Electro-Deformation of Fused Cells in a Microfluidic Array Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Media
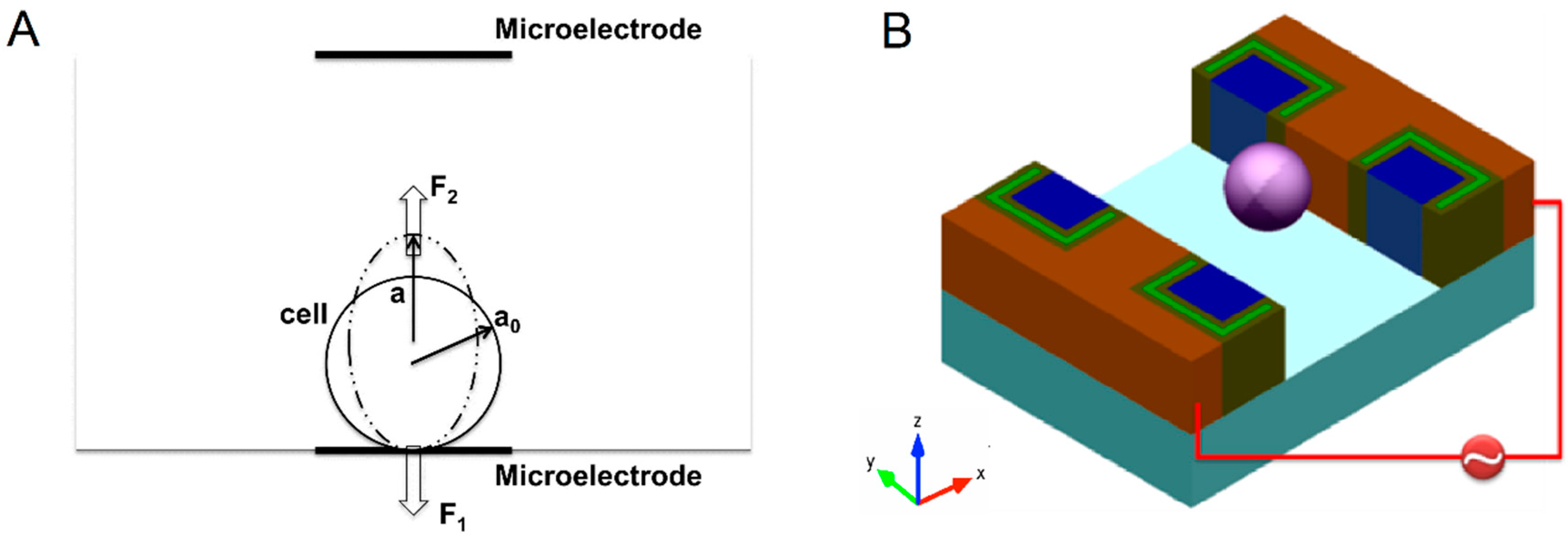
2.2. Fabrication of the Microfluidic Device, Operation, and Data Analysis
2.3. Numerical Simulation of ED Process
3. Results and Discussion
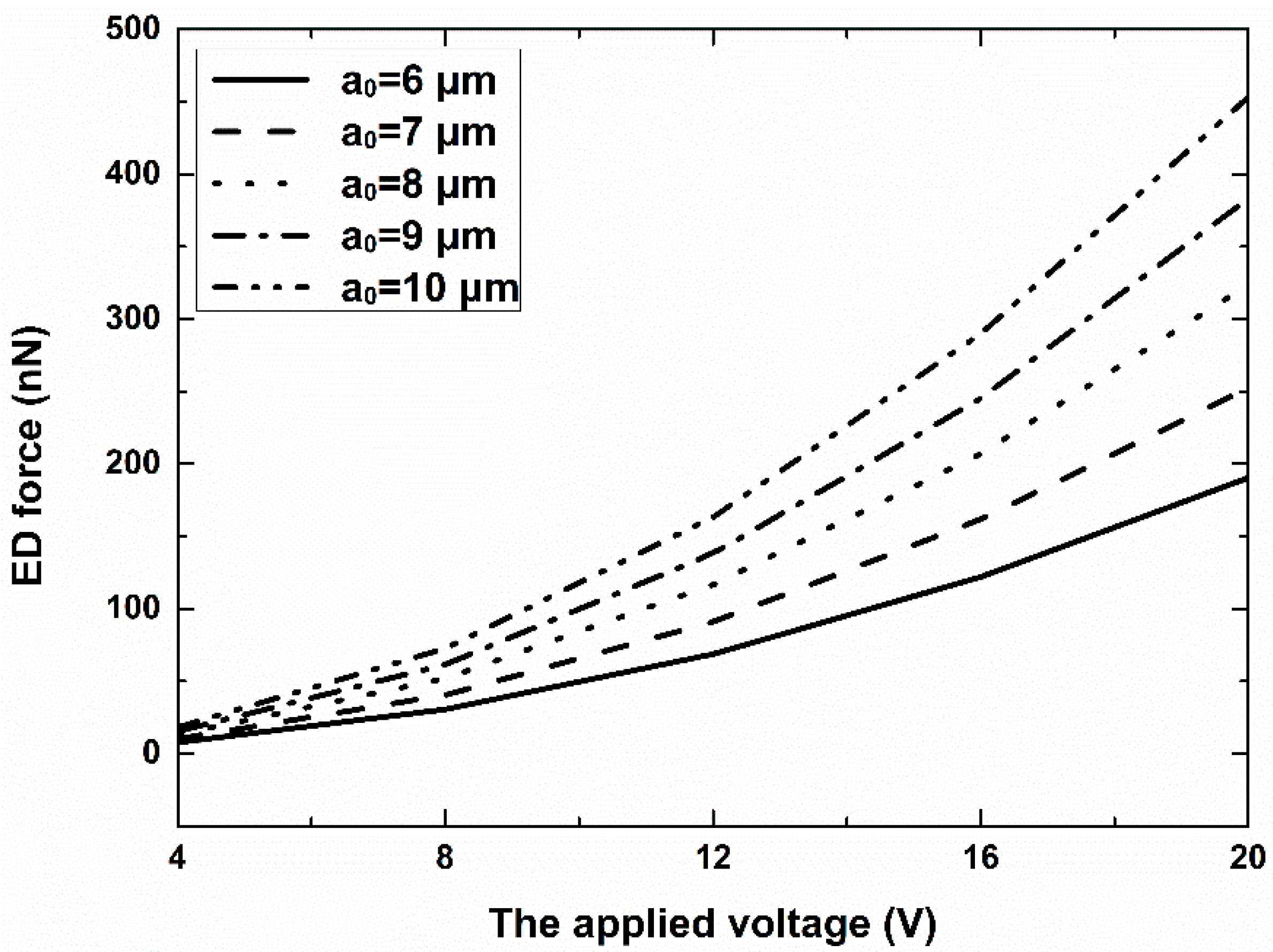
3.1. Simulation Results
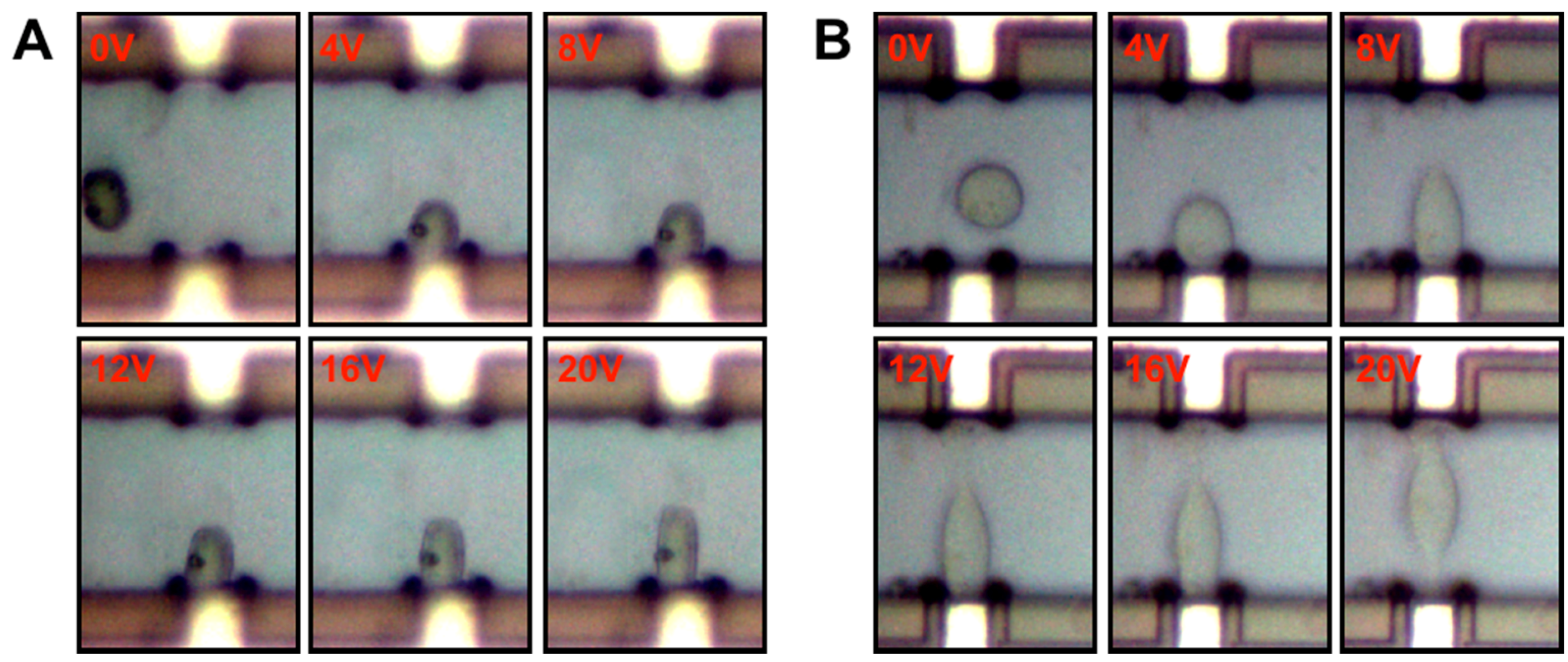
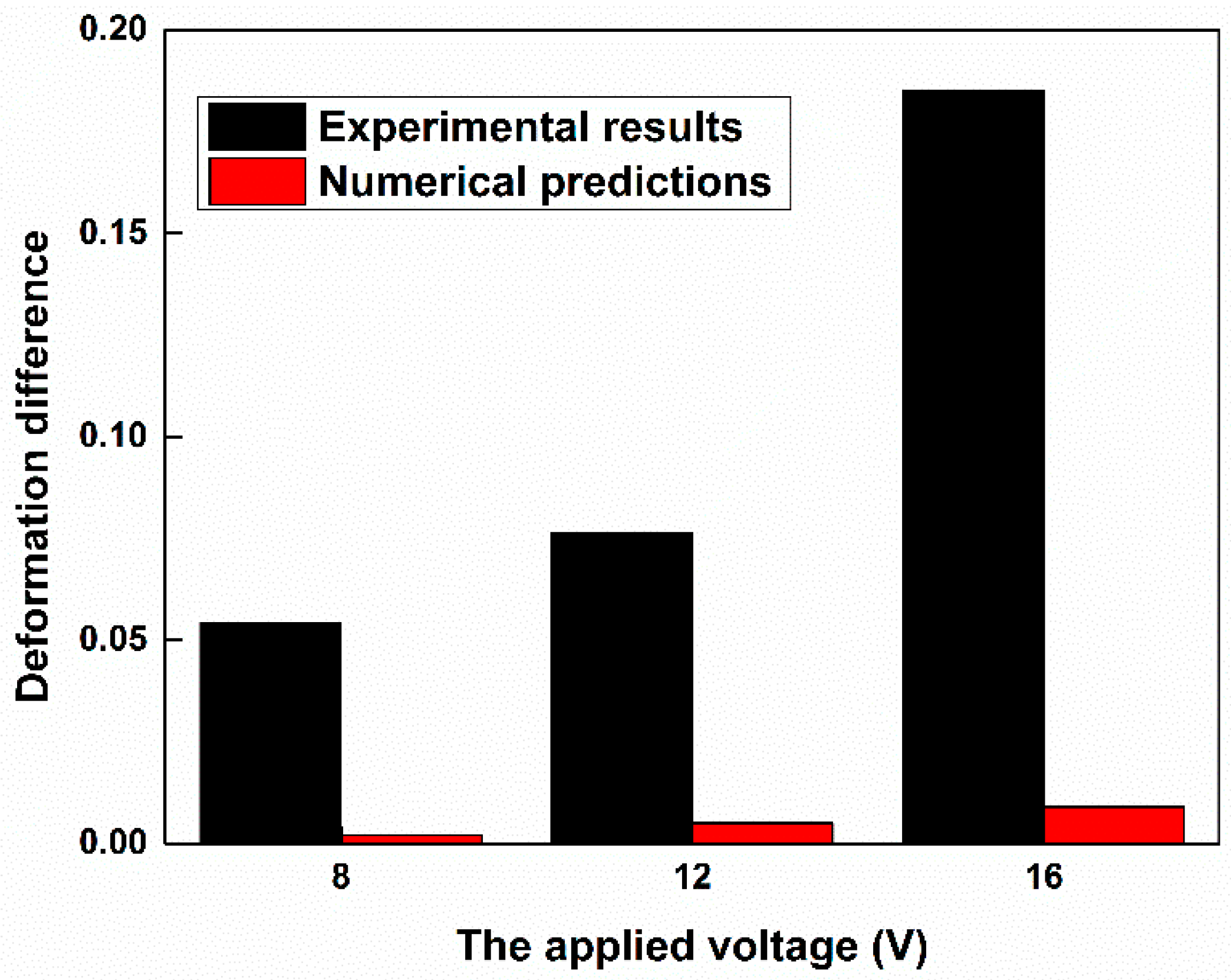
3.2. Cell Elongation
3.3. Comparison of the Fused and Unfused Stem Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AC | Alternating current |
| ED | Electro-deformation |
| DEP | Dielectrophoresis |
| ER | Electrorotation |
| MPA | Micropipette aspiration |
| AFM | Atomic force microscopy |
| CCD | charge-coupled device |
| mESC | Mouse embryonic stem cell |
| H-DMEM | High-glucose dulbecco's modified eagle medium |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| SOI | Silicon-on-insulator |
References
- Chen, E.H.; Olson, E.N. Unveiling the mechanisms of cell-cell fusion. Science 2005, 308, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, D.J.; Tanasijevic, B.; Kaur, A.; Obergfell, C.; O’Neill, R.J.; Krueger, W.; Rasmussen, T.P. Genome-wide reprogramming in hybrids of somatic cells and embryonic stem cells. Stem Cells 2007, 25, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.A.; Atienza, J.; Melton, D.A.; Eggan, K. Developmental biology: Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 2005, 309, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Melancon, J.M.; Foster, T.P.; Kousoulas, K.G. Genetic analysis of the herpes simplex virus type 1 UL20 protein domains involved in cytoplasmic virion envelopment and virus-induced cell fusion. J. Virol. 2004, 78, 7329–7343. [Google Scholar] [CrossRef] [PubMed]
- Dessain, S.K.; Adekar, S.P.; Stevens, J.B.; Carpenter, K.A.; Skorski, M.L.; Barnoski, B.L.; Goldsby, R.A.; Weinberg, R.A. High efficiency creation of human monoclonal antibody-producing hybridomas. J. Immunol. Methods 2004, 291, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Trevor, K.T.; Cover, C.; Ruiz, Y.W.; Akporiaye, E.T.; Hersh, E.M.; Landais, D.; Taylor, R.R.; King, A.D.; Walters, R.E. Generation of dendritic cell-tumor cell hybrids by electrofusion for clinical vaccine application. Cancer Immunol. Immunother. 2004, 53, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Niemela, P.S.; Miettinen, M.S.; Monticelli, L.; Hammaren, H.; Bjelkmar, P.; Murtola, T.; Lindahl, E.; Vattulainen, I. Membrane proteins diffuse as dynamic complexes with lipids. J. Am. Chem. Soc. 2010, 132, 7574–7575. [Google Scholar] [CrossRef] [PubMed]
- Javanainen, M.; Hammaren, H.; Monticelli, L.; Jeon, J.-H.; Miettinen, M.S.; Martinez-Seara, H.; Metzler, R.; Vattulainen, I. Anomalous and normal diffusion of proteins and lipids in crowded lipid membranes. Faraday Discuss. 2013, 161, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.M.; Fontes, A.; Barjas-Castro, M.L.; Barbosa, L.C.; Costa, F.F.; Cesar, C.L.; Saad, S.T.O. Optical tweezers for measuring red blood cell elasticity: Application to the study of drug response in sickle cell disease. Eur. J. Haematol. 2003, 70, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Dao, M.; Suresh, S.; Sow, C.H.; Chew, K.T. Large deformation of living cells using laser traps. Acta Mater. 2004, 52, 1837–1845. [Google Scholar] [CrossRef]
- Lincoln, B.; Erickson, H.M.; Schinkinger, S.; Wottawah, F.; Mitchell, D.; Ulvick, S.; Bilby, C.; Guck, J. Deformability-based flow cytometry. Cytometry A 2004, 59, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Glenister, F.K.; Coppel, R.L.; Cowman, A.F.; Mohandas, N.; Cooke, B.M. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 2002, 99, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Kirmizis, D.; Logothetidis, S. Atomic force microscopy probing in the measurement of cell mechanics. Int. J. Nanomed. 2010, 5, 137–145. [Google Scholar] [CrossRef]
- Engelhardt, H.; Gaub, H.; Sackmann, E. Viscoelastic properties of erythrocyte membranes in high-frequency electric fields. Nature 1984, 307, 378–380. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.A.; Buschmann, M.D.; Wertheimer, M.R. Mechanical properties of mammalian cells in suspension measured by electro-deformation. J. Micromech. Microeng. 2010, 20, 065007. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Thibault, M.; Buschmann, M.D.; Wertheimer, M.R. Electromechanical deformation of mammalian cells in suspension depends on their cortical actin thicknesses. J. Biomech. 2012, 45, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Doh, I.; Lee, W.C.; Cho, Y.H.; Pisano, A.P.; Kuypers, F.A. Deformation measurement of individual cells in large populations using a single-cell microchamber array chip. Appl. Phys. Lett. 2012, 100, 173702–1737023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Abdelgawad, M.; Yu, L.; Shakiba, N.; Chien, W.-Y.; Lu, Z.; Geddie, W.R.; Jewett, M.A.S.; Sun, Y. Electrodeformation for single cell mechanical characterization. J. Micromech. Microeng. 2011, 21, 054012. [Google Scholar] [CrossRef]
- Gimsa, J. A comprehensive approach to electro-orientation, electrodeformation, dielectrophoresis, and electrorotation of ellipsoidal particles and biological cells. Bioelectrochemistry 2001, 54, 23–31. [Google Scholar] [CrossRef]
- Qu, Y.; Hu, N.; Xu, H.; Yang, J.; Xia, B.; Zheng, X.; Yin, Z.Q. Somatic and stem cell pairing and fusion using a microfluidic array device. Microfluid. Nanofluid. 2011, 11, 633–641. [Google Scholar] [CrossRef]
- Hu, N.; Yang, J.; Qian, S.; Zhang, X.; Joo, S.W.; Zheng, X. A cell electrofusion microfluidic chip using discrete coplanar vertical sidewall microelectrodes. Electrophoresis 2012, 33, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.N.; Chen, D.Y.; Zhao, Y.; Wei, C.; Zhao, X.T.; Yue, W.T.; Long, R.; Wang, J.B.; Chen, J. A constriction channel based microfluidic system enabling continuous characterization of cellular instantaneous young’s modulus. Sens. Actuators B Chem. 2014, 202, 1183–1189. [Google Scholar] [CrossRef]
- Kotnik, T.; Miklavčič, D.; Slivnik, T. Time course of transmembrane voltage induced by time-varying electric fields—A method for theoretical analysis and its application. Bioelectrochem. Bioenerg. 1998, 45, 3–16. [Google Scholar] [CrossRef]


| Parameter | Value/Range | Reference |
|---|---|---|
| Medium conductivity () | 0.001 S·m−1 | Measured |
| Medium permittivity () | 80 | [23] |
| Cytoplasmic conductivity () | 0.3 S·m−1 | [23] |
| Cytoplasmic permittivity () | 70 | [18] |
| Cell membrane conductivity () | 5 × 10−7 S·m−1 | [23] |
| Cell membrane permittivity () | 10 | [18] |
| Cell membrane thickness () | 5 nm | [23] |
| Young’s modulus | 600 Pa | |
| Poisson’s ratio of cell () | 0.499 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Chen, M.; Yin, D.; Yang, Z.; Chen, X.; Wang, Z.; Xu, J.; Li, Y.; Qiu, J.; et al. Electro-Deformation of Fused Cells in a Microfluidic Array Device. Micromachines 2016, 7, 204. https://doi.org/10.3390/mi7110204
Liu Y, Zhang X, Chen M, Yin D, Yang Z, Chen X, Wang Z, Xu J, Li Y, Qiu J, et al. Electro-Deformation of Fused Cells in a Microfluidic Array Device. Micromachines. 2016; 7(11):204. https://doi.org/10.3390/mi7110204
Chicago/Turabian StyleLiu, Yan, Xiaoling Zhang, Mengdi Chen, Danfen Yin, Zhong Yang, Xi Chen, Zhenyu Wang, Jie Xu, Yuanyi Li, Jun Qiu, and et al. 2016. "Electro-Deformation of Fused Cells in a Microfluidic Array Device" Micromachines 7, no. 11: 204. https://doi.org/10.3390/mi7110204







