Influence of Surface Treatments on Urea Detection Using Si Electrolyte-Gated Transistors with Different Gate Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Fabrication of EGTs
2.3. Electrical Characterization
2.4. Functionalization of EGTs
3. Results and Discussion
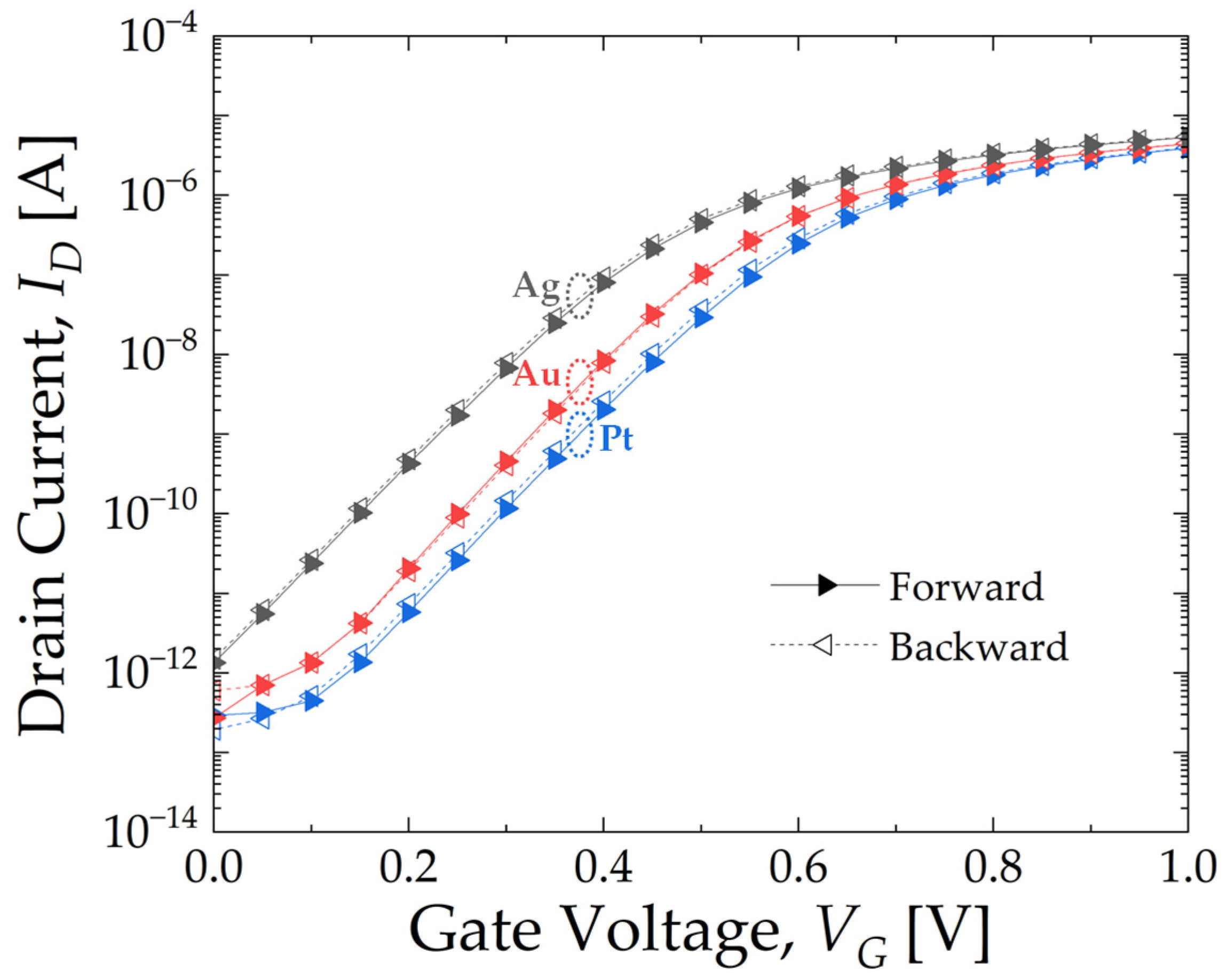
3.1. Intrinsic Electrical Characteristics
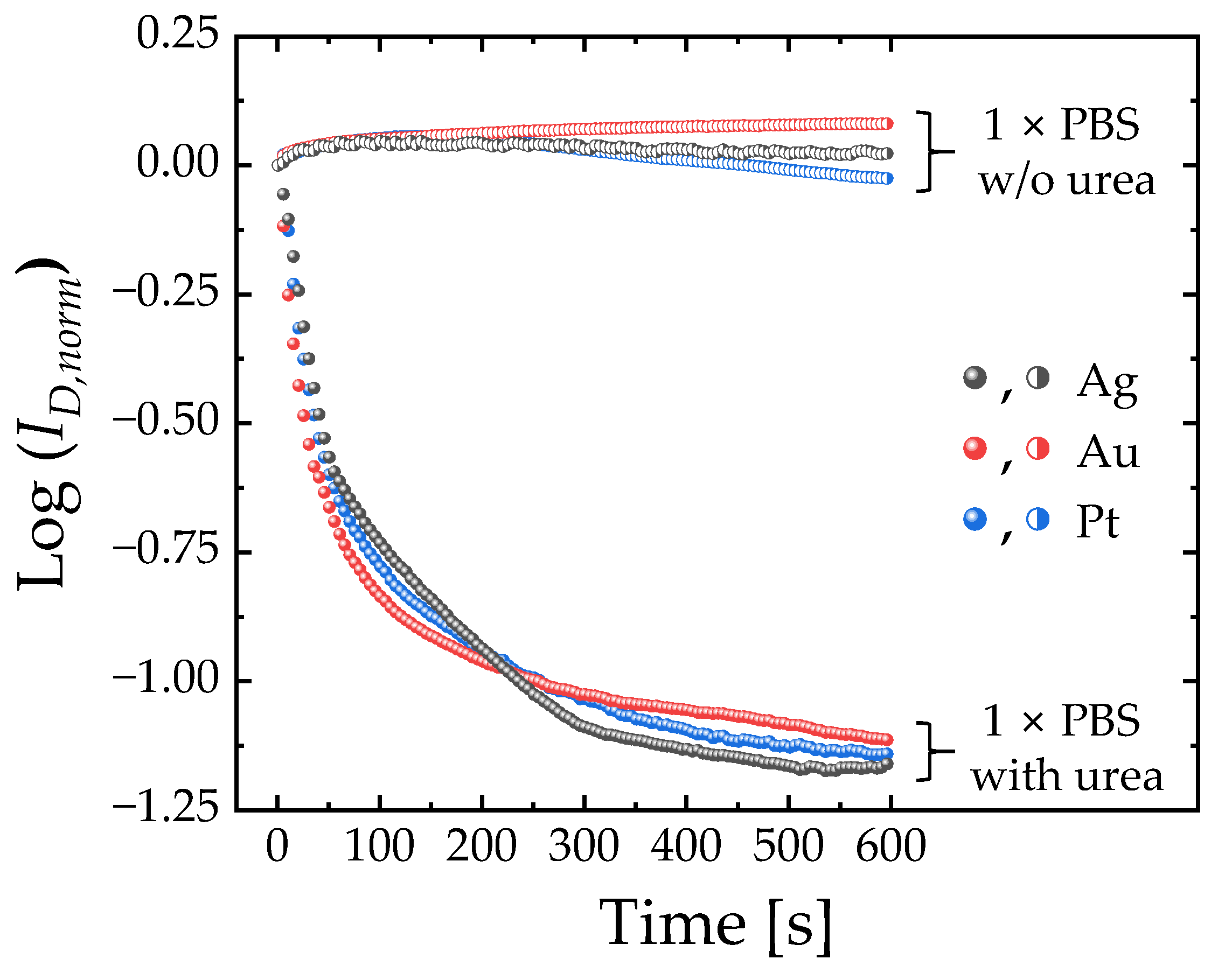
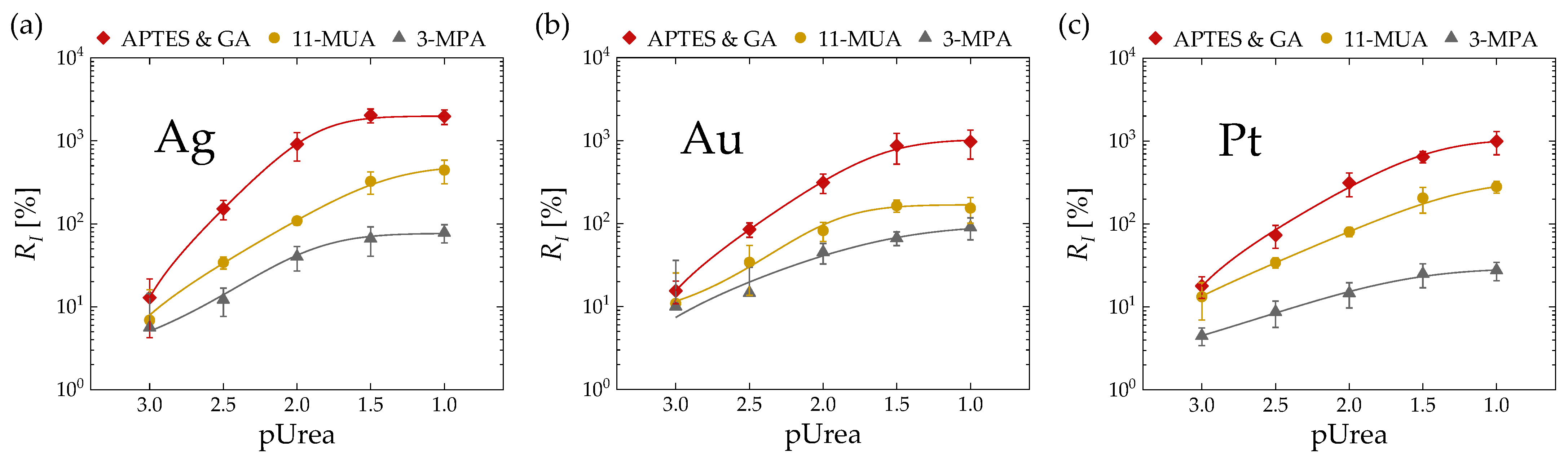
3.2. Urea Sensing Characteristics
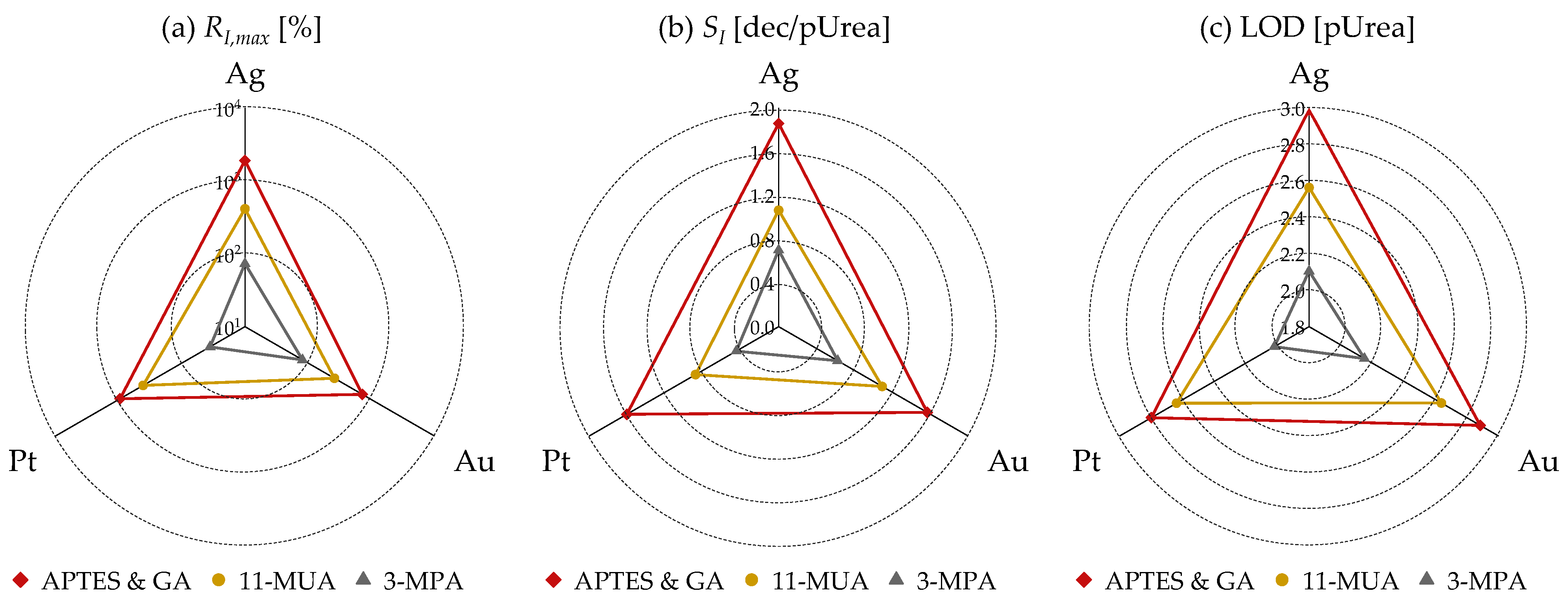
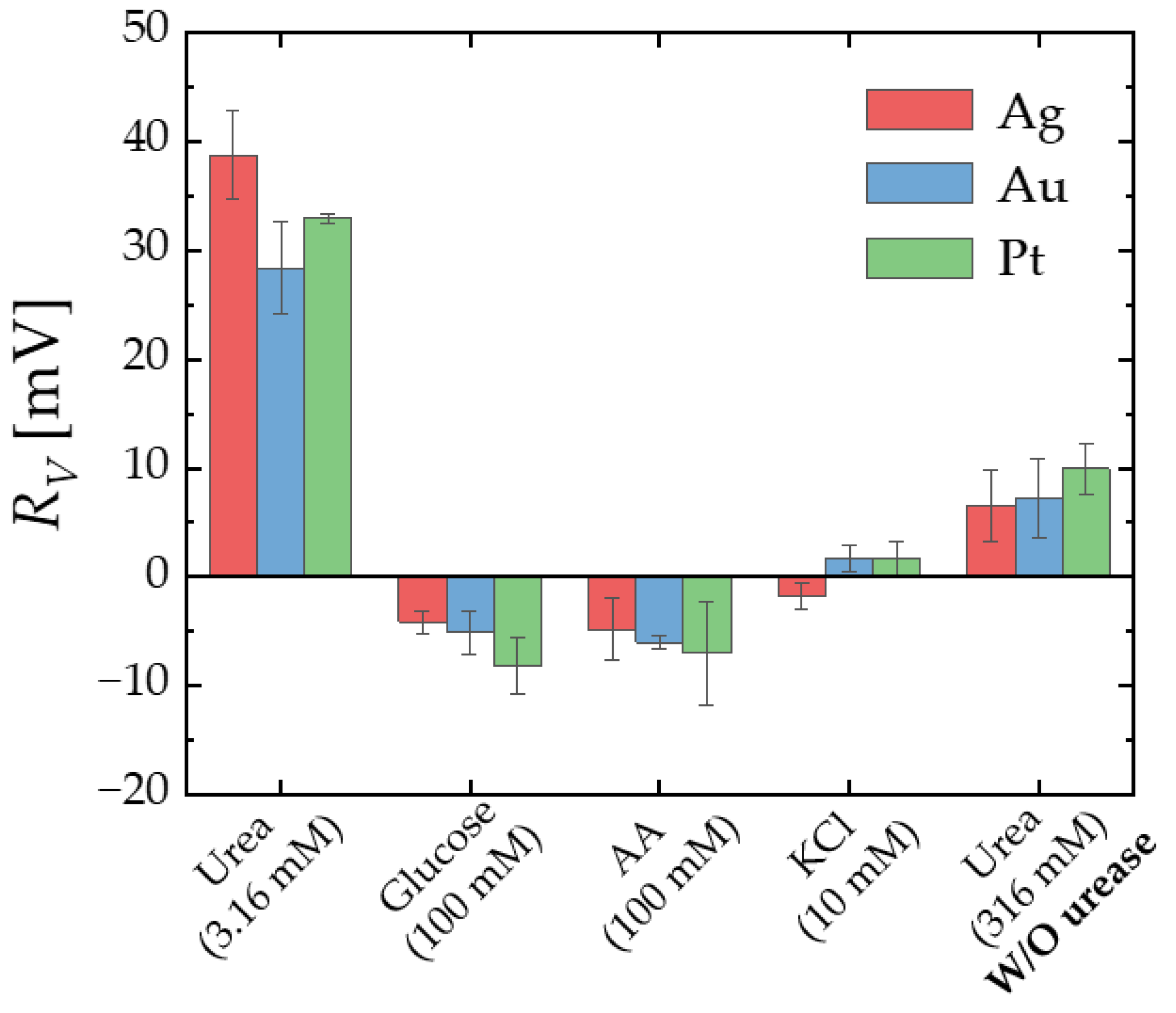
3.3. Selectivity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-Nanowire-Based CMOS-Compatible Field-Effect Transistor Nanosensors for Ultrasensitive Electrical Detection of Nucleic Acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-modulated transistor detects odorant binding protein chiral interactions. Nat. Commun. 2015, 6, 6010. [Google Scholar] [CrossRef] [PubMed]
- Macchia, E.; Manoli, K.; Holzer, B.; Di Franco, C.; Ghittorelli, M.; Torricelli, F.; Alberga, D.; Mangiatordi, G.F.; Palazzo, G.; Scamarcio, G.; et al. Single-molecule detection with a millimetre-sized transistor. Nat. Commun. 2018, 9, 3223. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Kim, D.; Jin, B.; Kim, S.-A.; Choi, W.; Shin, S.; Park, J.; Shim, W.-B.; Kim, K.; Lee, J.-S. An Ultrasensitive Silicon-Based Electrolyte-Gated Transistor for the Detection of Peanut Allergens. Biosensors 2022, 12, 24. [Google Scholar] [CrossRef]
- Macchia, E.; Sarcina, L.; Picca, R.A.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. Ultra-low HIV-1 p24 detection limits with a bioelectronic sensor. Anal. Bioanal. Chem. 2019, 412, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, F.; Adrahtas, D.Z.; Bao, Z.; Berggren, M.; Biscarini, F.; Bonfiglio, A.; Bortolotti, C.A.; Frisbie, C.D.; Macchia, E.; Malliaras, G.G.; et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. Methods Prim. 2021, 1, 66. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.; Choi, W.; Do, J.; Son, J.; Kim, K.; Jang, S.; Lee, J.-S. Sensing Characteristics of SARS-COV-2 Spike Protein Using Aptamer-Functionalized Si-Based Electrolyte-Gated Field-Effect Transistor (EGT). Biosensors 2024, 14, 124. [Google Scholar] [CrossRef]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Campos, R.; Borme, J.; Guerreiro, J.R.; Machado, G., Jr.; Cerqueira, M.F.; Petrovykh, D.Y.; Alpuim, P. Attomolar Label-Free Detection of DNA Hybridization with Electrolyte-Gated Graphene Field-Effect Transistors. ACS Sens. 2019, 4, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Hajian, R.; Balderston, S.; Tran, T.; Deboer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.-Y.; Nokes, J.; Athaiya, M.; et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, D.; Nikiforidis, G.; Savva, A.; Giugni, A.; Wustoni, S.; Palanisamy, T.; Chen, X.; Maria, I.P.; Di Fabrizio, E.; Costa, P.M.F.J.; et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 2019, 19, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Walt, D.R. Digital Concentration Readout of Single Enzyme Molecules Using Femtoliter Arrays and Poisson Statistics. Nano Lett. 2006, 6, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Jin, B.; Shin, S.; Do, J.; Son, J.; Kim, K.; Lee, J.-S. Highly Sensitive Detection of Urea Using Si Electrolyte-Gated Transistor with Low Power Consumption. Biosensors 2023, 13, 565. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Botewad, S.N.; Gaikwad, D.K.; Girhe, N.B.; Thorat, H.N.; Pawar, P.P. Urea Biosensors: A Comprehensive Review. Biotechnol. Appl. Biochem. 2023, 70, 485–501. [Google Scholar] [CrossRef]
- Salem, M.; Mauguen, Y.; Prangé, T. Revisiting glutaraldehyde cross-linking: The case of the Arg–Lys intermolecular doublet. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 225–228. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How To Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2011, 28, 416–423. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.J.; Krogh, E.T. Diphenylacetaldehyde and Its Enol: Determination of the Keto Enol and Hydration Equilibrium-Constants and the Pkas of the Aldehyde, Enol, and Hydrate. Comparison with Sterically Hindered Systems. J. Am. Chem. Soc. 1988, 110, 2600–2607. [Google Scholar] [CrossRef]
- Nolte, A.J.; Chung, J.Y.; Walker, M.L.; Stafford, C.M. In situ Adhesion Measurements Utilizing Layer-by-Layer Functionalized Surfaces. ACS Appl. Mater. Interfaces 2009, 1, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, Z.; Lei, C.; Lei, J.; Zhou, Y. An integrated giant magnetoimpedance biosensor for detection of biomarker. Biosens. Bioelectron. 2014, 58, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, C.; Chiu, L.-Y.; Abbasi, K.; Tolbert, B.S.; Sauvé, G.; Yen, Y.; Liu, C.-C. Application of bioconjugation chemistry on biosensor fabrication for detection of TAR-DNA binding protein 43. Biosens. Bioelectron. 2018, 117, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-T.; Jiang, B.-S.; Lo, Y.-H.; Huang, J.-J. Investigation of streptavidin-ligand biochemical interactions by IGZO thin film transistors integrated with microfluidic channels. Sens. Actuators B Chem. 2018, 262, 418–424. [Google Scholar] [CrossRef]
- Cecchet, F.; Rudolf, P.; Rapino, S.; Margotti, M.; Paolucci, F.; Baggerman, J.; Brouwer, A.M.; Kay, E.R.; Wong, J.K.Y.; Leigh, D.A. Structural, Electrochemical, and Photophysical Properties of a Molecular Shuttle Attached to an Acid-Terminated Self-Assembled Monolayer. J. Phys. Chem. B 2004, 108, 15192–15199. [Google Scholar] [CrossRef]
- Yao, D.-J.; Yang, Y.-R.; Tai, C.-C.; Hsiao, W.-H.; Ling, Y.-C. A biochemical sensing system using an 11-MUA/calix[6]arene bilayer to sense amine vapors. J. Micromech. Microeng. 2007, 17, 1435–1441. [Google Scholar] [CrossRef]
- Jennings, G.K.; Munro, J.C.; Yong, T.-H.; Laibinis, P.E. Effect of Chain Length on the Protection of Copper by n-Alkanethiols. Langmuir 1998, 14, 6130–6139. [Google Scholar] [CrossRef]
- Zheng, H.; Hirose, Y.; Kimura, T.; Suye, S.-I.; Hori, T.; Katayama, H.; Arai, J.-I.; Kawakami, R.; Ohshima, T. L-Proline sensor based on layer-by-layer immobilization of thermostable dye-linked l-proline dehydrogenase and polymerized mediator. Sci. Technol. Adv. Mater. 2006, 7, 243–248. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Picraux, L.B.; van Zee, R.D.; Yao, Y.; Tour, J.M. Energy-level alignment and work function shifts for thiol-bound monolayers of conjugated molecules self-assembled on Ag, Cu, Au, and Pt. Chem. Phys. Lett. 2007, 442, 390–393. [Google Scholar] [CrossRef]
- Shoorideh, K.; Chui, C.O. Optimization of the Sensitivity of FET-Based Biosensors via Biasing and Surface Charge Engineering. IEEE Trans. Electron Devices 2012, 59, 3104–3110. [Google Scholar] [CrossRef]
- Hideshima, S.; Hinou, H.; Ebihara, D.; Sato, R.; Kuroiwa, S.; Nakanishi, T.; Nishimura, S.-I.; Osaka, T. Attomolar Detection of Influenza A Virus Hemagglutinin Human H1 and Avian H5 Using Glycan-Blotted Field Effect Transistor Biosensor. Anal. Chem. 2013, 85, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Lu, N.; Wang, Y.; Li, T. Robust ultrasensitive tunneling-FET biosensor for point-of-care diagnostics. Sci. Rep. 2016, 6, 22554. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, S.D.; Langton, S.R. Kinetic properties of Helicobacter pylori urease compared with jack bean urease. FEMS Microbiol. Lett. 1992, 99, 15–22. [Google Scholar] [CrossRef]
- Silva, G.O.; Mulato, M. Urea Detection Using Commercial Field Effect Transistors. ECS J. Solid State Sci. Technol. 2018, 7, Q3014–Q3019. [Google Scholar] [CrossRef]




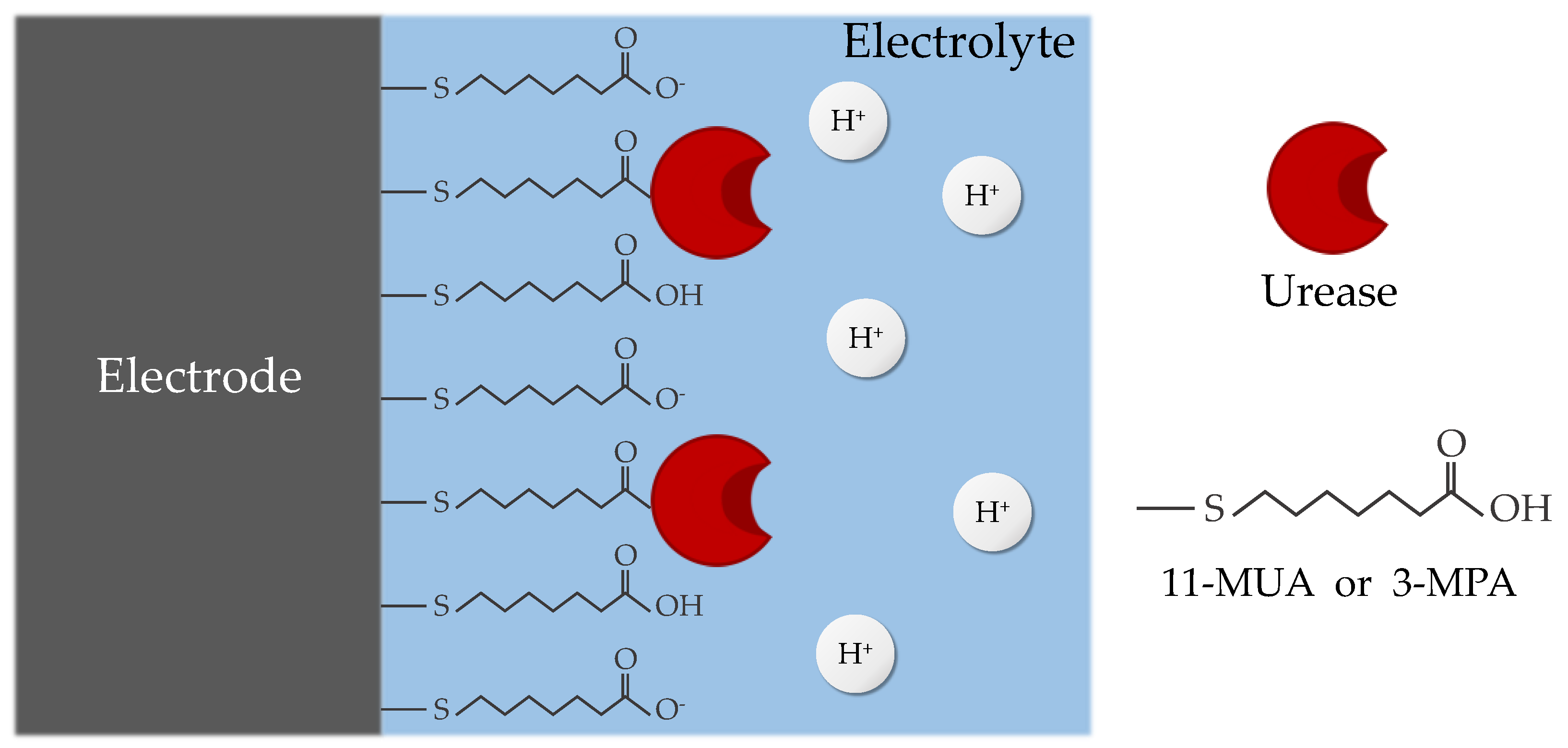
| Material | Functional Group | Length | pKa | |
|---|---|---|---|---|
| Binding with Gate | Binding with Receptor | |||
| APTES and GA [19,20,21,22] | Ethoxy group (OCH2CH3–) | Amine group (NH2–) | 1.5 nm | 10 |
| 11-MUA [23,24,25,26,27] | Thiol group (SH–) | Carboxyl group (COOH–) | 1.6 nm | 6.5 |
| 3-MPA [28,29] | Thiol group (SH–) | Carboxyl group (COOH–) | 0.6 nm | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Shin, S.; Do, J.; Son, J.; Kim, K.; Lee, J.-S. Influence of Surface Treatments on Urea Detection Using Si Electrolyte-Gated Transistors with Different Gate Electrodes. Micromachines 2024, 15, 621. https://doi.org/10.3390/mi15050621
Choi W, Shin S, Do J, Son J, Kim K, Lee J-S. Influence of Surface Treatments on Urea Detection Using Si Electrolyte-Gated Transistors with Different Gate Electrodes. Micromachines. 2024; 15(5):621. https://doi.org/10.3390/mi15050621
Chicago/Turabian StyleChoi, Wonyeong, Seonghwan Shin, Jeonghyeon Do, Jongmin Son, Kihyun Kim, and Jeong-Soo Lee. 2024. "Influence of Surface Treatments on Urea Detection Using Si Electrolyte-Gated Transistors with Different Gate Electrodes" Micromachines 15, no. 5: 621. https://doi.org/10.3390/mi15050621







