Infrared Ellipsometric Study of Hydrogen-Bonded Long-Chain Thiolates on Gold: Towards Resolving Structural Details
Abstract
: A set of newly synthesized aryl-substituted amides of 16-mercaptohexadecanoic acid (R = 4-OH; 3,5-di-OH) are self-assembled on Au(111) substrate. Self assembled monolayers (SAMs) formed by these molecules are studied by ellipsometry from infrared to visible spectral range. Best fit calculations based on the three-phase optical model are employed in order to determine the average tilt angle of the hydrocarbon chains. The data revealed that the SAMs reside in a crystalline-like environment as the long methylene chains predominantly exist in all-trans conformation. The calculated tilt angle of the hydrocarbon chain is decreased by approximately 12° in comparison with the one for the correspondent long-chain n-alkyl thiols. Strong hydrogen bonded networks were detected between the amide proton and the carbonyl oxygen as well as between hydroxyl groups in the end aryl substituents. The transition dipole moments of the C=O, N-H and O-H modes are oriented almost parallel to the gold surface.1. Introduction
Self assembled monolayers (SAMs) of thiolates on metallic surfaces have been thoroughly studied for more than two decades because of their proven application potential [1-3]. The possibility to manipulate the physical and chemical surface properties at molecular level revealed new areas for varied applications of nanoscale-based materials. An area of growing interest is the use of functionalized SAMs as building blocks in fabrication of molecular electronic components and nanostructure-based devices [4-8]. A typical problem associated with fabrication of such components is preserving of the molecular ordering within monolayer which in turn will increase their reliability and prolonged use. The packing and stability of SAMs formed by thiolates are governed by strong gold-sulfur bonds and favor van der Waals interactions between the adjacent methylene chains [2,3]. Although they secure enough steadiness at ambient conditions, some technical applications require increased stability against thermal desorption or vacuum. Additional stabilization can be attained provided that a hydrogen-bonded network can be created between the hydrocarbon chains. Amide groups incorporated in the alkyl chains have been the most often investigated functionalities in this respect, because they are known to form strong intermolecular hydrogen bonds between the amide proton and the carbonyl oxygen atoms [9-17]. An important requirement imposed on a SAM capable of building intermolecular hydrogen bonded network, is to preserve the packing and the conformational ordering of the parent thiolate. Inceptive studies have shown that hydrogen bonds between “buried” amide groups yield considerable conformational disorder within monolayer [10,11,13]. Further studies were focused on secondary amide containing SAMs where the spacer between the head sulfur atom and the amide moiety was kept constant while the alkyl tail length was systematically varied [15]. The data revealed that the spacer was perfectly oriented in all cases while the overlayers are only ordered when at least 15 carbon atoms are present in the alkyl tail. In another study, well ordered SAMs were obtained by reaction of a long-chain OH-terminated alkanethiol template monolayer on gold with vapor-phase alkyl isocyanates [16]. Reflection-absorption infrared spectroscopy (RAIRS) data disclosed interchain hydrogen bonded carbamate network and well ordered underlayer, while the overlayer becomes well ordered when the aliphatic tail exceeds five carbons [16]. In an analogous study Kim et al. [17] investigate the monolayer orientation of two model disulfides containing urea moiety and chemisorbed on gold surface. Both disulfides contain short spacers—ethylene and benzene ring respectively—and equally long alkyl chains comprised by 18 carbons. RAIRS spectra gave evidence for strong hydrogen-bonded network between urea moieties and crystalline-like packing of the alkyl chains owing to the reestablished van der Waals interactions [17].
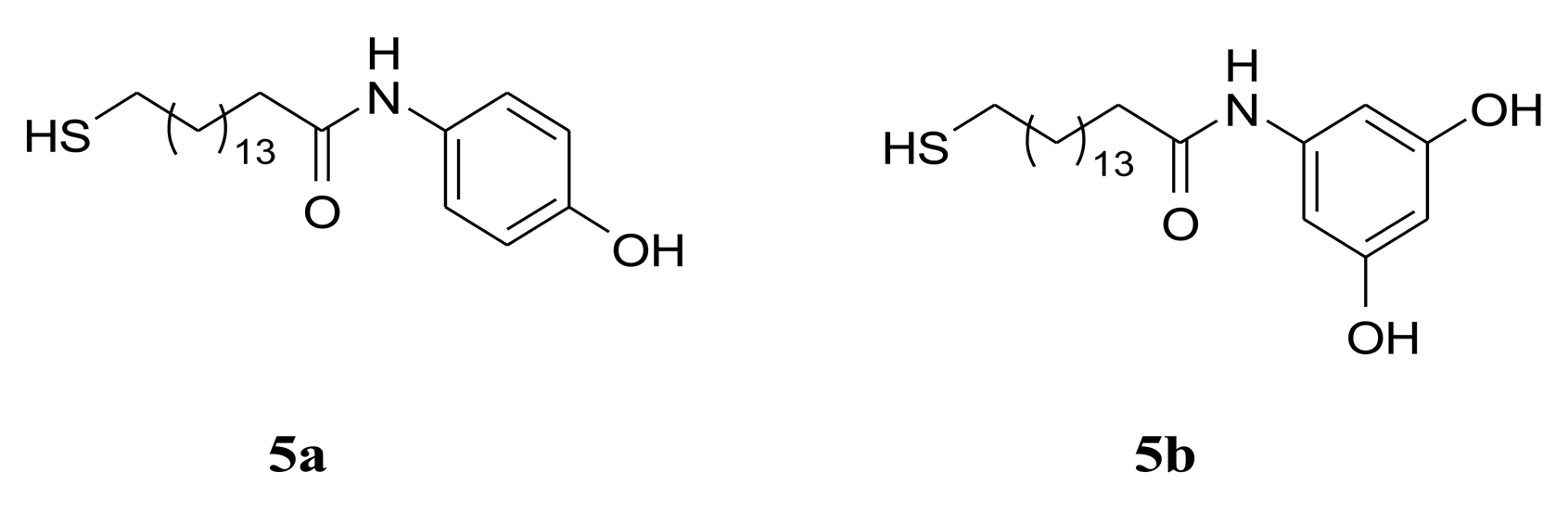
In our previous study [18], we reported on newly synthesized aryl substituted amides of 16-mercaptohexadecanoic acid (R = 4-OCH3; 3,5-di-OCH3) chemisorbed on Au(111) surface. The distance between the head sulfur atom and the amide group was substantially extended in comparison with the previously studied SAMs [8-17] and contained 16 carbons, as the endmost substituents were the voluminous 4-methoxy and 3,5-dimetoxy substituted phenyl rings. Incorporation of an amide group allowed formation of SAMs with extensive hydrogen-bonded networks, as it was determined by comparison of IR absorptions in solid state with IR ellipsometric data [18]. Although the hydrogen bonding reduced the tilt of the methylene chains, it did not affect their conformational ordering. The amide-bridged SAMs preserved an overall all trans conformation with virtually no gauche defects [18]. The strong head gold-sulfur bonds and the amide hydrogen bonds at the end of the alkyl chains restricted the conformational freedom and ensured enough stiffness of the whole structure to an extent that it sustained the steric hindrance caused by voluminous end substituents.
In this study we attempted to find additional stabilization for long-chain amide-bridged SAM by means of building of a second hydrogen bonded network between the endmost substituents. Synthesized structural analogues of the compounds were purposefully already studied in [18] as the methoxy groups were replaced by hydroxyl groups. Thus, in addition to the existing amide hydrogen bonding, this approach allowed formation of additional hydrogen bonded networks between the endmost substituted phenyl rings. Similarly, albeit comparatively short-chain SAMs formed by 3,4-dihydroxyphenylalanine-terminated propanethiol and tyrosine-terminated propanethiol have been studied by XPS, NEXAFS and RAIRS, but the authors did not comment on the influence of the second hydrogen bonded network on the SAM orientation [19,20]. A recent study of unusually long oligo(ethyleneglycol)-containing alkylthiolates on gold, disclosed enhanced thermal stability of the monolayers in comparison with aliphatic thiolates because of strong intermolecular hydrogen bonding [21].
2. Results and Discussion
2.1. Ellipsometry from Infrared to Visible Spectral Range
Spectroscopic ellipsometry was used to determine optical properties and thickness of the SAMs studied. Each monolayer was characterized by a complex refractive index and effective thickness which were adjusted in the course of an iterative procedure. The correspondent values of the blank gold substrate, that serve as input parameters in the calculations were determined by independent measurements. Each ellipsometric measurement must be performed over one and the same blank and subsequently covered substrate. Monolayers in the nanometer scale are very sensitive to the substrate roughness which might be dissimilar for different substrates and also can vary from spot to spot. Ellipsometric measurements randomly performed over different blank and covered substrates, or even different spots on one sample, may experience problems in the fitting procedure.
The thickness evaluation was performed by Vis-ellipsometry using the classical model of an isotropic film on a semi-infinite substrate. A value of 1.4 was accepted for the film high frequency refractive index n∞ of 5a and 5b (Scheme 1), which was already used for the thickness evaluation of structurally analogous methoxy-substituted thiols [18]. The fitting procedure converged with a thickness of 2.85 nm for the monolayer formed by the 4-hydroxy substituted thiol and 2.78 nm for the 3,5-dihydroxy substituted thiol. It is worth to note that the calculated effective thicknesses were found for monolayers obtained from 10−4 M thiol concentrations. The typically used 10−3 M thiol concentration yielded formation of stacked double layered structure because of pairing via OH⋯OH bonds prior to the self assembling.
2.2. Orientation of the Methylene Chains and Substituted Phenyl Rings
The SAM orientation analysis was based on the comparison of IR absorption in solid state with the ellipsometric spectra of the film. Theoretically simulated ellipsometric spectra by means of a suitable optical model can serve as quantitative measures in respect to the complex dielectric function, effective thickness and molecular orientation within the film. Best-fit simulations of the amplitudes and the band shapes in the tan ψ spectra in conjunction with surface selection rule for metallic surfaces were used to evaluate the average molecular tilt angles and the orientation of the end substituents.
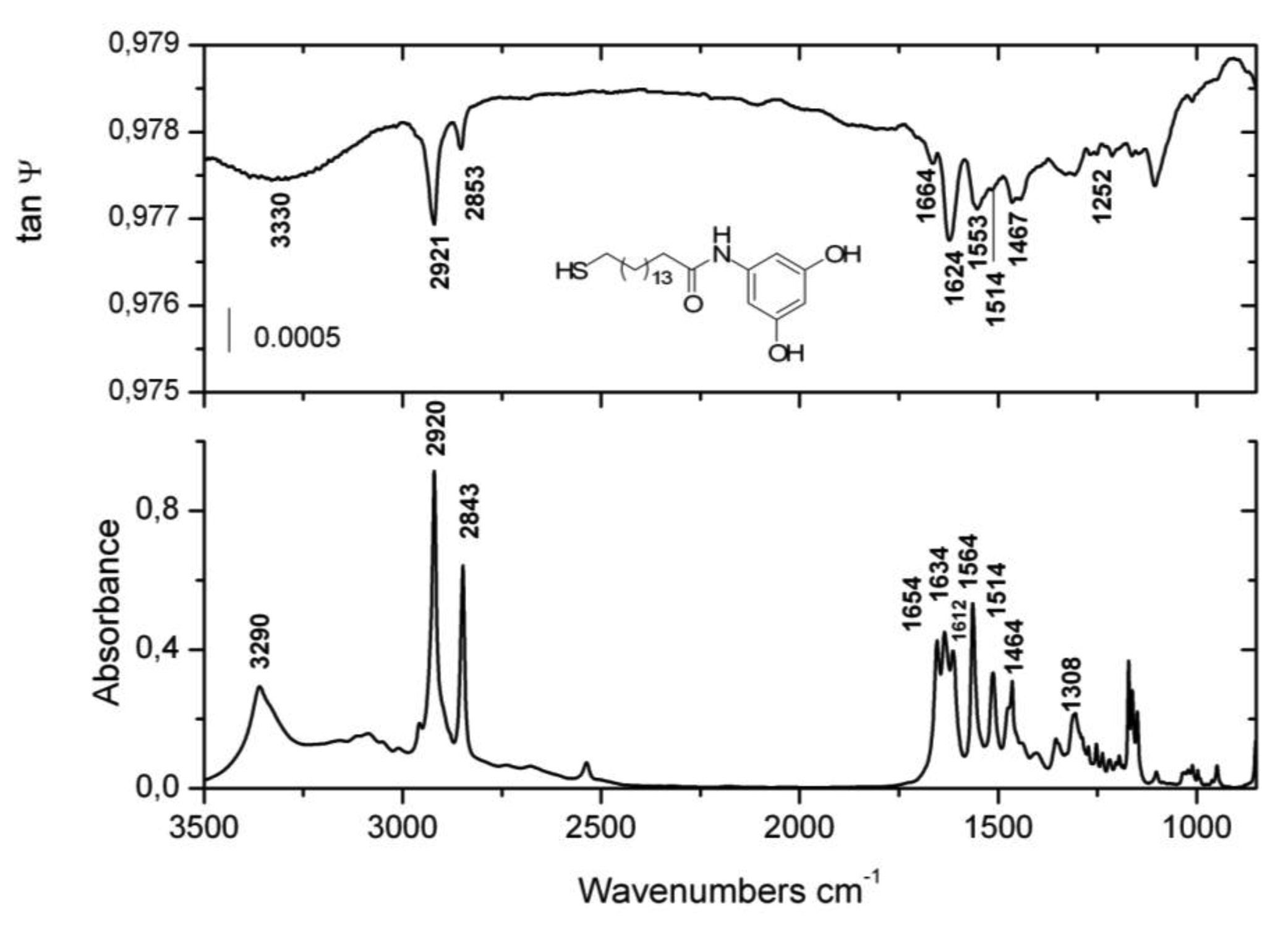
Ellipsometric tan ψ spectra of 5a and 5b along with their IR absorption counterparts in solid state are displayed in Figure 1 and 2 respectively. The structurally sensitive IR bands and their assignments are listed in Table 1 following available literature data of related molecules [22-24].
The IR absorption spectra displayed in Figures 1 and 2 will serve as reference for tanψ spectra of the adsorbates. According to the surface selection rule for metallic surfaces, only these bands whose transition dipole moments are projected onto the surface normal are visible in tan ψ spectra of thin films.
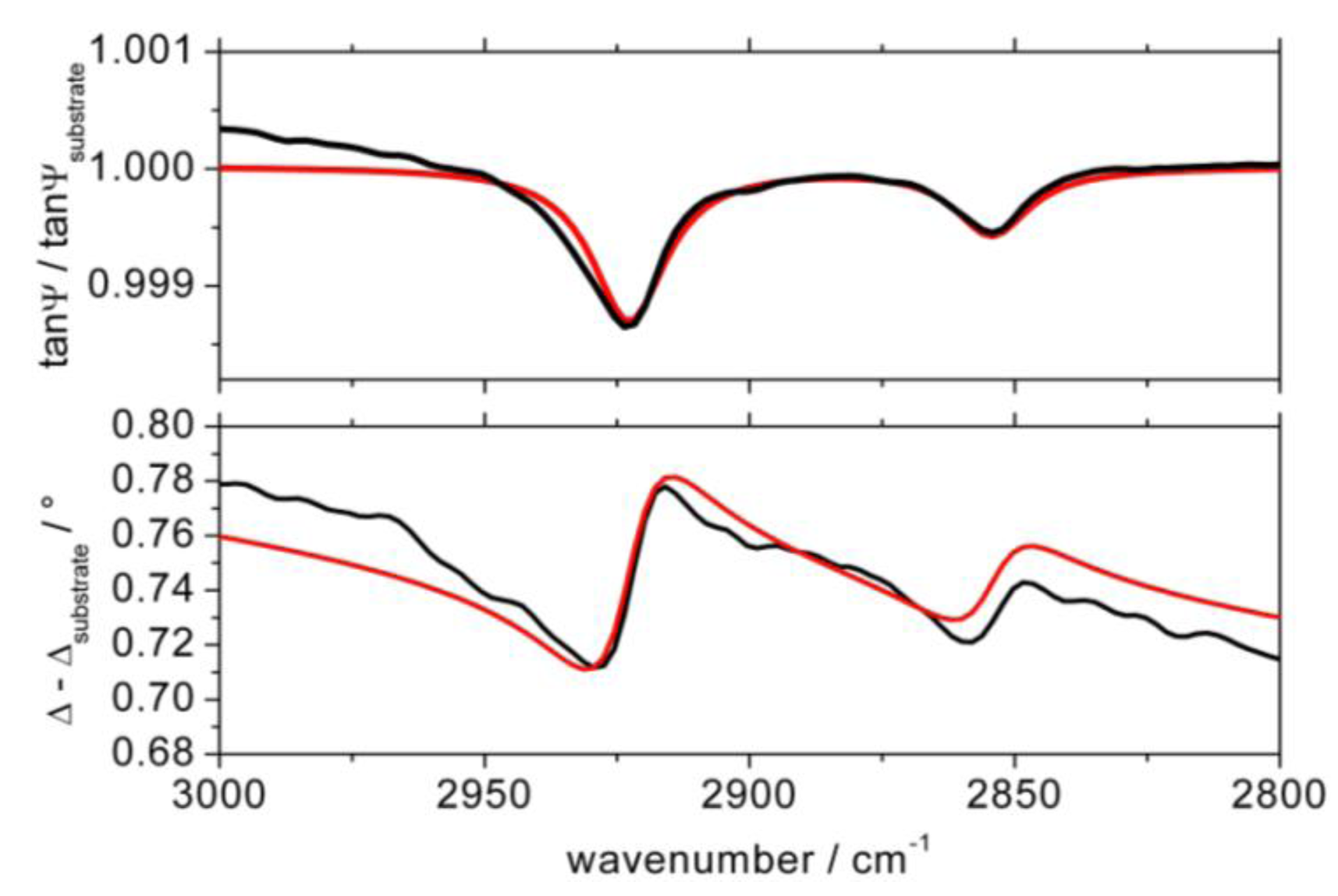
The examination of the adsorbate spectra shows that the hydrocarbon chains predominantly exist in all trans conformation with very few gauche defects. The wavenumbers of the antisymmetric νas(CH2) stretching modes for SAMs 5a and 5b very slightly deviate from these in solid state indicating crystalline lateral interactions between long alkyl chains [25].
The most intense bands in the fingerprint region for 5a (Figure 1) are located at 1,551 cm−1 and 1,517 cm−1. The first band can undoubtedly be assigned to amide II vibration of a hydrogen-bonded trans conformer of a secondary amide [10-18,21,22]. It mostly involves in-plane N-H bending and torsional N-C=O modes and, for non-hydrogen bonded species, appears at 1,513 cm−1 in solution [18]. Its enhanced intensity can be ascribed to a large contribution of the NH in-plane bending mode whose transition dipole moment is in the plane and along the molecular axis that might be oriented close to the surface normal [9,13-15,18-20]. The band at 1,517 cm−1 can be cogently assigned to a 19a mode of p-disubstituted benzene derivatives where the transition dipole moment is oriented along the both substituents [18-20,22,23] and is an important marker for probing the phenyl rings orientation [18,25,26]. The band 8a located at 1,608 cm−1 in the adsorbate (1,611 cm−1 in solid state), whose vibrational mode has the same transition dipole moment direction [23,24], is also of the same kind. The amide I band (predominantly C=O stretching) emerges as a very weak feature at 1,660 cm−1. Its peak position is slightly up-shifted from its counterpart absorption in crystal state and both are shifted down by about 25 cm−1 from that in solution indicating participation in hydrogen bonds. The significant reduction of ν(C=O) band intensity suggest close to parallel orientation of the carbonyl bonds to the gold surface. Another convincing proof for existing hydrogen bonded network between amide moieties is the location of the N-H stretching vibration. It appears at 3,430 cm−1 in solution and upon forming of hydrogen-bonded network a broad and intense band down-shifted by approximately 130 cm−1 emerges in KBr spectra. Besides the obvious peak at 3,312 cm−1 associated with amide hydrogen bonding, there is an apparent shoulder up-shifted by 60 cm−1 which is due to the OH⋯OH hydrogen bonds. The curve fitting procedure found a band at 3,378 cm−1 responsible for the OH hydrogen bonding. The same spectral region in the adsorbate spectrum is covered by one broad and low intense band centered around 3,321 cm−1. It presumably comprises the overlapped hydrogen bonded networks of both amide and hydroxyl groups resulting in one wide band contour. In the previously studied SAMs, formed by methoxyphenyl analogues of 5a and 5b [18], there was no spectral band in this region suggesting parallel orientation of the amide hydrogen bonds. Obviously the second hydrogen bonded network between the hydroxyl groups, slightly changes the average hydrocarbon tilt which is the reason for emergence of this band.
The inspection of the adsorbate spectrum of 5b also clearly demonstrates that the hydrocarbon chains are hydrogen bonded. Amide I band is observed as very weak feature and its wavenumber is consistent with the correspondent band in the hydrogen bonded polycrystalline sample. Its considerably reduced intensity is a hint for close to parallel orientation of the C=O bond to the metallic surface. Another prominent band is amide II band at 1,553 cm−1. This band gains intensity from the higher contribution of N-H in-plane bending mode. Its transition dipole moment is parallel to the long molecular axis implying nearly perpendicular chain's orientation. Broad and relatively more intense band than this for 5a is observed at 3,330 cm−1. The augmented intensity can be attributed to the presence of a second hydroxyl group.
The amide bridged alkanethiols require different organizational structure when are chemisorbed on Au(111) than the correspondent n-alkyl thiols. Previous considerations [10-14,18] suggested that trans conformation of a second amide yields formation of linear hydrogen bonds with reduced chain tilts as a consequence of the lengthened interchain distances. The imposed steric constrains are not fully commensurate with the hexagonal gold-sulfur lattice (√3 × √3)R30° generally accepted for bonding of thiols on Au(111) [1-3] and some increase in S-S spacing necessary to accommodate the hydrocarbon chains brings about chain tilt reduction [10-14,18]. Our model calculations carried out on 5a (Figure 3) determined average tilt of 19° for the hydrocarbon chains. It is lowered by 10-12° in comparison with the n-alkyl thiols with the same chain length [1-3], but is in accord with analogous results reported by others [10-15,17]. Recent DFT calculations performed on an amide bridged oligo(ethylene glycol) terminated thiol confirmed these conclusions and provided some geometrical parameters for the monolayers [28]. Presuming hexagonal (√3 × √3)R30° lattice model with fixed 5 Å spacing between sulfur atoms while the bond lengths and dihedral angles have been left free to change, the authors predicted significant decrease of the chain tilt by ∼10–15° and corresponding reduction of the O⋯H distances to 1.9 Å. The optimized bond angles of C=O and N-H with respect to the long axis were calculated close to 90° which is in accord with the existing experimental data [28].
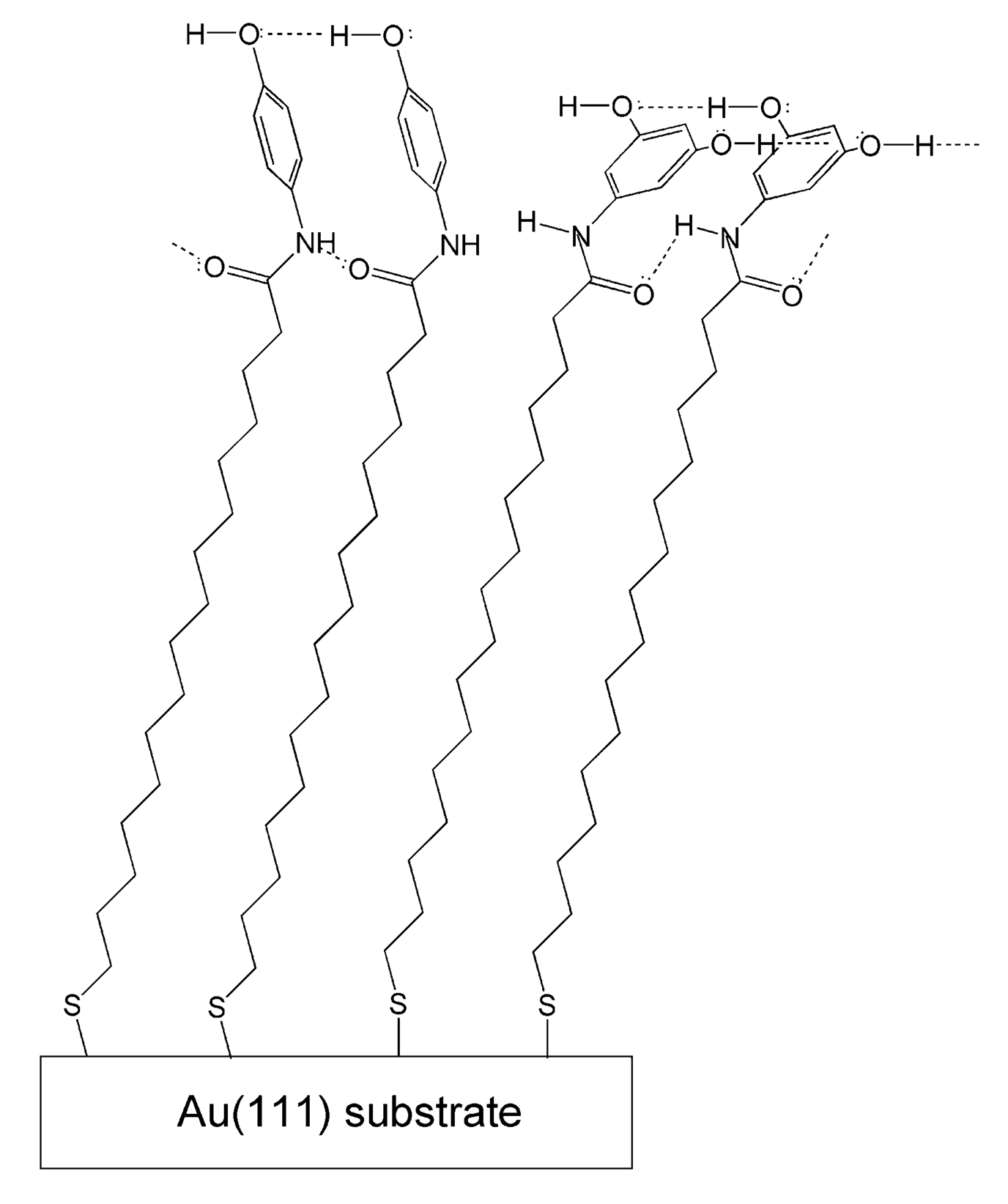
The intensity changes in some distinct characteristic bands in tanψ spectra can serve as suitable markers for determination of the phenyl rings orientation. Bands, such as 8a and 19a (Table 1, Figure 1), for p-di-substituted benzene derivatives have transition dipole moments oriented along the line connecting both substituents, while 8b and 19b are perpendicularly polarized in the same plane [22-24]. Both 8a and 19a show markedly increased intensity in the spectrum of 5a, which is associated with larger projections of their transition dipole moments onto surface normal. Alternatively, the band 19b at 1,381 cm−1 in KBr is not discernible in the adsorbate spectrum implying almost vertical orientation of the phenyl rings. These observations are in agreement with the results obtained by Uvdal et al. [20] for the orientation alignment of tyrosine-terminated propanethiol self assembled on a gold surface.
Different phenyl ring orientation is observed for 3,5-di-hydroxyphenyl substituted thiol 5b. The transition dipole moments of 19a and 19b modes for 1,3,5-three substituted benzene are mutually orthogonal, as 19a is polarized along the line connecting positions 1,4 while 19b is perpendicular to it in the phenyl ring plane [23,24]. Both vibrations have medium intensities in absorption, as 19a is located at 1,464 cm−1 and 19b at 1,514 cm−1. Although the intensity of 19a in the tanψ spectrum prevails over that of 19b, the appearance of both bands in adsorbate spectrum indicates tilted orientation of the phenyl ring. Other characteristic features also are the degenerated modes 8a at 1,614 cm−1 and 8b at 1,636 cm−1 in the bulk spectrum. While similar to mode 19a, the transition dipole moment of 8a is polarized along the line connecting positions 1,4, the correspondent dipole moment direction for 8b is dominated by a radial component and is oblique to 8a [23,24]. Both modes are represented as one very strong band at 1,624 cm−1 in the adsorbate spectrum implying tilted orientation of the three-substituted phenyl rings. Apparently the additional hydrogen bonded network forces the phenyl rings of 5b to adopt tilted orientation that minimizes the unfavorable van der Waals interactions. The specific orientations of the terminal phenyl rings of 5a and 5b are schematically presented in Figure 4.
The built secondary hydrogen bonded networks through the OH groups in 5a and 5b substantially increased the thermal stability of the monolayers. Test measurements performed after heating the samples in an oven for 30 min at 60 °C, 80 °C and 105 °C revealed no detectable changes in the structure and conformational ordering within the monolayers. Both 5a and 5b showed equal thermal stability at the above conditions notwithstanding the different number of the OH groups. The parent methoxyphenyl substituted thiols [18] appeared less stable as some aggravation in the conformational ordering was observed after heating the sample above 80 °C.
Amide bridged SAMs having secondary hydrogen bonded network turn out to be suitable candidates for use as building blocks in molecular electronics or in nanostructure based devices when the working conditions require elevated temperatures.
3. Experimental Section
3.1. Synthesis of the Model Compounds
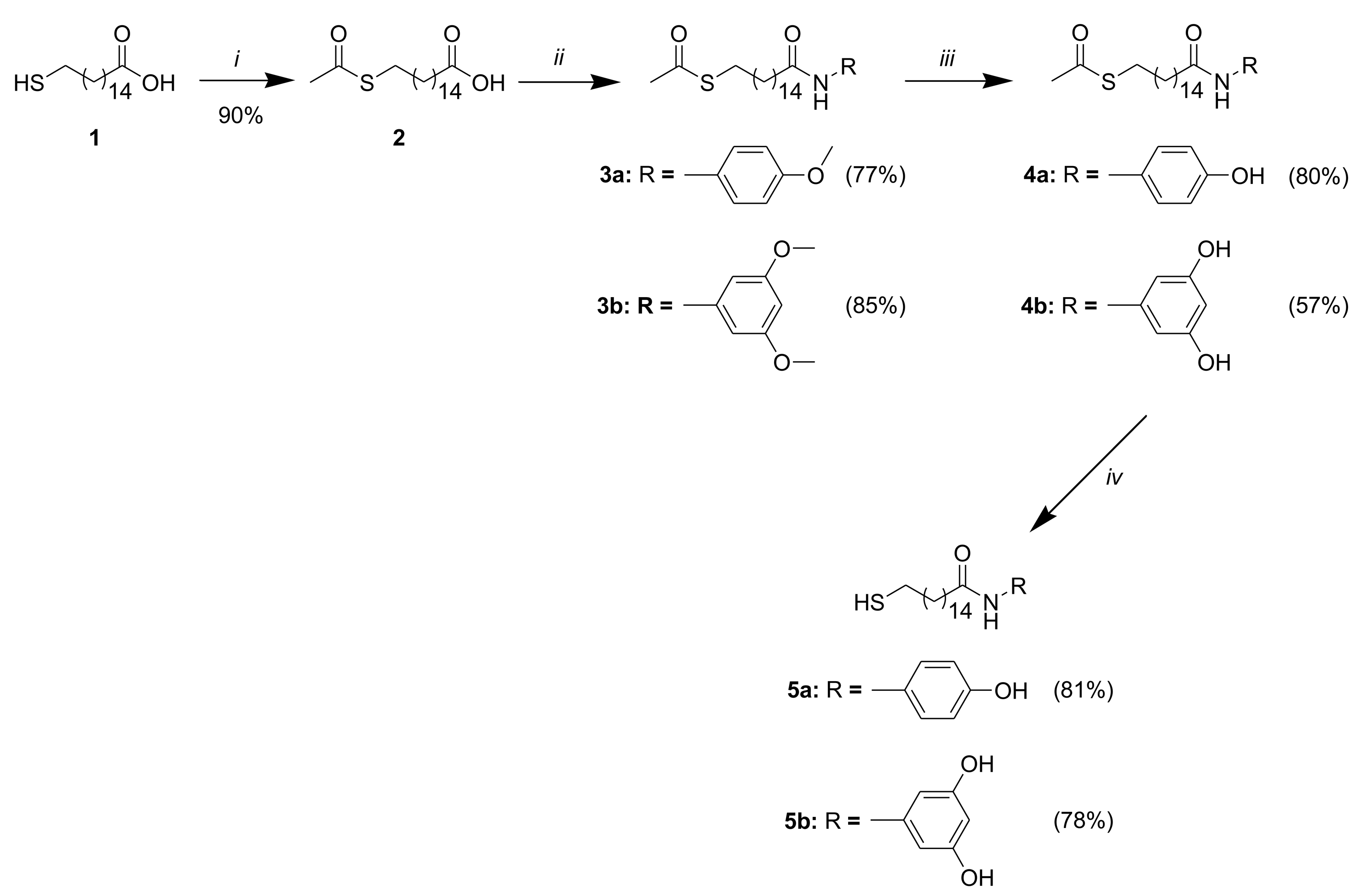
The synthesis of the amides 5a, 5b is depicted in Scheme 2. The protection of the thiol group in 1 was achieved with Zn dust and acetyl chloride in AcOH with high yield [29]. The coupling reaction of the acid 2 with 4-methoxyalniline and 3,5-dimethoxyaniline was carried out in CH2Cl2 using EDCI and HOBT as activating reagents. The addition of 0.5 eq DMAP increased the yields of the amides 3a and 3b to 77% and 85% respectively. The transformation of the methoxy group of 3a, 3b into OH-group was realized with BBr3 in CH2Cl2 at 20 °C. The compounds 4a and 4b were isolated in 80% and 57% yield respectively. The thioacetates 4a, 4b were subsequently deprotected chemoselectively to the target compounds 5a, 5b under basic condition in high yields [29]. The structure of all synthesized compounds was confirmed by IR, 1H- and 13C-NMR spectra.
3.2. Monolayer Preparation
Gold substrates (200 nm vacuum evaporated gold on microscope slides with 2 nm intermediate Ti layer) were purchased from Ssens bv (The Netherlands). Spectroscopy grade methylene chloride was obtained from Merck and used as received. Glassware was cleaned with piranha solution (30% H2O2:H2SO4 3:7) for 30 min at 90 °C.
The gold substrates were first annealed for a few seconds on an acetylene burner (2,300 °C) in order to remove possible organic contaminants. The annealed substrates were cooled down in a stream of Ar and then immersed in a 10−4 M solution of the respective thiol in a mixture 10:1 CH2Cl2:CH3OH (Merck) at room temperature overnight. Before the ellipsometric measurements, the samples were rinsed thoroughly with methylene chloride/methanol and blown dry with Ar. Such a procedure effectively removes the physisorbed molecules from the surface.
3.3. Infrared Spectroscopic Ellipsometry (IRSE)
The SAMs were examined by infrared spectroscopic ellipsometry. The measurements were performed with the IR ellipsometer, described in detail elsewhere [30]. All spectra were recorded in the 4,000–800 cm−1 range with a resolution of 4 cm−1. The sample size (10 × 19 mm) restricted the incidence angles to 70°, because larger angles would have increased the error due to the opening angle in our setup. A special chamber purged with dry air maintained low humidity during the measurements. Data collection included set of intensity measurements taken with the analyzer fixed at 45° in relation to the plane of incidence and the front polarizer consecutively set at 0° and 90° and then at 45° and 135°. Good signal to noise ratios were achieved after averaging 4 cycles of measurements where 256 scans were co-added for each set. The whole measurement procedure was fully automated using Opus Macro software (BRUKER).
The optical response of the sample is quantified by means of the complex reflection coefficient ρ expressed in terms of the ellipsometric parameters tanψ and Δ, according to the relation.
3.4. Vis-Ellipsometry
The visible ellipsometric measurements were performed by variable angle spectroscopic ellipsometer SE-801-E, SENTECH GmbH, Germany. The thicknesses were determined from a best-fit on three angles measurements at 60°, 65° and 70° in the spectral range 400–700 nm.
3.5. Calculations
The procedure applied for calculation of SAM optical constants and thickness was based on the classical three phase model. The calculations were applied to simulate the ellipsometric tanψ and Δ spectra where the vibrational band shapes were approximated by independent Lorenzian oscillators with wavenumbers (ν̃i0), parameters for the oscillator strengths (Fi) and full width at half maximum FWHM (Γi) to yield the complex dielectric function ε̂ = ε′ + ıε″ with
According to the uniaxial symmetry assumed for the SAMs studied, the components of the dielectric function ε are related by εx = εy ≠ εz.
The tilt angle of the hydrocarbon chains for SAM formed by 4-hydroxyphenyl amide 5a was evaluated following the method described previously [32,33].
4. Conclusions
Novel terminally hydroxyphenyl substituted long-chain alkanethiols containing amide groups 5a and 5b (Scheme 1) were synthesized and their self assembled monolayers on Au(111) were studied by means of infrared and visible ellipsometry. The amide group was incorporated in the end of the alkyl chain, as the spacer between the head sulfur atom and the amide moiety comprises 16 carbon atoms. Extensive hydrogen bonded networks were detected between alkyl chains via amide groups as well as between hydroxyl-substituted phenyl rings. The hydrogen bonding reduced the chain tilt by approximately 12° but did not affect the chain conformation. The comparison of the bulk IR spectra in solid state with the tanψ spectra of the adsorbates revealed that the alkyl chains predominantly exist in all trans conformation with only a few gauche defects. The strong head gold-sulfur bonds and the terminal amide hydrogen bonds substantially restrict the conformation freedom of the hydrocarbon chains and give rise to more rigid structure. The hydrogen bonding between the hydroxyl substituted phenyl rings adds additional stiffness to the monolayer, but yields different orientation of the endmost aryl substituents. The hydrogen bonding between para-substituted phenyl rings fixes their orientation close to vertical, while the phenyl rings in meta-di-substituted species are tilted. The additional H-bonded networks for both species can result in substantially enhanced thermal stability in comparison with the parent amide bridged SAMs [18].

| Compound | Tanψ (film) (cm−1) | KBr (cm−1) | Assignmenta |
|---|---|---|---|
| 5a | 3,321 | 3,312 | νNH str |
| 2,919 | 2,917 | νas(CH2) | |
| 2,851 | 2,850 | νs(CH2) | |
| 1,660 | 1,653 | ν(C=O) | |
| Amide I | |||
| 1,608 | 1,611 | ν(CC)Ar 8a | |
| 1,551 | 1,548 | NH, N-C=O | |
| Amide II | |||
| 1,518 | 1,517 | ν(CC)Ar 19a | |
| 1,250 | 1,250 | Amide III | |
| 5b | 3,330 | 3,360 | νNH str |
| 2,921 | 2,920 | νas(CH2) | |
| 2,853 | 2,849 | νs(CH2) | |
| 1,664 | 1,654 | ν(C=O) | |
| Amide I | |||
| 1,624 | 1,636 | ν(CC)Ar 8b | |
| 1,614 | ν(CC)Ar 8a | ||
| 1,553 | 1,563 | NH, N-C=O | |
| Amide II | |||
| 1,514 | 1,514 | ν(CC)Ar 19b | |
| 1,467 | 1,464 | ν(CC)Ar 19a | |
Acknowledgments
The authors thank to C. Cobet and N. Esser, ISAS Berlin for making available the VIS ellipsometer and for cooperation as well as to I. Fischer for the technical assistance. The financial support by the Deutsche Forschungsgemeinschaft and the Bulgarian Academy of Sciences (contract 436 BUL 113/127), the Senatsverwaltung für Wissenschaft, Forschung und Kultur des Landes Berlin, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie is gratefully acknowledged.
References
- Dubois, L.H.; Nuzzo, R.G. Synthesis, structure and properties of model organic surfaces. Annu. Rev. Phys. Chem. 1992, 43, 437–463. [Google Scholar]
- Schreiber, F. Structure and growth of self-assembling monolayers. Progr. Surf. Sci. 2000, 65, 151–256. [Google Scholar]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar]
- Li, Y.; Chang, S.C.; Williams, R.S. Self-assembly of alkanethiol molecules onto platinum and platinum oxide surfaces. Langmuir 2003, 19, 6744–6749. [Google Scholar]
- Shi, D.X.; Ji, W.; Lin, X.; He, X.B.; Lian, J.C.; Gao, L.; Cai, J.M.; Lin, H.; Du, S.X.; Lin, F.; et al. Role of lateral alkyl chains in modulation of molecular structures on metal surfaces. Phys. Rev. Lett. 2006, 96, 226101. [Google Scholar]
- Akkerman, H.B.; Blom, P.W.M.; de Leeuw, D.M.; de Boer, B. Towards molecular electronics with large-area molecular junctions. Nature 2006, 441, 69–72. [Google Scholar]
- Smits, E.C.P.; Mathijssen, S.G.J.; van Hal, P.A.; Setayesh, S.; Geuns, T.C.T.; Mutsaers, K.A.H.A.; Cantatore, E.; Wondergem, H.J.; Werzer, O.; Resel, R.; et al. Bottom-up organic integrated circuits. Nature 2008, 455, 956–959. [Google Scholar]
- Maisch, S.; Buckel, F.; Effenberger, F. Preparation of high quality electrical insulator self-assembled monolayers on gold. Experimental investigation of the conduction mechanism through organic thin films. J. Am. Chem. Soc. 2005, 127, 17315–17322. [Google Scholar]
- Lenk, T.J.; Hallmark, V.M.; Hoffmann, C.L.; Rabolt, J.F.; Castner, D.G.; Erdelen, C.; Ringsdorf, H. Structural investigation of molecular-organization in self-assembled monolayers of a semifluorinated amideethiol. Langmuir 1994, 10, 4610–4617. [Google Scholar]
- Tam-Chang, S.-W.; Biebuych, H.A.; Whitesides, G.M.; Jeon, N.; Nuzzo, R.G. Self-assembled monolayers on gold generated from alkanethiols with the structure RNHCOCH(2)SH. Langmuir 1995, 11, 4371–4382. [Google Scholar]
- Clegg, R.S.; Hutchison, J.E. Hydrogen-bonding, self-assembled monolayers: Ordered molecular films for study of through-peptide electron transfer. Langmuir 1996, 12, 5239–5243. [Google Scholar]
- Chechik, V.; Schönherr, H.; Vansco, G.J.; Stirling, C.J.M. Self-assembled monolayers of branched thiols and disulfides on gold: Surface coverage, order and chain orientation. Langmuir 1998, 14, 3003–3010. [Google Scholar]
- Clegg, R.S.; Reed, S.M.; Hutchison, J.E. Self-assembled monolayers stabilized by three-dimensional networks of hydrogen bonds. J. Am. Chem. Soc. 1998, 120, 2486–2487. [Google Scholar]
- Clegg, R.S.; Reed, S.M.; Smith, R.K.; Barron, B.L.; Rear, J.A.; Hutchison, J.E. The interplay of lateral and tiered interactions in stratified self-organized molecular assemblies. Langmuir 1999, 15, 8876–8883. [Google Scholar]
- Clegg, R.S.; Hutchison, J.E. Control of monolayer assembly structure by hydrogen bonding rather than by adsorbate-substrate templating. J. Am. Chem. Soc. 1999, 121, 5319–5327. [Google Scholar]
- Ferguson, M.K.; Low, E.R.; Morris, J.R. Well-ordered self-assembled monolayers created via vapor-phase reactions on a monolayer template. Langmuir 2004, 20, 3319–3323. [Google Scholar]
- Kim, J.H.; Shin, S.S.; Kim, S.B.; Hasegawa, T. Hydrogen-bonding networks of dialkyl disulfides containing the urea moiety in self-assembled monolayers. Langmuir 2004, 20, 1674–1679. [Google Scholar]
- Angelova, P.N.; Hinrichs, K.; Philipova, I.L.; Kostova, K.V.; Tsankov, D.T. Monolayer orientation of omega-substituted amide-bridged alkanethiols on gold. J. Phys. Chem. C 2010, 114, 1253–1259. [Google Scholar]
- Petoral, R.M., Jr; Uvdal, K. Structural investigation of 3,4-dihydroxyphenylalanine-terminated propanethiol assembled on gold. J. Phys. Chem. B 2003, 107, 13396–13402. [Google Scholar]
- Uvdal, K.; Ekeroth, J.; Konradson, P.; Liedberg, B. Tyrosine derivatives assembled on gold. J. Colloid Interface Sci. 2003, 260, 361–366. [Google Scholar]
- Valiokas, R.; Östblom, M.; Björefors, F.; Liedberg, B.; Shi, J.; Konradsson, P. Structural and kinetic properties of laterally stabilized, oligo(ethylene glycol)-containing alkylthiolates on gold: A modular approach. Biointerphases 2006, 1, 22–34. [Google Scholar]
- Roeges, N.P.G. A Guide to the Complete Interpretation of Infrared Spectra of Organic Molecules; Wiley: Chichester, UK, 1994; pp. 313–323. [Google Scholar]
- Varsányi, G. Vibrational Spectra of Benzene Derivatives; Akadémiai Kiadó: Budapest, Hungary, 1969; pp. 142–168. [Google Scholar]
- Varsányi, G. Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives; John Wiley &Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Snyder, R.G.; Strauss, H.L.; Elliger, C.A. C-H stretching modes and the structure of normal-alkyl chains. 1. Long, disordered chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar]
- Angelova, P.; Hinrichs, K.; Esser, N.; Kostova, K.; Tsankov, D. Self-assembly of terminally aryl-substituted long-chain alkanethiols on silver. Vib. Spectrosc. 2007, 45, 55–60. [Google Scholar]
- Angelova, P.; Hinrichs, K.; Kostova, K.; Tsankov, D. Orientation analysis of omega-substituted long-chain alkanethiols self-assembled on au substrate. J. Phys. Chem. C 2008, 112, 17683–17687. [Google Scholar]
- Malysheva, L.; Onipko, A.; Liedberg, B. Ab initio Modeling of amide-stabilized, oligo(ethylene glycol)-terminated self-assemblies: In-SAM molecular geometry, orientation, and hydrogen bonding. J. Phys. Chem. A 2008, 112, 1683–1687. [Google Scholar]
- Svedhem, S.; Hollander, C.-A.; Shi, J.; Konradsson, P.; Liedberg, B.; Svensson, S.C.T. Synthesis of a series of oligo(ethylene glycol)-terminated alkanethiol amides designed to address structure and stability of biosensing interfaces. J. Org. Chem. 2001, 66, 4494–4503. [Google Scholar]
- Röseler, A.; Korte, E.H. Infrared spectroscopic ellipsometry. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Chalmers, J., Eds.; Wiley: Chichester, UK, 2002; Volume 2, pp. 2–5. [Google Scholar]
- Rosu, D.M.; Jones, J.C.; Hsu, J.W.P.; Kavanagh, K.L.; Tsankov, D.; Schade, U.; Esser, N.; Hinrichs, K. Molecular orientation in octanedithiol and hexadecanethiol monolayers on GaAs and Au. Langmuir 2009, 25, 919–923. [Google Scholar]
- Chollet, P.-A.; Messier, J.; Rosilio, C. Infrared determination of the orientation of molecules in stearamide monolayers. J. Chem. Phys. 1976, 64, 1042–1050. [Google Scholar]
- Tolstoy, V.P.; Chernyshova, I.V.; Skryshevsky, V.A. Handbook of Infrared Spectroscopy of Ultrathin Films; Wiley & Sons Inc.: New York, NY, USA, 2003; pp. 266–280. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsankov, D.; Philipova, I.; Kostova, K.; Hinrichs, K. Infrared Ellipsometric Study of Hydrogen-Bonded Long-Chain Thiolates on Gold: Towards Resolving Structural Details. Micromachines 2011, 2, 306-318. https://doi.org/10.3390/mi2020306
Tsankov D, Philipova I, Kostova K, Hinrichs K. Infrared Ellipsometric Study of Hydrogen-Bonded Long-Chain Thiolates on Gold: Towards Resolving Structural Details. Micromachines. 2011; 2(2):306-318. https://doi.org/10.3390/mi2020306
Chicago/Turabian StyleTsankov, Dimiter, Irena Philipova, Kalina Kostova, and Karsten Hinrichs. 2011. "Infrared Ellipsometric Study of Hydrogen-Bonded Long-Chain Thiolates on Gold: Towards Resolving Structural Details" Micromachines 2, no. 2: 306-318. https://doi.org/10.3390/mi2020306




