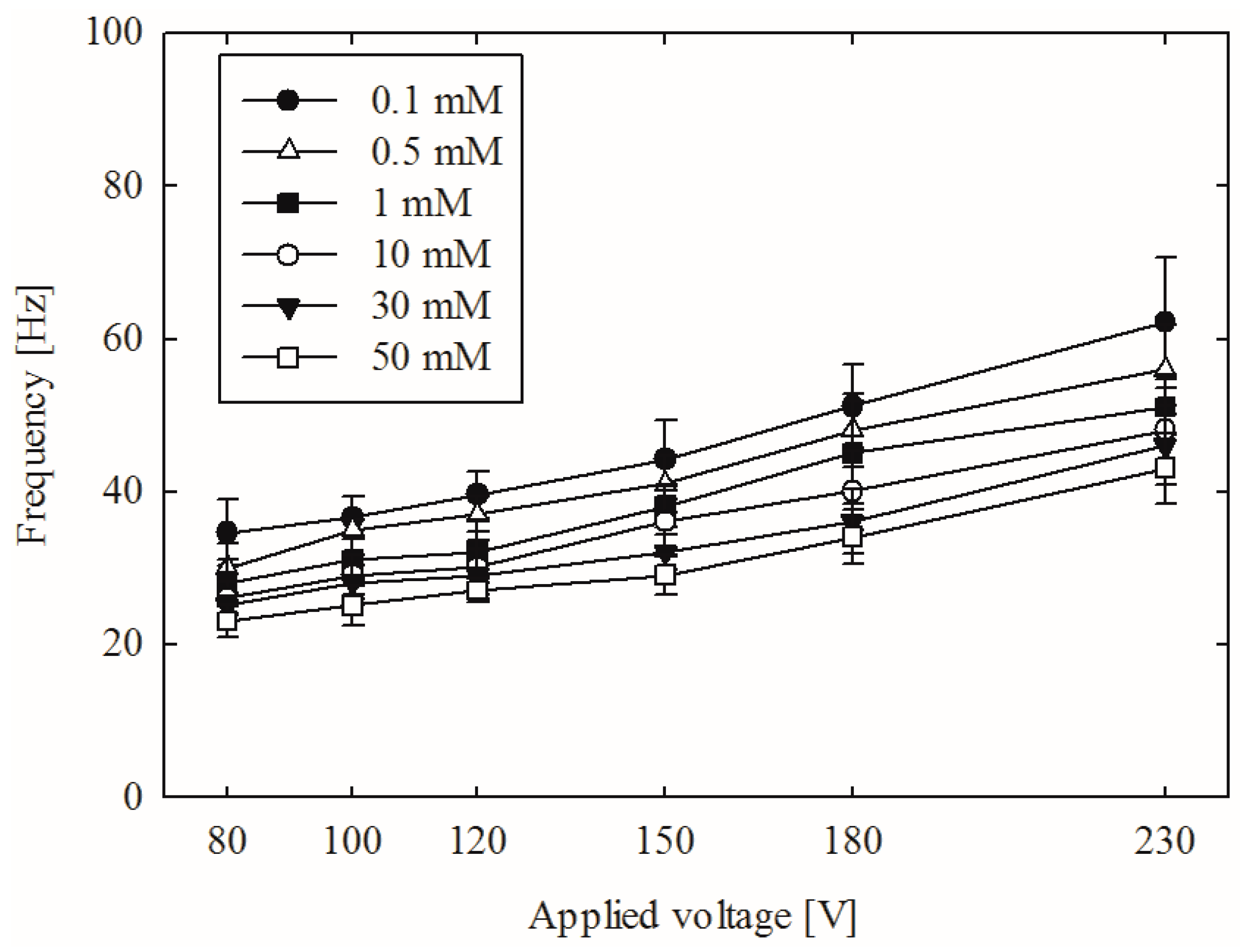
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choi, E.; Kwon, K.; Lee, S.J.; Kim, D.; Park, J. Non-equilibrium electrokinetic micromixer with 3D nanochannel networks. Lab Chip 2015, 15, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Kim, S.J. Electrokinetic flow-induced currents in silica nanofluidic channels. J. Colloid Interface Sci. 2009, 333, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Raj, A.; Zhu, L.; Masel, R.I.; Shannon, M.A. Non-equilibrium electrokinetic micro/nanofluidic mixer. Lab Chip 2008, 8, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Li, L.D.; Han, J. Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir 2009, 25, 7759–7765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, Y.-A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Wang, Y.-C.; Lee, J.H.; Jang, H.; Han, J. Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys. Rev. Lett. 2007, 99, 044501. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Burns, M.A.; Hasselbrink, E.F. Electrokinetic protein preconcentration using a simple glass/poly (dimethylsiloxane) microfluidic chip. Anal. Chem. 2006, 78, 4779–4785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, D. Millisecond-order rapid micromixing with non-equilibrium electrokinetic phenomena. Microfluid. Nanofluid. 2012, 12, 897–906. [Google Scholar] [CrossRef]
- Mani, A.; Zangle, T.A.; Santiago, J.G. On the propagation of concentration polarization from microchannel-nanochannel interfaces part I: Analytical model and characteristic analysis. Langmuir 2009, 25, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Oddy, M.H.; Santiago, J.G.; Mikkelsen, J.C. Electrokinetic instability micromixing. Anal. Chem. 2001, 73, 5822–5832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhe, J.; Dutta, P.; Chung, B.T. A microfluidic mixer utilizing electrokinetic relay switching and asymmetric flow geometries. J. Fluids Eng. Trans. ASME 2007, 129, 395–403. [Google Scholar] [CrossRef]
- Yan, D.; Yang, C.; Miao, J.; Lam, Y.; Huang, X. Enhancement of electrokinetically driven microfluidic t-mixer using frequency modulated electric field and channel geometry effects. Electrophoresis 2009, 30, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Song, Y.-A.; Kim, S.J.; Kim, M.; Han, J.; Kang, K.H. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip 2012, 12, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Kwak, R.; Kim, S.J.; Han, J. Continuous-flow biomolecule and cell concentrator by ion concentration polarization. Anal. Chem. 2011, 83, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Plecis, A.; Nanteuil, C.; Haghiri-Gosnet, A.-M.; Chen, Y. Electropreconcentration with charge-selective nanochannels. Anal. Chem. 2008, 80, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Dukhin, S.S. Nonequilibrium electric surface phenomena. Adv. Colloid Interface Sci. 1993, 44, 1–134. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, G.B.; Fu, L.M.; Lee, K.H.; Yang, R.J. Electrokinetically driven active micro-mixers utilizing zeta potential variation induced by field effect. J. Micromech. Microeng. 2004, 14, 1390–1398. [Google Scholar] [CrossRef]
- Pu, Q.S.; Yun, J.S.; Temkin, H.; Liu, S.R. Ion-enrichment and ion-depletion effect of nanochannel structures. Nano Lett. 2004, 4, 1099–1103. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.J.; Cheow, L.F.; Li, L.D.; Kang, K.H.; Han, J. Massively parallel concentration device for multiplexed immunoassays. Lab Chip 2011, 11, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, S.M.; Manukyan, G.; Staicu, A.; Rubinstein, I.; Zaltzman, B.; Lammertink, R.G.H.; Mugele, F.; Wessling, M. Direct observation of a nonequilibrium electro-osmotic instability. Phys. Rev. Lett. 2008, 101, 236101. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.-H.; Chang, C.-C.; Yang, R.-J. Electrokinetic micromixing of charged and non-charged samples near nano-/microchannel junction. Microfluid. Nanofluid. 2013, 14, 839–844. [Google Scholar] [CrossRef]
- Yossifon, G.; Chang, H.-C. Selection of nonequilibrium overlimiting currents: Universal depletion layer formation dynamics and vortex instability. Phys. Rev. Lett. 2008, 101, 254501. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kwon, K.; Jeon, T.-J.; Kim, S.M.; Kim, D. Quantification of vortex generation due to non-equilibrium electrokinetics at the micro/nanochannel interface: Particle tracking velocimetry. Micromachines 2016. submitted. [Google Scholar]
- Posner, J.D.; Perez, C.L.; Santiago, J.G. Electric fields yield chaos in microflows. Proc. Natl. Acad. Sci. USA 2012, 109, 14353–14356. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-D.; Yang, R.-J. Formation of Ionic Depletion/Enrichment Zones in a Hybrid Micro-/Nano-channel. Microfluid. Nanofluid. 2008, 5, 631–638. [Google Scholar] [CrossRef]
- Song, S.; Singh, A.K. On-chip sample preconcentration for integrated microfluidic analysis. Anal. Bioanal. Chem. 2006, 384, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Han, J. Fabrication and characterization of 20 nm planar nanofluidic channels by glass-glass and glass-silicon bonding. Lab Chip 2005, 5, 837–844. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Jeon, T.-J.; Kim, S.M.; Kim, D. Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Spectral Analysis. Micromachines 2016, 7, 109. https://doi.org/10.3390/mi7070109
Lee SJ, Jeon T-J, Kim SM, Kim D. Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Spectral Analysis. Micromachines. 2016; 7(7):109. https://doi.org/10.3390/mi7070109
Chicago/Turabian StyleLee, Seung Jun, Tae-Joon Jeon, Sun Min Kim, and Daejoong Kim. 2016. "Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Spectral Analysis" Micromachines 7, no. 7: 109. https://doi.org/10.3390/mi7070109
APA StyleLee, S. J., Jeon, T.-J., Kim, S. M., & Kim, D. (2016). Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Spectral Analysis. Micromachines, 7(7), 109. https://doi.org/10.3390/mi7070109