OLED Hybrid Integrated Polymer Microfluidic Biosensing for Point of Care Testing
Abstract
:1. Introduction
2. Introduction to OLEDs
3. Design, Fabrication, Packaging and Integration
3.1. Design and Fabrication of Polymer based Microfluidic Chip
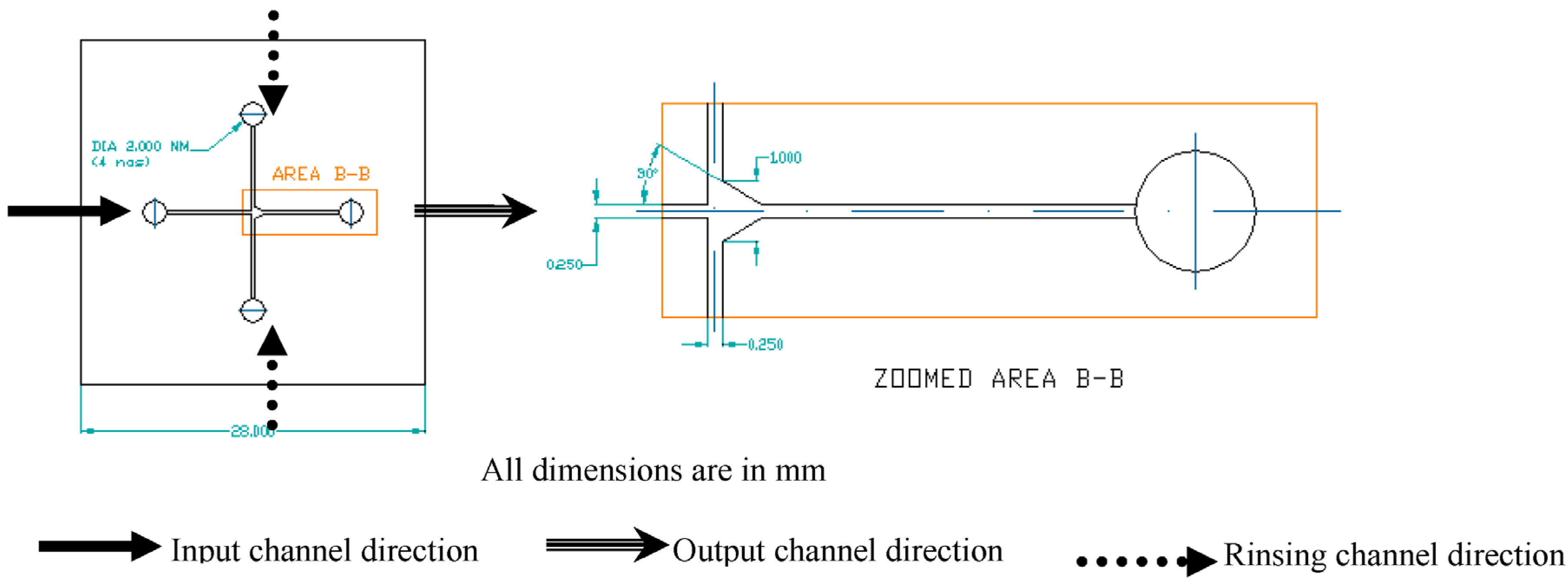
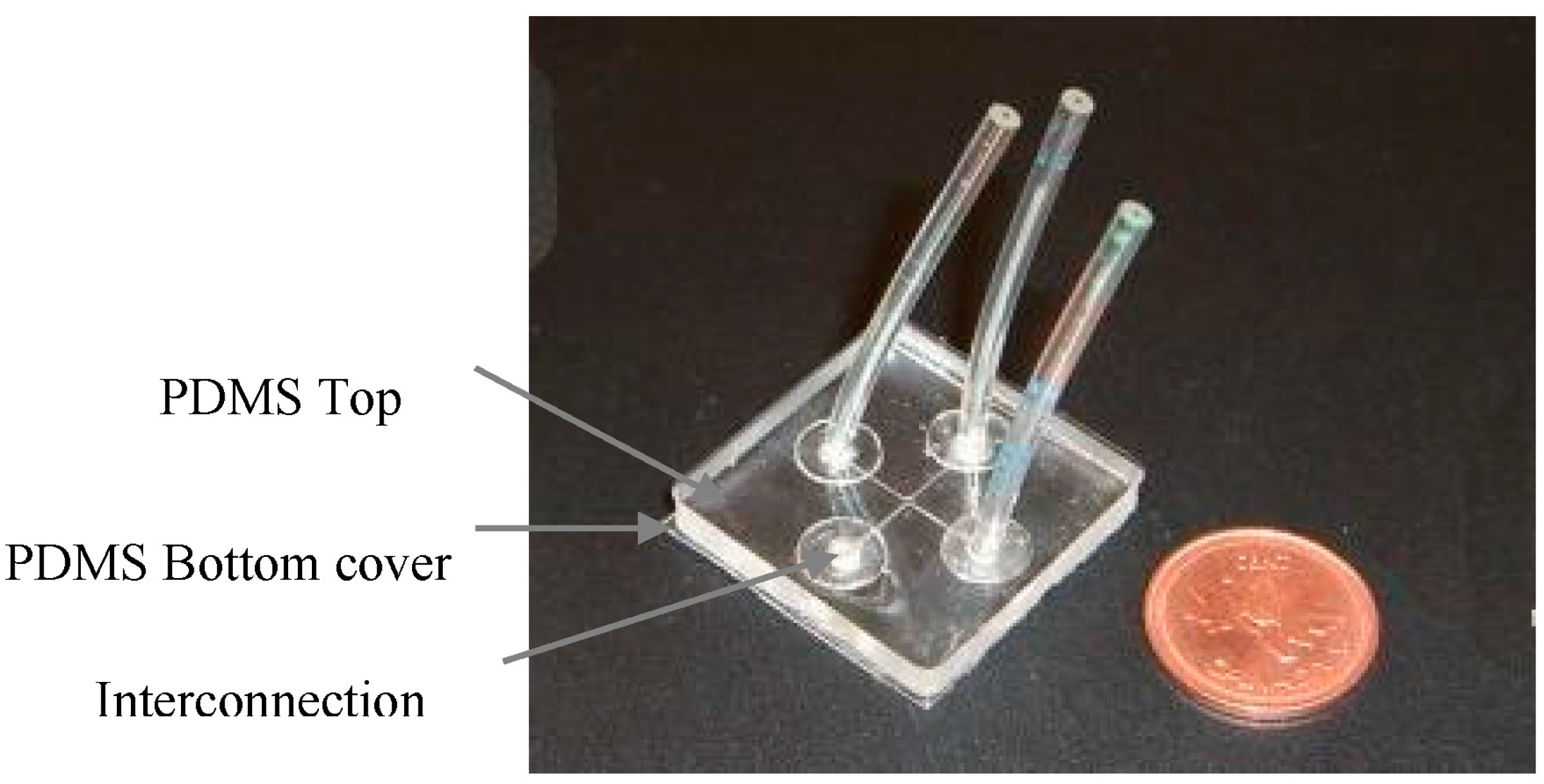
3.2. Design and Fabrication of OLED Chip as Excitation Source
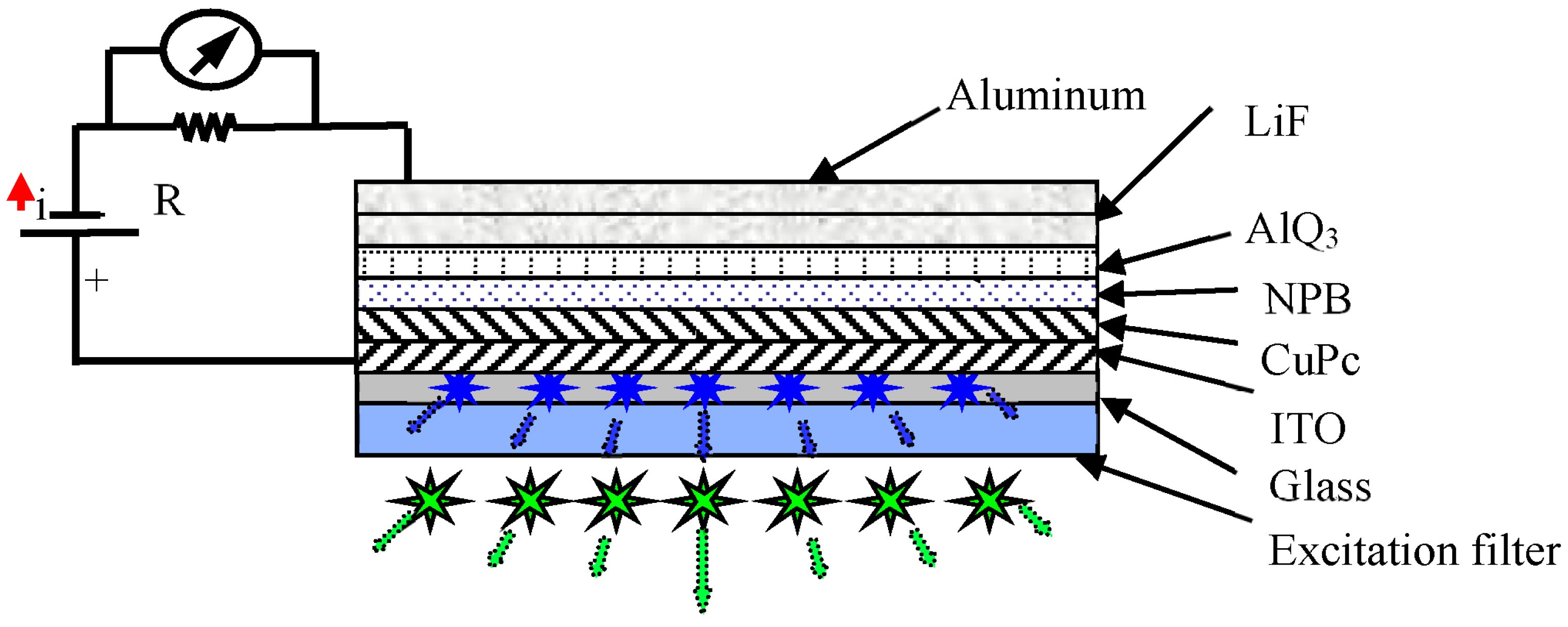
3.3. Bio-Organic Optical Integration and Detection System
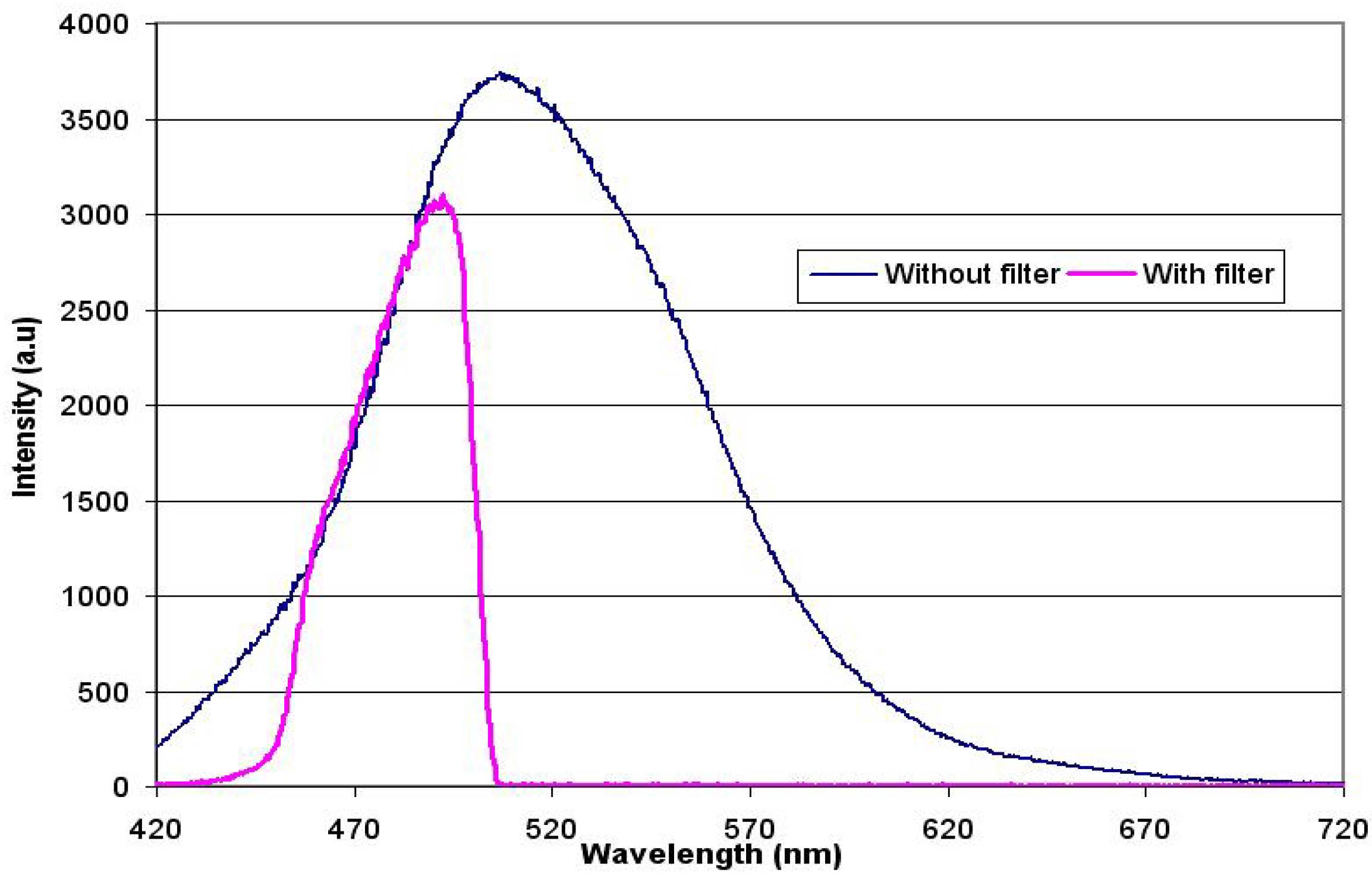
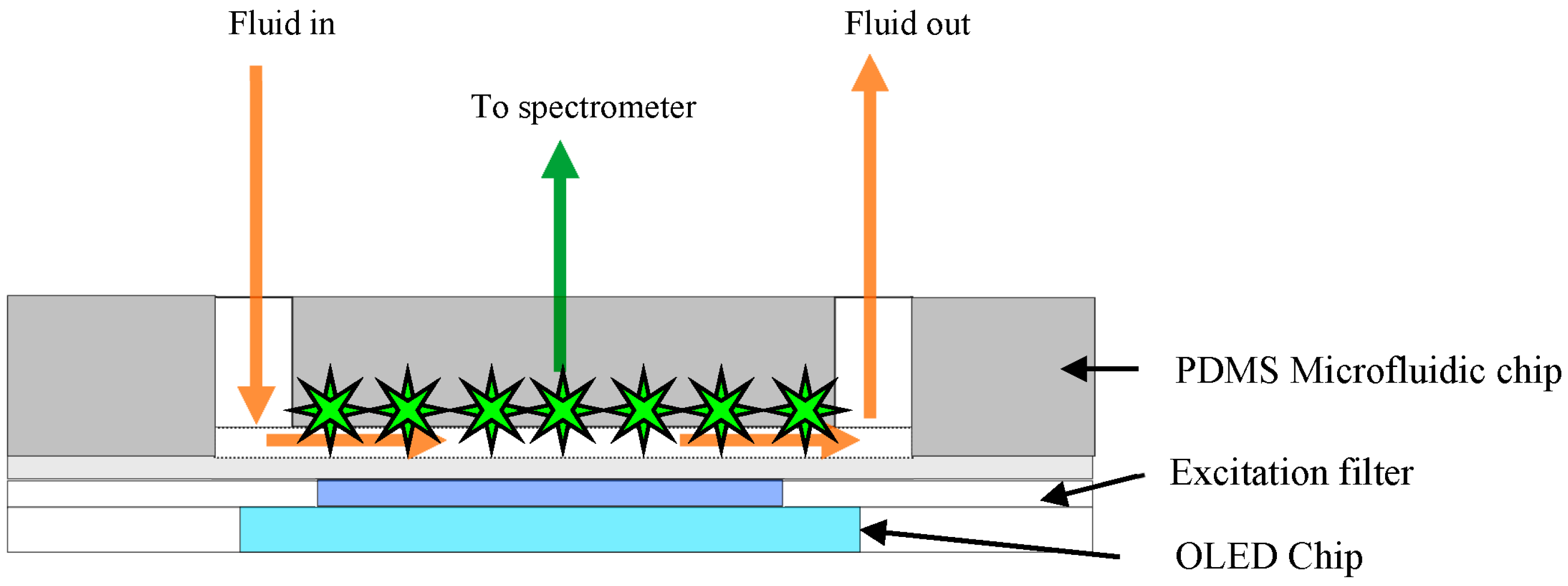
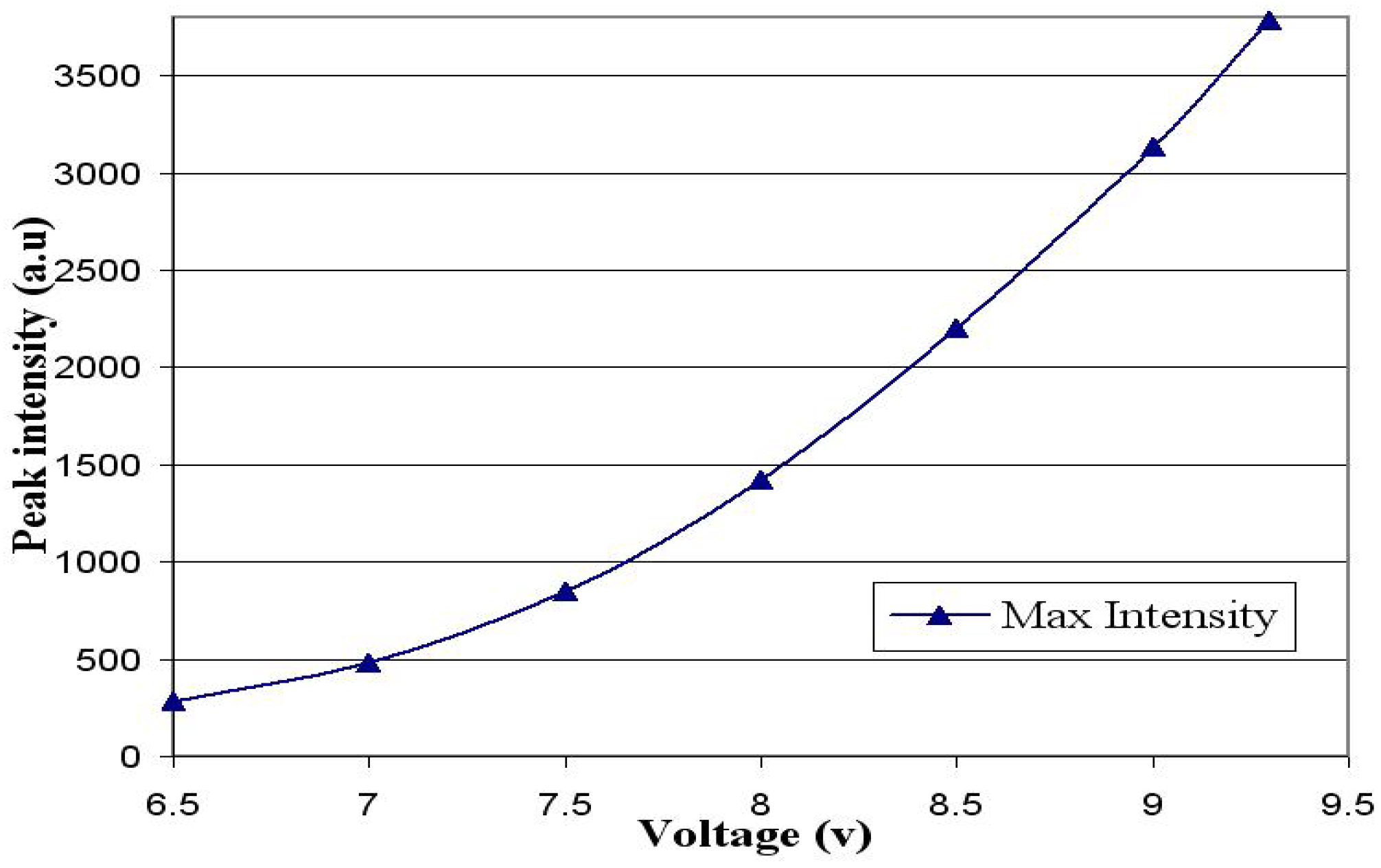
4. Stability of OLED Emission at Different Voltages

5. Bio-Optical Testing on Microfluidic Chip
5.1. Selection and Preparation of Enzymes
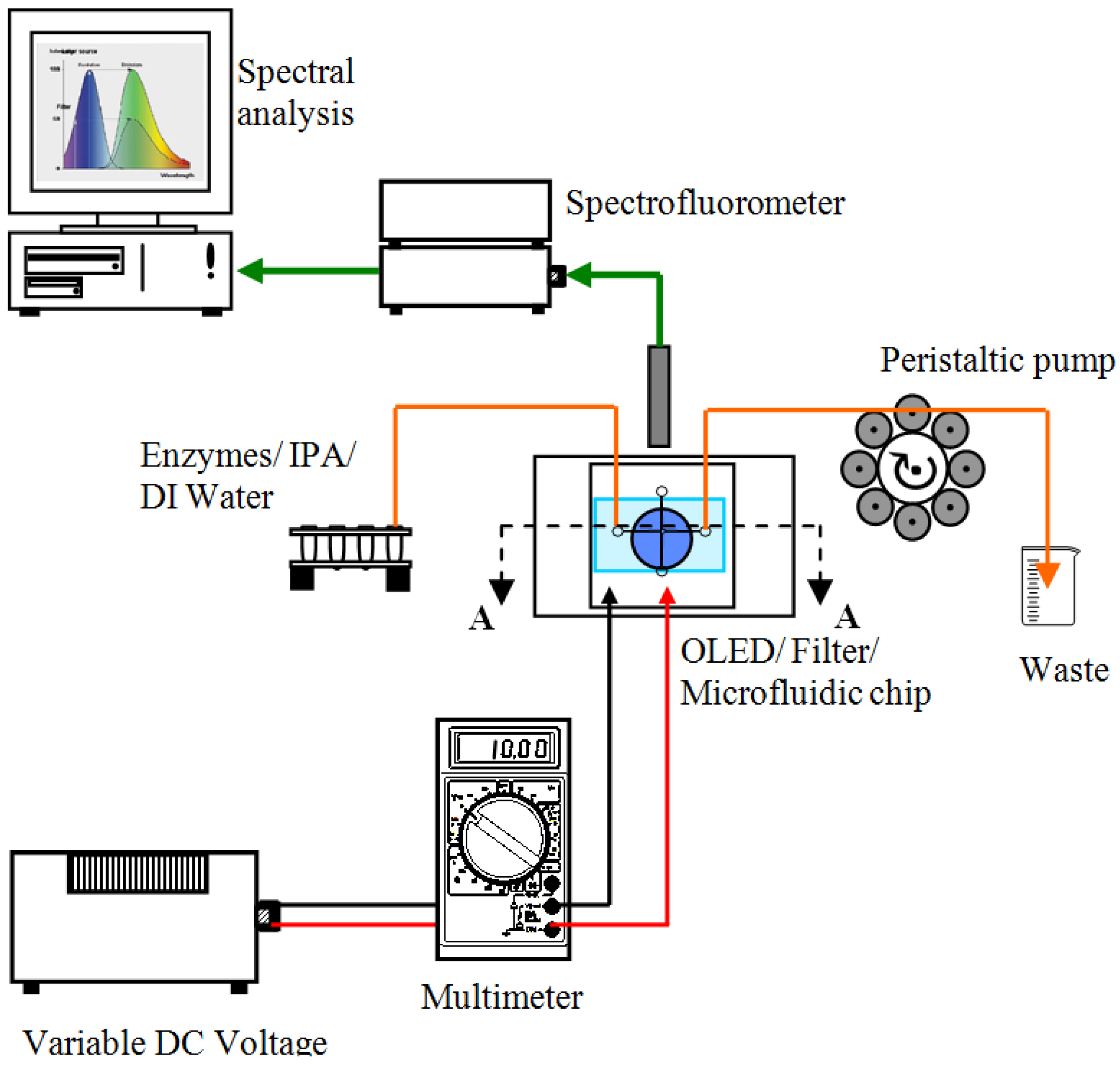

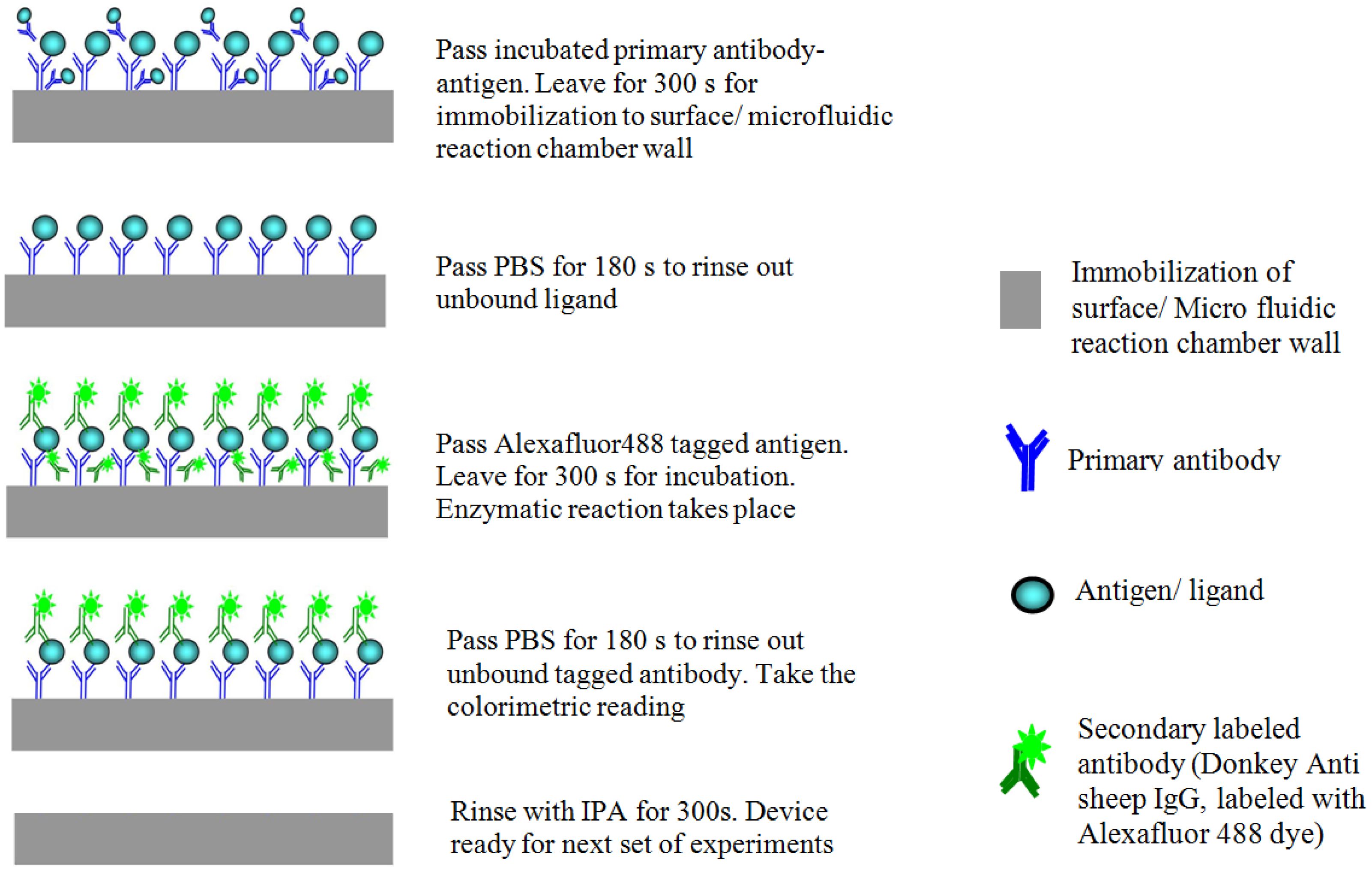
5.2. Bio-Optical Detection Methodology
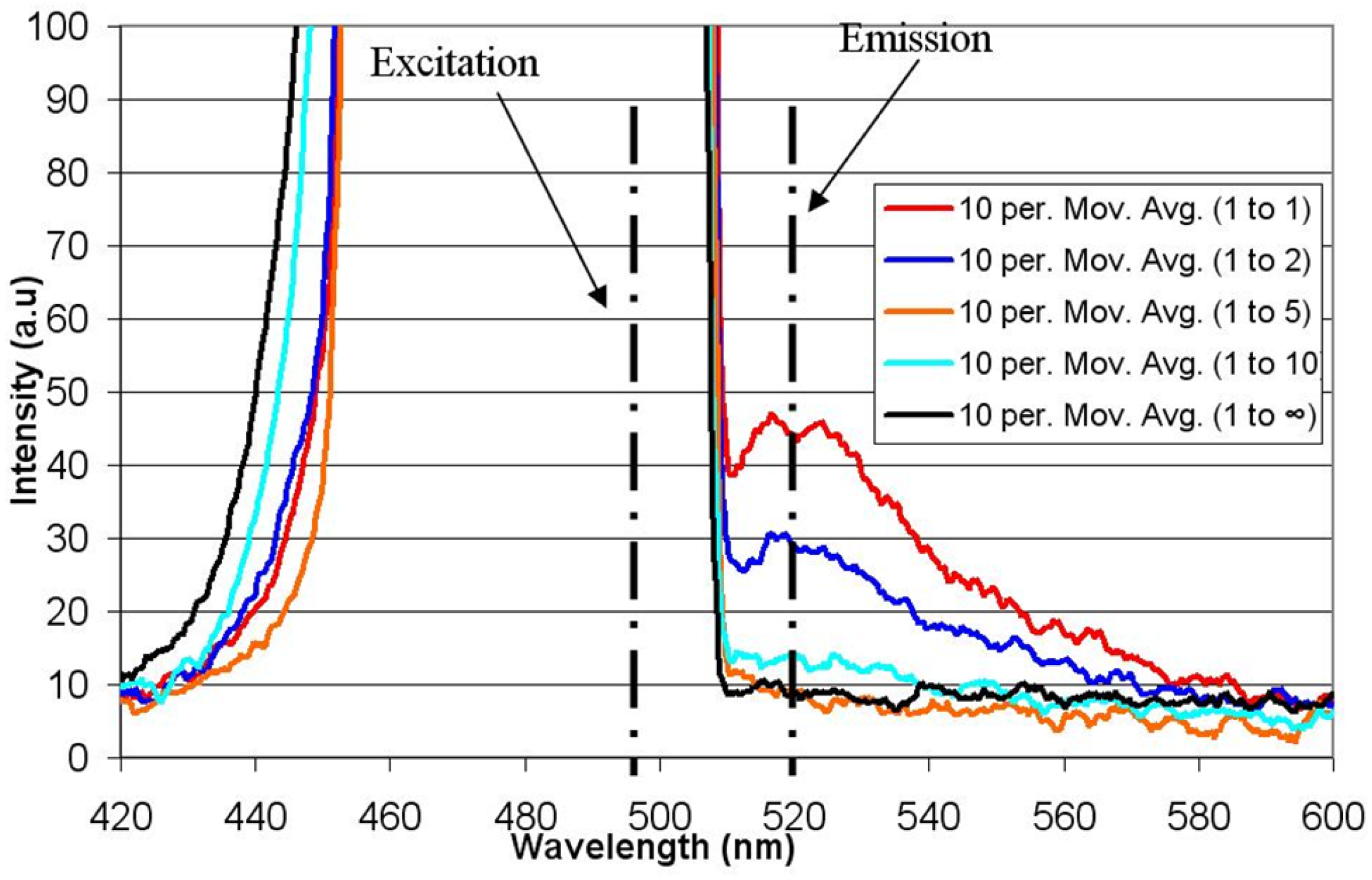
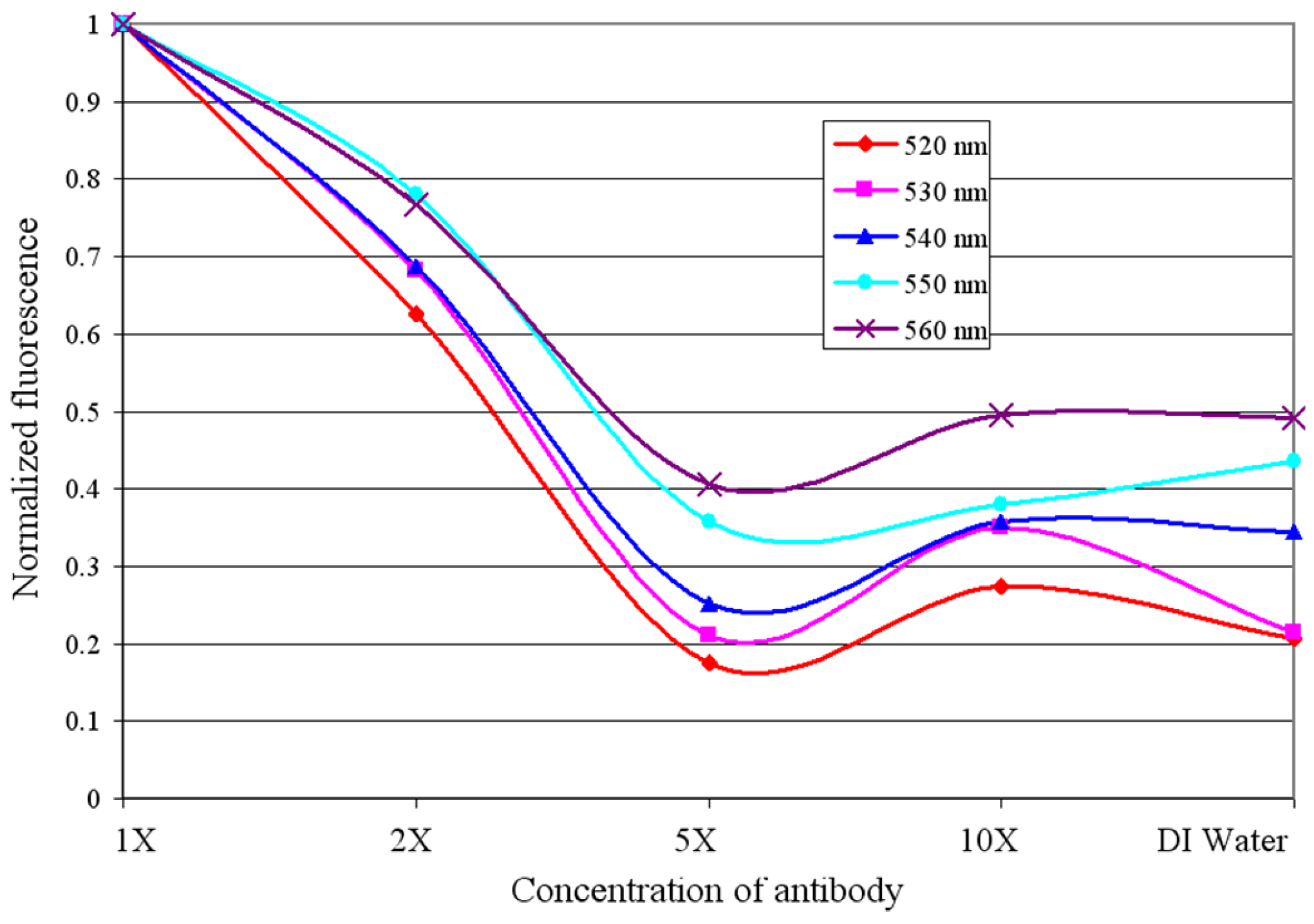
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahn, C.H.; Choi, J.; Beaucage, G. Disposable smart lab on a chip for point-of-care clinical diagnostics. Proc. IEEE 2004, 92, 154–173. [Google Scholar] [CrossRef]
- Allen, P.B.; Rodriguez, I.; Kuyper, C.L.; Lorenz, R.M.; Spicar-Mihalic, P.; Kuo, J.S.; Chiu, D.T. Selective Electroless and Electrolytic Deposition of Metal for Applications in Microfluidics: Fabrication of a Microthermocouple. Anal. Chem. 2003, 75, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Altendorf, E.; Zebert, D.; Holl, M.; Yager, P. Differential blood cell counts obtained using a microchannel based flow cytometer. In Proceedings of IEEE International Conference on Solid-State Sensors and Actuators (TRANSDUCERS’97), Chicago, IL, USA, 16–19 June 1997; pp. 531–534.
- Bashir, R. State-of-the-art in detection, opportunities and prospects. Adv. Drug Deliv. Rev. 2004, 56, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Bessoth, F.G.; Naji, O.P.; Eijkel, J.C.T.; Manz, A. Towards an on-chip gas chromatograph: The development of a gas injector and a dc plasma emission detector. J. Anal. At. Spectrom. 2002, 17, 794–799. [Google Scholar] [CrossRef]
- Brehmer, M.; Conrad, L.; Funk, L. New developments in soft lithography. J. Dispers. Sci. Technol. 2003, 24, 291–304. [Google Scholar] [CrossRef]
- Camou, S.; Kitamura, M.; Arakawa, Y.; Fuji, T. Integration of OLED light source and optical fibers on a PDMS based microfluidic device for on-chip fluorescence detection. In Proceedings of 7th International Conference on Miniaturized Chemical and Biochemical Analysis Systems, Squaw Valley, CA, USA, 5–9 October 2003; pp. 383–386.
- Chabinyc, M.L.; Chiu, D.T.; McDonald, J.C. An integrated fluorescence detection system in poly(dimethylsiloxane) for microfluidic applications. Anal. Chem. 2001, 73, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Oh, K.W.; Thomas, J.H. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab Chip 2002, 2, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Coté, G.L.; Lec, R.M.; Pishko, M.V. Emerging biomedical sensing technologies and their applications. IEEE Sens. J. 2003, 3, 251–266. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 Processing for Low-Cost Microstructures Fabrication without Cleanroom Facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Effenhauser, C.S.; Bruin, G.J.M.; Paulus, A.; Ehrat, M. Integrated capillary electrophoresis on flexible silicone microdevices: Analysis of DNA restriction fragments and detection of single DNA molecules on microchips. Anal. Chem. 1997, 69, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Integrated microfluidic devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]
- Fintschenko, Y.; Wilson, G.S. Flow injection immunoassays: A review. Microchim. Acta 1998, 129, 7–18. [Google Scholar] [CrossRef]
- Hofmann, O.; Wang, X.; deMello, J.C.; Bradley, D.D.; deMello, A.J. Towards microalbuminuria determination on a disposable diagnostic microchip with integrated fluorescence detection based on thin-film organic light emitting diodes. Lab Chip 2005, 5, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.M.; Mogensen, K.B.; Kutter, J.P.; Geschke, O. A biochemical microdevice with an integrated chemiluminescence detector. Sens. Actuators B Chem. 2003, 90, 15–21. [Google Scholar] [CrossRef]
- Lapos, J.A.; Manica, D.P.; Ewing, A.G. Dual fluorescence and electrochemical detection on an electrophoresis microchip. Anal. Chem. 2002, 74, 3348–3353. [Google Scholar] [CrossRef] [PubMed]
- Laser, D.; Santiago, J. A review of micropumps. J. Micromech. Microeng. 2004, 14, 35–64. [Google Scholar] [CrossRef]
- Li, J.; Thibault, P.; Bings, N.H. Integration of microfabricated devices to capillary electrophoresis-electrospray mass spectrometry using a low dead volume connection: Application to rapid analyses of proteolytic digests. Anal. Chem. 1999, 71, 3036–3045. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chiem, N.; Ocvirk, G.; Tang, T.; Fluri, K.; Harrison, D.J. Microfabrication of a planar absorbance and fluorescence cell for integrated capillary electrophoresis devices. Anal. Chem. 1996, 68, 1040–1046. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Linder, V.; Verpoorte, E.; Thormann, W.; de Rooij, N.F.; Sigrist, H. Surface biopassivation of replicated poly(dimethylsiloxane) microfluidic channels and application to heterogeneous immunoreaction with on-chip fluorescence detection. Anal. Chem. 2001, 73, 4181–4189. [Google Scholar] [CrossRef] [PubMed]
- Massin, C.; Vincent, F.; Homsy, A. Planar microcoil-based microfluidic NMR probes. J. Magn. Resonance 2003, 164, 242–255. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zmija, J.; Malachowski, M.J. Organic light emitting diodes operation and application in displays. Arch. Mater. Sci. Eng. 2009, 40, 5–12. [Google Scholar]
- Woolley, A.T.; Lao, K.; Glazer, A.N.; Mathies, R.A. Capillary electrophoresis chips with integrated electrochemical detection. Anal. Chem. 1998, 70, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Yao, B.; Luo, G.; Wang, L. A microfluidic device using a green organic light emitting diode as an integrated excitation source. Lab Chip 2005, 5, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, E.; De Rooij, N. Microfluidics meets MEMS. Proc. IEEE 2003, 91, 930–953. [Google Scholar] [CrossRef]
- Weigl, B.H.; Bardell, R.L.; Cabrera, C.R. Lab-on-a-chip for drug development. Adv. Drug Deliv. Rev. 2003, 55, 349–377. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, A.; Packirisamy, M.; Izquierdo, R. OLED Hybrid Integrated Polymer Microfluidic Biosensing for Point of Care Testing. Micromachines 2015, 6, 1406-1420. https://doi.org/10.3390/mi6091406
Acharya A, Packirisamy M, Izquierdo R. OLED Hybrid Integrated Polymer Microfluidic Biosensing for Point of Care Testing. Micromachines. 2015; 6(9):1406-1420. https://doi.org/10.3390/mi6091406
Chicago/Turabian StyleAcharya, Ashwin, Muthukumaran Packirisamy, and Ricardo Izquierdo. 2015. "OLED Hybrid Integrated Polymer Microfluidic Biosensing for Point of Care Testing" Micromachines 6, no. 9: 1406-1420. https://doi.org/10.3390/mi6091406






