Recent Progress and Challenges of Implantable Biodegradable Biosensors
Abstract
:1. Introduction
2. Materials
2.1. Substrates and Insulators
2.1.1. Paper
2.1.2. Silk
2.1.3. Gelatin and Shellac
2.1.4. Polymers (Synthetic Polymers)
2.2. Active Layer
2.2.1. Inorganic Semiconductors
2.2.2. Organic Semiconductors
2.2.3. Integration of Transistors
2.3. Dielectrics
2.4. Electrodes and Interconnects
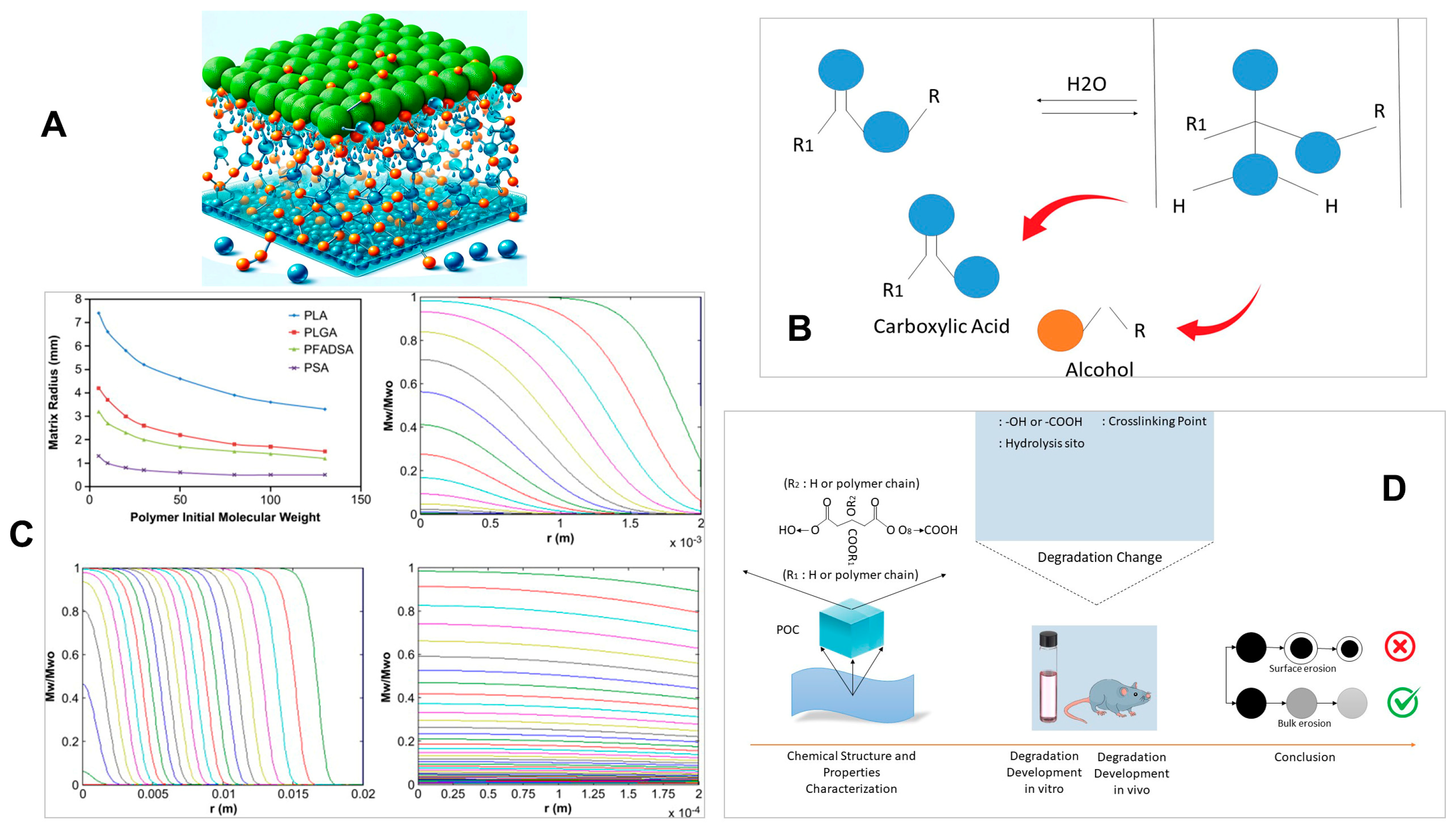
3. Biodegradation Mechanisms
3.1. Polymers
3.2. Silicon-Based Materials
3.3. Metals
4. Sensing Mechanisms of Biodegradable Biosensors
4.1. Resistive Sensors
4.2. Capacitive Sensors
4.3. Piezoelectric Sensors
4.4. Triboelectric Sensors
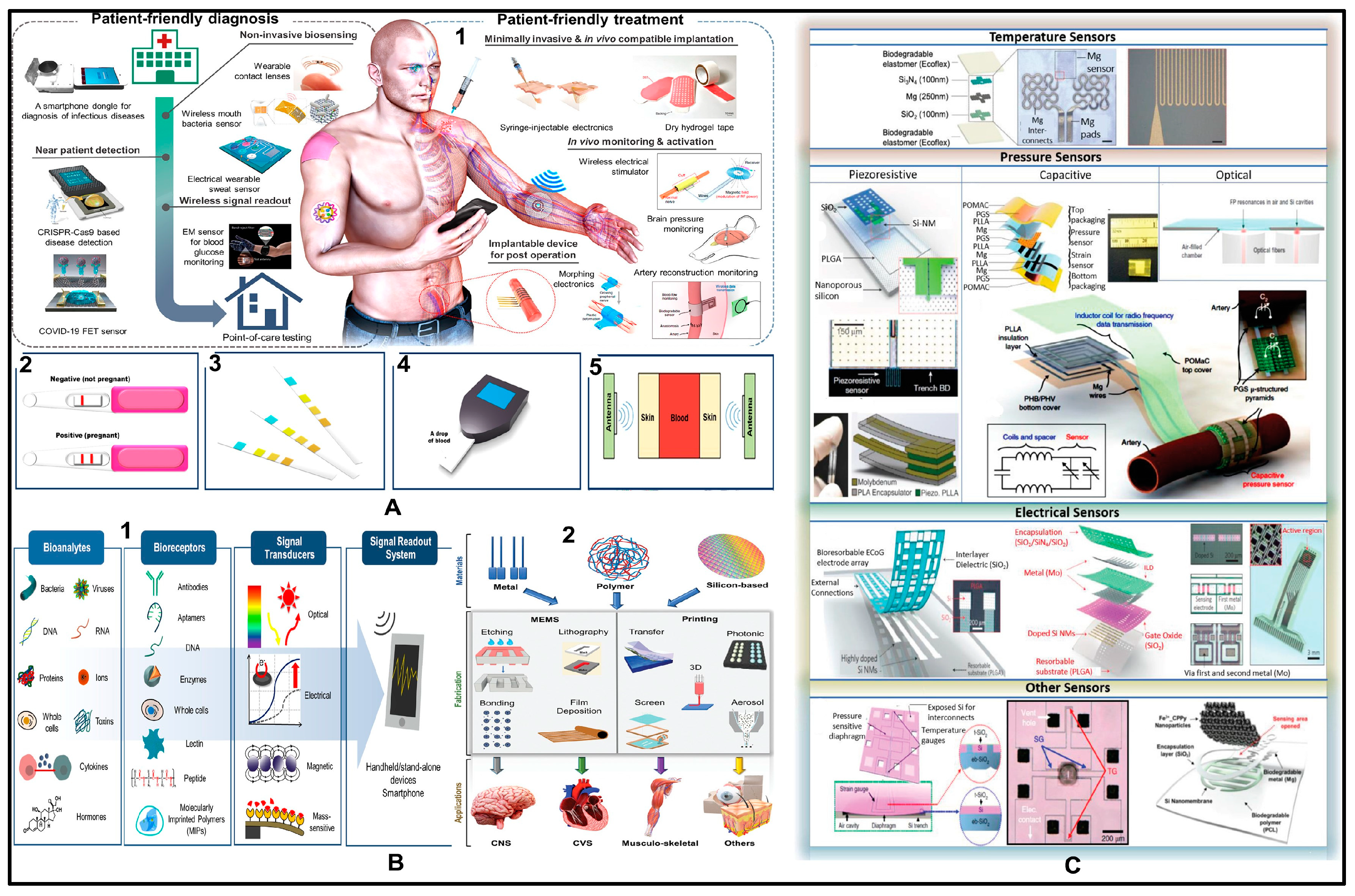
5. Applications of Implantable Biosensors
5.1. Biomarkers
5.1.1. Glucose
5.1.2. Lactate
5.1.3. Glutamate
5.2. Central Nervous System
5.3. Cardiovascular System
5.4. Musculoskeletal System
5.5. Force Sensing
5.6. Other Applications
6. Challenges and Future Directions
6.1. Power Supply
6.2. Data Communication
6.3. Materials
6.4. Fabrication
6.5. Implanting into Body
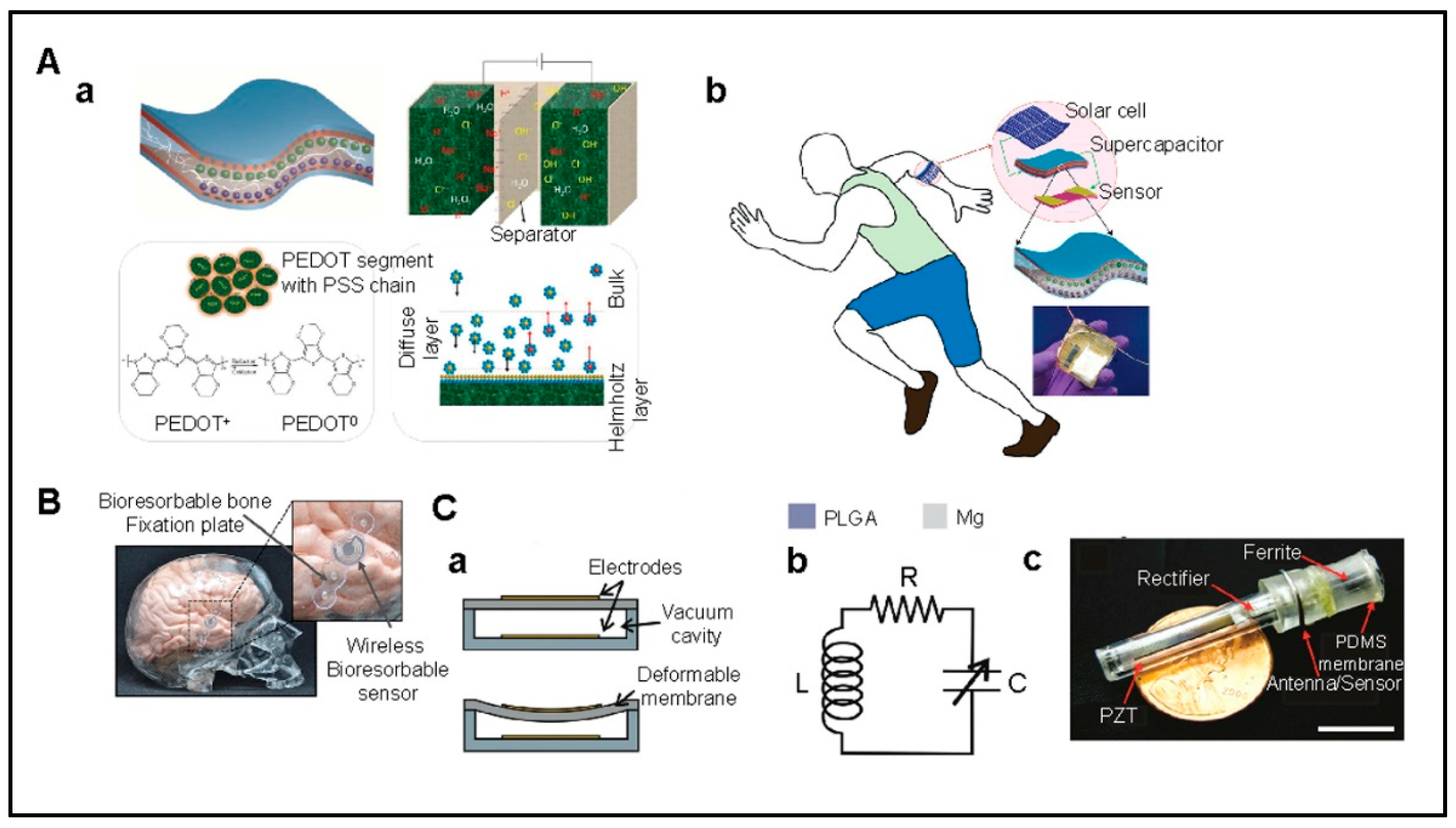
6.6. Long-Term Performance and Calibration
6.7. Integrated Design Considerations
6.8. Future Directions
6.8.1. Material Science Advances
6.8.2. Nanotechnology Integration
6.8.3. Wireless and Energy Harvesting Technologies
6.8.4. Personalized Medicine
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gough, D.A.; Armour, J.C. Development of the implantable glucose sensor. What are the prospects and why is it taking so long? Diabetes 1995, 44, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Rodger, D.C.; Saati, S.; Humayun, M.S.; Tai, Y.-C. Microfabricated Implantable Parylene-Based Wireless Passive Intraocular Pressure Sensors. J. Microelectromech. Syst. 2008, 17, 1342–1351. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Wazni, O.M.; Callahan, T.; Kanj, M.; Hakim, A.H.; Wolski, K.; Wilkoff, B.L.; Saliba, W.; Lindsay, B.D. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: The iTransmit study. Heart Rhythm. 2015, 12, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Huang, D.T.; Nielsen, J.C. The role of implantable cardioverter-defibrillators and sudden cardiac death prevention: Indications, device selection, and outcome. Eur. Heart J. 2019, 41, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Francis, J.; Maron, B.J. Role of implantable cardioverter defibrillators in the treatment of hypertrophic cardiomyopathy. Indian Pacing Electrophysiol. J. 2005, 5, 72–75. [Google Scholar] [PubMed]
- Kringelbach, M.L.; Jenkinson, N.; Owen, S.L.; Aziz, T.Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 2007, 8, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Arshak, A.; Arshak, K.; Waldron, D.; Morris, D.; Korostynska, O.; Jafer, E.; Lyons, G. Review of the potential of a wireless MEMS and TFT microsystems for the measurement of pressure in the GI tract. Med. Eng. Phys. 2005, 27, 347–356. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical Systems (MEMS) for Biomedical Applications. Micromachines 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, O. A brief history of cardiac pacing. Images Paediatr. Cardiol. 2006, 8, 17–81. [Google Scholar] [PubMed]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwödiauer, R.; Mumyatov, A.; Fergus, J.W.; et al. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Choi, Y.; Yin, R.; Pfenniger, A.; Arora, R.; Efimov, I.; A Rogers, J. Abstract 10305: A Bioresorbable Pacemaker with Transient Closed-Loop System. Circ. 2022, 146, A10305. [Google Scholar] [CrossRef]
- French, P. In-Vivo Microsystems: A Review. Sensors 2020, 20, 4953. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Joseph, L.L.; Kollem, S.; Vijumon, V.; Ajayan, J. Biodegradable sensors: A comprehensive review. Measurement 2023, 219, 113261. [Google Scholar] [CrossRef]
- Araromi, O.A.; Graule, M.A.; Dorsey, K.L.; Castellanos, S.; Foster, J.R.; Hsu, W.-H.; Passy, A.E.; Vlassak, J.J.; Weaver, J.C.; Walsh, C.J.; et al. Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature 2020, 587, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanizamani, F.; Moulahoum, H.; Celik, E.G.; Timur, S. Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Appl. Sci. 2023, 13, 4630. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, G.; Xu, J.; Han, M.; Wang, Z.; Li, T.; Long, M.; Wang, L.; Huang, W.; Wu, Y. Development of Poly(Glycerol Sebacate) and Its Derivatives: A Review of the Progress over the past Two Decades. Polym. Rev. 2022, 63, 613–678. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Coyle, S.; Lau, K.T.; Moyna, N.; O’Gorman, D.; Diamond, D.; Di Francesco, F.; Costanzo, D.; Salvo, P.; Trivella, M.G.; De Rossi, D.E.; et al. BIOTEX—Biosensing Textiles for Personalised Healthcare Management. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.N.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Mariello, M.; Kim, K.; Wu, K.; Lacour, S.P.; Leterrier, Y. Recent Advances in Encapsulation of Flexible Bioelectronic Implants: Materials, Technologies, and Characterization Methods. Adv. Mater. 2022, 34, e2201129. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Hernandez, A.L.; Unluturk, B.D.; Quintero, S.A.; de Barros, N.R.; Apu, E.H.; Bin Shams, A.; Ostrovidov, S.; Li, J.; Contag, C.; et al. Biodegradable Implantable Sensors: Materials Design, Fabrication, and Applications. Adv. Funct. Mater. 2021, 31, 2104149. [Google Scholar] [CrossRef]
- Eder, F.; Klauk, H.; Halik, M.; Zschieschang, U.; Schmid, G.; Dehm, C. Organic electronics on paper. Appl. Phys. Lett. 2004, 84, 2673–2675. [Google Scholar] [CrossRef]
- Russo, A.; Ahn, B.Y.; Adams, J.J.; Duoss, E.B.; Bernhard, J.T.; Lewis, J.A. Pen-on-Paper Flexible Electronics. Adv. Mater. 2011, 23, 3426–3430. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, V.; Bharti, D.; Mahato, A.K.; Varun, I.; Tiwari, S.P. Solution-Processed Organic Field-Effect Transistors with High Performance and Stability on Paper Substrates. ACS Appl. Mater. Interfaces 2019, 11, 8357–8364. [Google Scholar] [CrossRef] [PubMed]
- Zschieschang, U.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Klauk, H. Organic Electronics on Banknotes. Adv. Mater. 2010, 23, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.C.; Rowehl, J.A.; Lunt, R.R.; Xu, J.; Wang, A.; Boyce, C.M.; Im, S.G.; Bulović, V.; Gleason, K.K. Direct monolithic integration of organic photovoltaic circuits on unmodified paper. Adv. Mater. 2011, 23, 3500–3505. [Google Scholar] [CrossRef]
- Kim, D.Y.; Steckl, A.J. Electrowetting on Paper for Electronic Paper Display. ACS Appl. Mater. Interfaces 2010, 2, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Rolland, J.P.; Kumar, S.; Beattie, P.D.; Jain, S.; Noubary, F.; Wong, V.L.; Pohlmann, R.A.; Ryan, U.S.; Whitesides, G.M. A Paper-Based Multiplexed Transaminase Test for Low-Cost, Point-of-Care Liver Function Testing. Sci. Transl. Med. 2012, 4, 152ra129. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S.; Burns, M.A.; Johnson, B.N.; Brahmasandra, S.; Handique, K.; Webster, J.R.; Krishnan, M.; Sammarco, T.S.; et al. Electrochemical Detection for Paper-based Microfluidics We Report the First Demonstration of Electrochemical Detection for Paper-based Microfluidic Devices. Photoli- Thography Was Used to Make Microfluidic Channels on Filter Paper, and Screen-Printing Technology Was Used to Fabricate Electrodes on t. Semantic Scholar. 15 July 2009. Available online: https://www.semanticscholar.org/paper/Electrochemical-Detection-for-Paper-based-We-Report-Dungchai-Chailapakul/3e8bdfcb6d371fb90e91c938b7905a1c0c1ca8d1 (accessed on 1 March 2024).
- Fang, X.; Zhao, Q.; Cao, H.; Liu, J.; Guan, M.; Kong, J. Rapid detection of Cu2+ by a paper-based microfluidic device coated with bovine serum albumin (BSA)–Au nanoclusters. Anal. 2015, 140, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M. “Green” electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2013, 43, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, X.M.; Yu, X.; Cui, F.Z. Preparation of Hydroxyapatite-Fibroin Nanocomposites. Key Eng. Mater. 2005, 288–289, 191–194. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Cronin-Golomb, M.; Georgakoudi, I.; Kaplan, D.L.; Omenetto, F.G. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules 2008, 9, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.; Gopinath, A.; Kaplan, D.L.; Dal Negro, L.; Omenetto, F.G. Nano- and Micropatterning of Optically Transparent, Mechanically Robust, Biocompatible Silk Fibroin Films. Adv. Mater. 2008, 20, 3070–3072. [Google Scholar] [CrossRef]
- Murphy, A.R.; John, P.S.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Toffanin, S.; Capelli, R.; Camassa, L.M.; Ferroni, S.; Kaplan, D.L.; Omenetto, F.G.; Muccini, M.; Zamboni, R. A silk platform that enables electrophysiology and targeted drug delivery in brain astroglial cells. Biomaterials 2010, 31, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Sarma, M.G.; Borthakur, B.B.; Bora, U. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015, 62, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.L.; Lee, A.; Zalka, A. Commonly used suture materials in skin surgery. Am. Fam. Physician 1991, 44, 2123–2128. [Google Scholar] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Hwang, S.-W.; Marelli, B.; An, B.; Moreau, J.E.; Yang, M.; Brenckle, M.A.; Kim, S.; Kaplan, D.L.; Rogers, J.A.; et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 2014, 111, 17385–17389. [Google Scholar] [CrossRef]
- Amsden, J.J.; Perry, H.; Boriskina, S.V.; Gopinath, A.; Kaplan, D.L.; Negro, L.D.; Omenetto, F.G. Spectral analysis of induced color change on periodically nanopatterned silk films. Opt. Express 2009, 17, 21271–21279. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki-Modaress, M.; Mirzadeh, H.; Zandi, M.; Rajabi-Zeleti, S.; Sodeifi, N.; Aghdami, N.; Mofrad, M.R.K. Gelatin/chondroitin sulfate nanofibrous scaffolds for stimulation of wound healing: In-vitro and in-vivo study. J. Biomed. Mater. Res. Part A 2017, 105, 2020–2034. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Remijn, C.; Cabero, A.M.; Heussen, P.C.; Hoorn, J.W.S.T.; Velikov, K.P. Novel All-Natural Microcapsules from Gelatin and Shellac for Biorelated Applications. Adv. Funct. Mater. 2013, 23, 4710–4718. [Google Scholar] [CrossRef]
- Macdonald, A.R.; Charlton, F.; Corrigan, D.K. Accelerating the development of implantable neurochemical biosensors by using existing clinically applied depth electrodes. Anal. Bioanal. Chem. 2022, 415, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Burugapalli, K.; Wijesuriya, S.; Far, M.Y.; Song, W.; Moussy, F.; Zheng, Y.; Ma, Y.; Wu, Z.; Li, K. Electrospun polyurethane-core and gelatin-shell coaxial fibre coatings for miniature implantable biosensors. Biofabrication 2013, 6, 015002. [Google Scholar] [CrossRef] [PubMed]
- Neupane, M.P.; Park, I.S.; Bae, T.S.; Yi, H.K.; Uo, M.; Watari, F.; Lee, M.H. Titania nanotubes supported gelatin stabilized gold nanoparticles for medical implants. J. Mater. Chem. 2011, 21, 12078–12082. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Głowacki, E.D.; Schwabegger, G.; Leonat, L.; Akpinar, H.Z.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Natural resin shellac as a substrate and a dielectric layer for organic field-effect transistors. Green Chem. 2013, 15, 1473–1476. [Google Scholar] [CrossRef]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an implantable material in medicine and dentistry. J. Oral Implant. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Ramshaw, J.A.; Werkmeister, J.A.; Glattauer, V. Collagen-based Biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Chew, D.J.; Minev, I.R.; Fawcett, J.W.; Lacour, S.P. Concurrent recordings of bladder afferents from multiple nerves using a microfabricated PDMS microchannel electrode array. Lab Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Ahn, J.-H.; Choi, W.M.; Kim, H.-S.; Kim, T.-H.; Song, J.; Huang, Y.Y.; Liu, Z.; Lu, C.; Rogers, J.A. Stretchable and Foldable Silicon Integrated Circuits. Nature 2008, 320, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.H.; Alam, F.; Ahmed, A.; Ahad, M. Precise calibration of optical fiber sensor for ammonia sensing using multivariate analysis. In SPIE Commercial + Scientific Sensing and Imaging; SPIE: Orlando, FL, USA, 2018. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Ren, M.; Ding, C.; Chen, P.; Gu, Q.; Wu, Q. Evaluation of PHBHHx and PHBV/PLA fibers used as medical sutures. J. Mater. Sci. Mater. Med. 2013, 25, 561–571. [Google Scholar] [CrossRef]
- Bao, W.; Zhou, J.; Luo, J.; Wu, D. PLGA microspheres with high drug loading and high encapsulation efficiency prepared by a novel solvent evaporation technique. J. Microencapsul. 2006, 23, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Baek, S.-K.; Park, J.-H.; Betz, A.R.; Choi, S.-O. Fabrication of Circular Obelisk-Type Multilayer Microneedles Using Micro-Milling and Spray Deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Cyr, K.M.; Matsumoto, A.; Langer, R.; Borenstein, J.T.; Kaplan, D.L. Silk Fibroin Microfluidic Devices. Adv. Mater. 2007, 19, 2847–2850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Papadimitrakopoulos, F.; Burgess, D.J. A Review of the Development of a Vehicle for Localized and Controlled Drug Delivery for Implantable Biosensors. J. Diabetes Sci. Technol. 2008, 2, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Mirji, N.; Mishra, S.; Maji, D. PDMS micromachining for flexible 3D microdevice realization using Kapton Tape as hard mask. In Proceedings of the TENCON 2019—2019 IEEE Region 10 Conference (TENCON), Kerala, India, 17–20 October 2019; pp. 57–61. [Google Scholar]
- Terry, S.; Eckerle, J.; Kornbluh, R.; Low, T.; Ablow, C. Silicon pressure transducer arrays for blood-pressure measurement. Sensors Actuators A Phys. 1990, 23, 1070–1079. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Park, G.; Edwards, C.; Corbin, E.A.; Kang, S.-K.; Cheng, H.; Song, J.-K.; Kim, J.-H.; Yu, S.; Ng, J.; et al. Dissolution Chemistry and Biocompatibility of Single-Crystalline Silicon Nanomembranes and Associated Materials for Transient Electronics. ACS Nano 2014, 8, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Goosen, J.; French, P.; Sarro, P. Pressure, flow and oxygen saturation sensors on one chip for use in catheters. In Proceedings of the IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems (Cat. No.00CH36308), Munich, Germany, 23–27 January 2000; pp. 537–540. [Google Scholar]
- Heimann, R.B. Silicon Nitride, a Close to Ideal Ceramic Material for Medical Application. Ceramics 2021, 4, 208–223. [Google Scholar] [CrossRef]
- Alam, F.; Jalal, A.H.; Forouzanfar, S.; Karabiyik, M.; Baboukani, A.R.; Pala, N. Flexible and Linker-Free Enzymatic Sensors Based on Zinc Oxide Nanoflakes for Noninvasive L-Lactate Sensing in Sweat. IEEE Sens. J. 2020, 20, 5102–5109. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Saliba, W.; Khatib, M.; Zheng, Y.; Pieters, C.; Oved, H.; Silberman, E.; Zohar, O.; Hu, Z.; Kloper, V.; et al. Biodegradable, Biocompatible, and Implantable Multifunctional Sensing Platform for Cardiac Monitoring. ACS Sens. 2024, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hoseyni, S.M.; Das, R.; Awais, M.; Basdogan, I.; Beker, L. A Flexible and Biodegradable Piezoelectric-Based Wearable Sensor for Non-Invasive Monitoring of Dynamic Human Motions and Physiological Signals. Adv. Mater. Technol. 2023, 8, 2300347. [Google Scholar] [CrossRef]
- Kim, J.J.; Stafford, G.R.; Beauchamp, C.; Kim, S.A. Development of a Dental Implantable Temperature Sensor for Real-Time Diagnosis of Infectious Disease. Sensors 2020, 20, 3953. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Niroui, F.; Leung, K.T. High-Performance, Flexible Enzymatic Glucose Biosensor Based on ZnO Nanowires Supported on a Gold-Coated Polyester Substrate. ACS Appl. Mater. Interfaces 2010, 2, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.H.; Alam, F.; Roychoudhury, S.; Umasankar, Y.; Pala, N.; Bhansali, S. Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Rivnay, J. Transistors in Bioelectronics, in Introduction to Bioelectronics: Materials, Devices, and Applications; Stavrinidou, E., Proctor, C.M., Eds.; AIP Publishing LLC: Melville, NY, USA, 2022. [Google Scholar]
- Alireza, Z.; Maghami, M.H.; Farzad, A.; Amir, M.S. Implantable Biomedical Devices. In Biomedical Engineering; Radovan, H., Marek, P., Jaroslav, M., Eds.; IntechOpen: Rijeka, Croatia, 2012; Chapter 7. [Google Scholar]
- Cheng, L.; Hao, X.; Liu, G.; Zhang, W.; Cui, J.; Zhang, G.; Yang, Y.; Wang, R. A Flexible Pressure Sensor Based on Silicon Nanomembrane. Biosensors 2023, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ren, X.; Wang, Z.; Wang, X.; Roberts, R.C.; Chan, P.K.L. High performance organic transistor active-matrix driver developed on paper substrate. Sci. Rep. 2014, 4, 6430. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, G.; Liu, Y. Functional Organic Field-Effect Transistors. Adv. Mater. 2010, 22, 4427–4447. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Guo, Y.; Liu, Y. When Flexible Organic Field-Effect Transistors Meet Biomimetics: A Prospective View of the Internet of Things. Adv. Mater. 2019, 32, e1901493. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.D.; Loo, Y.-L. Engineering the organic semiconductor-electrode interface in polymer solar cells. J. Mater. Chem. 2010, 20, 6604–6611. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, L.; Chi, L. Organic Semiconductor Field-Effect Transistors Based on Organic-2D Heterostructures. Front. Mater. 2020, 7, 295. [Google Scholar] [CrossRef]
- Wei, S.; Yifan, Z.; Junsheng, Y. Polymer Dielectric in Organic Field-Effect Transistor. In Polymer Dielectrics; Boxue, D., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 1. [Google Scholar]
- Kwon, Y.-W.; Lee, C.H.; Choi, D.-H.; Jin, J.-I. Materials science of DNA. J. Mater. Chem. 2008, 19, 1353–1380. [Google Scholar] [CrossRef]
- Chang, J.; Wang, C.; Huang, C.; Tsai, T.; Guo, T.; Wen, T. Chicken Albumen Dielectrics in Organic Field-Effect Transistors. Adv. Mater. 2011, 23, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.H.; Nguyen, T.T.; Giang, K.M.; Do, X.T.; Nguyen, T.T.; Hoang, H.C.; Ta, V.D. Chicken albumen-based whispering gallery mode microlasers. Soft Matter 2020, 16, 9069–9073. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, J.; Wang, W.; Xuan, W.; Wang, X.; Zhang, Q.; Smith, C.G.; Luo, J. Transient Resistive Switching Devices Made from Egg Albumen Dielectrics and Dissolvable Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10954–10960. [Google Scholar] [CrossRef] [PubMed]
- Petritz, A.; Wolfberger, A.; Fian, A.; Griesser, T.; Irimia-Vladu, M.; Stadlober, B. Cellulose-Derivative-Based Gate Dielectric for High-Performance Organic Complementary Inverters. Adv. Mater. 2015, 27, 7645–7656. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, Y.; Teng, J.; Wang, J.; Nee, T. Preparation and characterization of pentacene-based organic thin-film transistors with PVA passivation layers. Thin Solid Films 2009, 517, 5318–5321. [Google Scholar] [CrossRef]
- MacDiarmid, A.; Okuzaki, H.; Lu, J.; Manohar, S.; Bashari, E.; Temple, D.; Pinto, N.; Epstein, A. Dependency of Conductivity of Selected Doped Conducting Polymers on Unusual. Through Space. Researchgate. 1 March 2002. Available online: https://www.researchgate.net/publication/252297426_Dependency_of_Conductivity_of_Selected_Doped_Conducting_Polymers_on_Unusual_Through_Space (accessed on 1 March 2024).
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Herve, T.; Sanaur, S.; Bernard, C.; Malliaras, G.G. Highly Conformable Conducting Polymer Electrodes for In Vivo Recordings. Adv. Mater. 2011, 23, H268–H272. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B. Melanin, the What, the Why and the How: An Introductory Review for Materials Scientists Interested in Flexible and Versatile Polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Vahidzadeh, E.; Kalra, A.P.; Shankar, K. Melanin-based electronics: From proton conductors to photovoltaics and beyond. Biosens. Bioelectron. 2018, 122, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lin, W.; Li, Z.; Zhang, R.; Wen, H.; Gao, B.; Chen, G.; Gao, P.; Yuen, M.M.F.; Wong, C.P.; et al. Water-Based Isotropically Conductive Adhesives: Towards Green and Low-Cost Flexible Electronics. Adv. Funct. Mater. 2011, 21, 4582–4588. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Lu, L. Wearable and Biodegradable Sensors for Human Health Monitoring. ACS Appl. Bio Mater. 2020, 4, 122–139. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.N.; Federspiel, W.J.; Little, S.R. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials 2009, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Peltoniemi, H.; Waris, E.; Suuronen, R.; Serlo, W.; Kellomäki, M.; Törmälä, P.; Waris, T. Developments in Craniomaxillofacial Surgery: Use of Self-Reinforced Bioabsorbable Osteofixation Devices. Plast. Reconstr. Surg. 2001, 108, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, J.; Soini, Y.M.; Törmälä, P.; Waris, T.J.; Ashammakhi, N. Self-reinforced polylactide/polyglycolide 80/20 screws take more than 1(1/2) years to resorb in rabbit cranial bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Schädlich, A.; Naolou, T.; Amado, E.; Schöps, R.; Kressler, J.; Mäder, K. Noninvasive in Vivo Monitoring of the Biofate of 195 kDa Poly(vinyl alcohol) by Multispectral Fluorescence Imaging. Biomacromolecules 2011, 12, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Darabi, M.A.; Khosrozadeh, A.; Wang, Y.; Ashammakhi, N.; Alem, H.; Erdem, A.; Chang, Q.; Xu, K.; Liu, Y.; Luo, G.; et al. An Alkaline Based Method for Generating Crystalline, Strong, and Shape Memory Polyvinyl Alcohol Biomaterials. Adv. Sci. 2020, 7, 1902740. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2003, 66A, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kunze, C.; Bernd, H.E.; Androsch, R.; Nischan, C.; Freier, T.; Kramer, S.; Kramp, B.; Schmitz, K.-P. In vitro and in vivo studies on blends of isotactic and atactic poly (3-hydroxybutyrate) for development of a dura substitute material. Biomaterials 2006, 27, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Ahadian, S.; Huyer, L.D.; Rito, M.L.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.J.; Kuzum, D.; Hwang, S.-W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yan, Y.; Bai, W.; Xue, Y.; Gamble, P.; Tian, L.; Kandela, I.; Haney, C.R.; Spees, W.; Lee, Y.; et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng. 2018, 3, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.-W.; Jain, H.; Kang, S.-K.; Su, Y.; et al. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Redlich, C.; Quadbeck, P.; Thieme, M.; Kieback, B. Molybdenum—A biodegradable implant material for structural applications? Acta Biomater. 2020, 104, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.J.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef]
- Luo, M.; Martinez, A.W.; Song, C.; Herrault, F.; Allen, M.G. A Microfabricated Wireless RF Pressure Sensor Made Completely of Biodegradable Materials. J. Microelectromech. Syst. 2013, 23, 4–13. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.; Hamzah, E.; Low, H.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C 2017, 73, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, X.; Chen, S.; Uzun, S.; Levitt, A.S.; Shuck, C.E.; Han, W.; Gogotsi, Y. Hydrophobic and Stable MXene–Polymer Pressure Sensors for Wearable Electronics. ACS Appl. Mater. Interfaces 2020, 12, 15362–15369. [Google Scholar] [CrossRef]
- Dahiya, A.S.; Thireau, J.; Boudaden, J.; Lal, S.; Gulzar, U.; Zhang, Y.; Gil, T.; Azemard, N.; Ramm, P.; Kiessling, T.; et al. Review—Energy Autonomous Wearable Sensors for Smart Healthcare: A Review. J. Electrochem. Soc. 2019, 167, 037516. [Google Scholar] [CrossRef]
- Stampfer, C.; Jungen, A.; Linderman, R.; Obergfell, D.; Roth, S.; Hierold, C. Nano-electromechanical displacement sensing based on single-walled carbon nanotubes. Nano Lett. 2006, 6, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lee, S.; Xue, Y.; Yan, Y.; Liu, T.; Kang, S.; Lee, Y.J.; Lee, S.H.; Seo, M.; Lu, D.; et al. Materials, Mechanics Designs, and Bioresorbable Multisensor Platforms for Pressure Monitoring in the Intracranial Space. Adv. Funct. Mater. 2020, 30, 1910718. [Google Scholar] [CrossRef]
- Cui, X.; Chen, J.; Zhu, Y.; Jiang, W. Lightweight and conductive carbon black/chlorinated poly(propylene carbonate) foams with a remarkable negative temperature coefficient effect of resistance for temperature sensor applications. J. Mater. Chem. C 2018, 6, 9354–9362. [Google Scholar] [CrossRef]
- Salvatore, G.A.; Sülzle, J.; Valle, F.D.; Cantarella, G.; Robotti, F.; Jokic, P.; Knobelspies, S.; Daus, A.; Büthe, L.; Petti, L.; et al. Biodegradable and Highly Deformable Temperature Sensors for the Internet of Things. Adv. Funct. Mater. 2017, 27, 1702390. [Google Scholar] [CrossRef]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Mirzajani, H.; Urey, H. IDE-Integrated Microneedle Arrays as Fully Biodegradable Platforms for Wearable/Implantable Capacitive Biosensing. IEEE Sens. Lett. 2023, 8, 1–4. [Google Scholar] [CrossRef]
- Park, K.; Son, J.H.; Hwang, G.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Signore, M.A.; Rescio, G.; De Pascali, C.; Iacovacci, V.; Dario, P.; Leone, A.; Quaranta, F.; Taurino, A.; Siciliano, P.; Francioso, L. Fabrication and characterization of AlN-based flexible piezoelectric pressure sensor integrated into an implantable artificial pancreas. Sci. Rep. 2019, 9, 17130. [Google Scholar] [CrossRef] [PubMed]
- Abu Ali, T.; Pilz, J.; Schäffner, P.; Kratzer, M.; Teichert, C.; Stadlober, B.; Coclite, A.M. Piezoelectric Properties of Zinc Oxide Thin Films Grown by Plasma-Enhanced Atomic Layer Deposition. Phys. Status Solidi (A) 2020, 217, 2000319. [Google Scholar] [CrossRef]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A., Jr.; Rödel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Li, J. Microstructure and Piezoelectric Properties of Lead Zirconate Titanate Nanocomposites Reinforced with In-Situ Formed ZrO2 Nanoparticles. Materials 2022, 15, 1389. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Kong, L.; Song, W.; Jiang, C.; Tian, S.; Yu, F.; Qin, L.; Wang, C.; Zhao, X. The electromechanical features of LiNbO3 crystal for potential high temperature piezoelectric applications. J. Mater. 2019, 5, 73–80. [Google Scholar] [CrossRef]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric Biomaterials for Sensors and Actuators. Adv. Mater. 2018, 31, e1802084. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Pan, H.; Tian, G.; Zhang, B.; Xiong, D.; Gao, Y.; Yan, C.; Chu, X.; Chen, N.; Zhong, S.; et al. Hierarchically structured PVDF/ZnO core-shell nanofibers for self-powered physiological monitoring electronics. Nano Energy 2020, 72, 104706. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, E.; Cui, X.; Xi, Y.; Ji, J.; Yuan, J.; Xu, L.; Liu, Z.; Li, Z. A Biodegradable Piezoelectric Sensor for Real-Time Evaluation of the Motor Function Recovery after Nerve Injury. Adv. Funct. Mater. 2024, 2400295. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine–Chitosan-Based Flexible Biodegradable Piezoelectric Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 9008–9016. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Peng, X.; Wang, Z.L. Fiber/Fabric-Based Piezoelectric and Triboelectric Nanogenerators for Flexible/Stretchable and Wearable Electronics and Artificial Intelligence. Adv. Mater. 2019, 32, e1902549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qin, B.; Fang, C.; Liu, L.; Poechmueller, P.; Yang, X. Serpentine liquid electrode based Dual-mode skin Sensors: Monitoring biomechanical movements by resistive or triboelectric mode. Chem. Eng. J. 2023, 479, 147898. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, e1501478. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, Z.; Li, J.; Guo, J. Textile-based triboelectric nanogenerators with high-performance via optimized functional elastomer composited tribomaterials as wearable power source. Nano Energy 2019, 65, 104012. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, J.; Lee, J.; Kim, K.; Park, I. Ultrathin, Biocompatible, and Flexible Pressure Sensor with a Wide Pressure Range and Its Biomedical Application. ACS Sens. 2020, 5, 481–489. [Google Scholar] [CrossRef]
- Hijazi, M. Sensitive and Selective Ammonia Gas Sensor Based on Molecularly Functionalized Tin Dioxide Working at Room Temperature, Capteur de gaz SnO2 Fonctionnalisé Fonctionnant à Température Ambiante, Sensible et Sélectif Pour la Détection D’ammoniac, Université de Lyon, 2017LYSEM030. 2017. Available online: https://theses.hal.science/tel-01848722 (accessed on 25 August 2017).
- Ksendzov, A.; Lin, Y. Integrated optics ring-resonator sensors for protein detection. Opt. Lett. 2005, 30, 3344–3346. [Google Scholar] [CrossRef] [PubMed]
- Palwai, S.; Batra, A.; Kotru, S.; Vaseashta, A. Electrospun Polyvinylidene Fluoride Nanofiber Membrane-Based Flexible Capacitive Tactile Sensors for Biomedical Applications. Surf. Eng. Appl. Electrochem. 2022, 58, 194–201. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2013, 6, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F.; et al. Super-stretchable, Transparent Carbon Nanotube-Based Capacitive Strain Sensors for Human Motion Detection. Sci. Rep. 2013, 3, 3048. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef]
- Wang, J.; Jiu, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; He, P.; Suganuma, K. A highly sensitive and flexible pressure sensor with electrodes and elastomeric interlayer containing silver nanowires. Nanoscale 2014, 7, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, L.; Wu, T.; Song, H.; Tang, C. Flexible piezoelectric pressure sensor with high sensitivity for electronic skin using near-field electrohydrodynamic direct-writing method. Extreme Mech. Lett. 2021, 48, 101279. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Yoo, Y.K.; Kang, S.; Kim, J.; Lee, J.H. Enhanced sensitivity of piezoelectric pressure sensor with microstructured polydimethylsiloxane layer. Appl. Phys. Lett. 2014, 104, 123701. [Google Scholar] [CrossRef]
- Rajala, S.; Siponkoski, T.; Sarlin, E.; Mettänen, M.; Vuoriluoto, M.; Pammo, A.; Juuti, J.; Rojas, O.J.; Franssila, S.; Tuukkanen, S. Cellulose Nanofibril Film as a Piezoelectric Sensor Material. ACS Appl. Mater. Interfaces 2016, 8, 15607–15614. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.S.; Yoon, H.; Kim, S. Textile-Based Triboelectric Nanogenerators for Self-Powered Wearable Electronics. Adv. Funct. Mater. 2018, 29, 1804533. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, J.; Yeh, M.-H.; Guo, H.; Li, Z.; Fan, X.; Zhang, T.; Zhu, L.; Wang, Z.L. Blow-driven triboelectric nanogenerator as an active alcohol breath analyzer. Nano Energy 2015, 16, 38–46. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lee, P.-W.; Tsao, Y.-H.; Lin, Z.-H. Utilization of self-powered electrochemical systems: Metallic nanoparticle synthesis and lactate detection. Nano Energy 2017, 42, 241–248. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Hou, T.-C.; Su, Y.; Hu, C.; Wang, Z.L. Triboelectric nanogenerator built inside clothes for self-powered glucose biosensors. Nano Energy 2013, 2, 1019–1024. [Google Scholar] [CrossRef]
- Abel, P.; von Woedtke, T. Biosensors for in vivo glucose measurement: Can we cross the experimental stage. Biosens. Bioelectron. 2002, 17, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.H.; Umasankar, Y.; Ahmed, A.; Pretto, E.A.; Bhansali, S. Towards a wearable fuel cell sensor for transdermal monitoring of isoflurane—An anesthetic. Anal. Methods 2019, 11, 2007–2012. [Google Scholar] [CrossRef]
- Clark, L.C.; Spokane, R.B.; Homan, M.M.; Sudan, R.; Miller, M. Long-term stability of electroenzymatic glucose sensors implanted in mice. An update. ASAIO Trans. 1988, 34, 259–265. [Google Scholar] [PubMed]
- Vaddiraju, S.; Kastellorizios, M.; Legassey, A.; Burgess, D.; Jain, F.; Papadimitrakopoulos, F. Needle-implantable, wireless biosensor for continuous glucose monitoring. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015; pp. 1–5. [Google Scholar]
- Yu, Y.; Nguyen, T.; Tathireddy, P.; Young, D.J.; Roundy, S. Wireless hydrogel-based glucose sensor for future implantable applications. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Heligon, S.G.; Brown, N.L.; Reichert, W.M.; Klitzman, B. Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo. Acta Biomater. 2016, 30, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, N.P.; Ganesana, M.; Karimi, A.; Leiter, J.C.; Andreescu, S. Platinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxia. Anal. Chem. 2015, 87, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Gough, D.A. A Continuous, Implantable Lactate Sensor. Anal. Chem. 1995, 67, 1536–1540. [Google Scholar] [CrossRef]
- Rahman, A.R.A.; Justin, G.; Guiseppi-Elie, A. Towards an implantable biochip for glucose and lactate monitoring using microdisc electrode arrays (MDEAs). Biomed. Microdevices 2008, 11, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- McLamore, E.; Mohanty, S.; Shi, J.; Claussen, J.; Jedlicka, S.; Rickus, J.; Porterfield, D. A self-referencing glutamate biosensor for measuring real time neuronal glutamate flux. J. Neurosci. Methods 2010, 189, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Leviton, A.; Paneth, N.; Reuss, M.L.; Susser, M.; Allred, E.N.; Dammann, O.; Kuban, K.; van Marter, L.J.; Pagano, M.; Hegyi, T.; et al. Maternal Infection, Fetal Inflammatory Response, and Brain Damage in Very Low Birth Weight Infants. Pediatr. Res. 1999, 46, 566. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Kimberly, W.T. Today’s Approach to Treating Brain Swelling in the Neuro Intensive Care Unit. Semin. Neurol. 2016, 36, 502–507. [Google Scholar] [CrossRef]
- Rangel-Castillo, L.; Gopinath, S.; Robertson, C.S. Management of Intracranial Hypertension. Neurol. Clin. 2008, 26, 521–541. [Google Scholar] [CrossRef]
- Zhang, X.; Medow, J.E.; Iskandar, B.J.; Wang, F.; Shokoueinejad, M.; Koueik, J.; Webster, J.G. Invasive and noninvasive means of measuring intracranial pressure: A review. Physiol. Meas. 2017, 38, R143–R182. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.L.H.; Czosnyka, M.; Jalloh, I.; Newcombe, V.F.J.; Helmy, A.; Shannon, R.J.; Budohoski, K.P.; Kolias, A.G.; Kirkpatrick, P.J.; Carpenter, T.A.; et al. Acknowledgments. J. Neurotrauma 2007, 24, S1-106. [Google Scholar] [CrossRef]
- Tharin, S.; Golby, A. Functional brain mapping and its applications to neurosurgery. Oper. Neurosurg. 2007, 60. Available online: https://journals.lww.com/onsonline/fulltext/2007/04001/functional_brain_mapping_and_its_applications_to.1.aspx (accessed on 30 April 2007). [CrossRef] [PubMed]
- Kim, H.; Yang, S.M.; Jang, T.; Oh, N.; Kim, H.; Hwang, S. Bioresorbable Silicon Nanomembranes and Iron Catalyst Nanoparticles for Flexible, Transient Electrochemical Dopamine Monitors. Adv. Health Mater. 2018, 7, e1801071. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and dopamine receptors in Alzheimer’s disease: A systematic review and network meta-analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A Sensitive and Biodegradable Pressure Sensor Array for Cardiovascular Monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simões, J.A. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: A review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
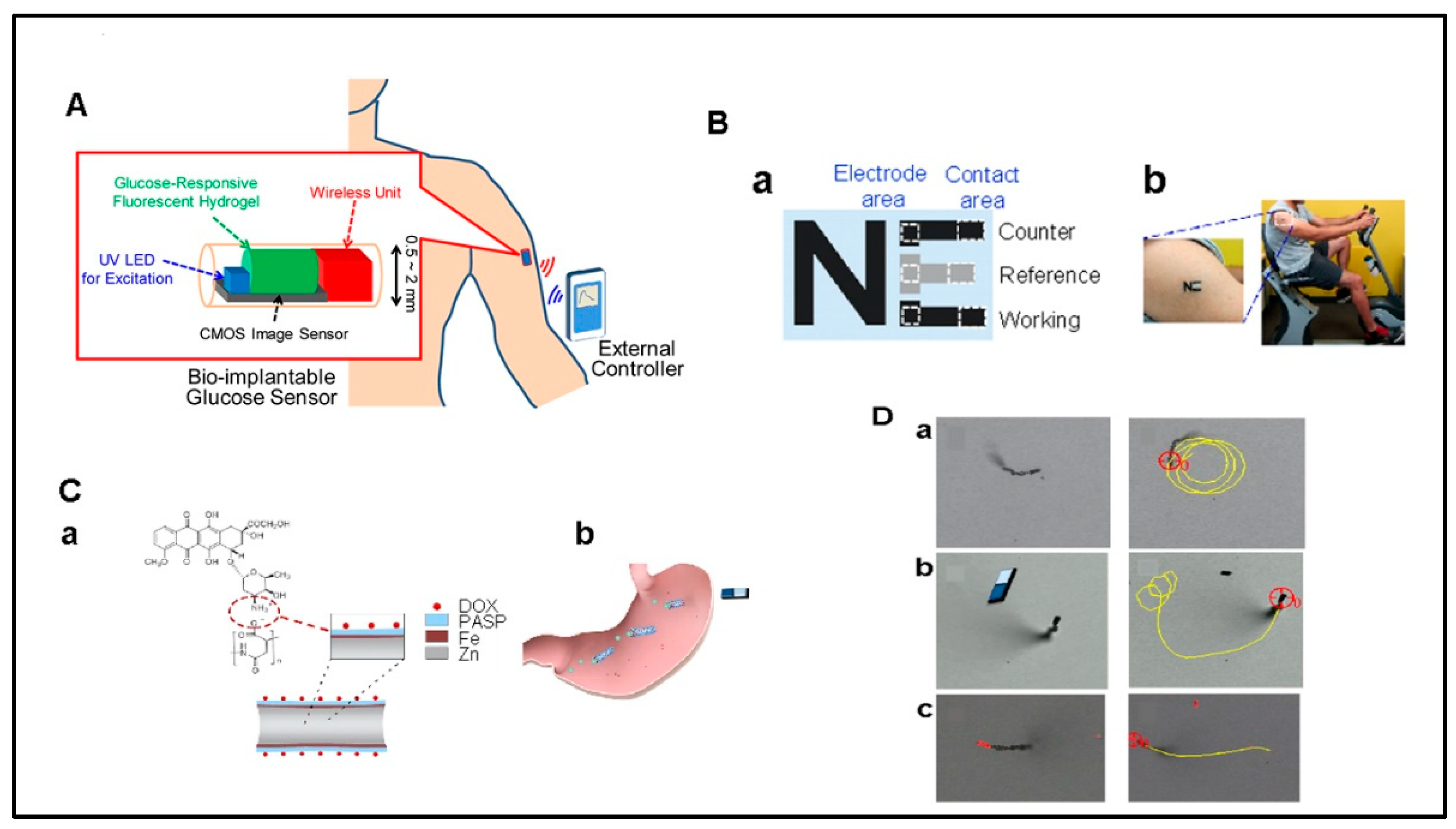
- Tokuda, T.; Takahashi, M.; Uejima, K.; Masuda, K.; Kawamura, T.; Ohta, Y.; Motoyama, M.; Noda, T.; Sasagawa, K.; Okitsu, T.; et al. CMOS image sensor-based implantable glucose sensor using glucose-responsive fluorescent hydrogel. Biomed. Opt. Express 2014, 5, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, T.; Li, J.; Yu, S.; Xu, Z.; Yin, M.; Wang, J.; Wang, X. Self-Propelled and Targeted Drug Delivery of Poly(aspartic acid)/Iron–Zinc Microrocket in the Stomach. ACS Nano 2019, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Zaszczyńska, A.; Gradys, A.; Sajkiewicz, P. Progress in the Applications of Smart Piezoelectric Materials for Medical Devices. Polymers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Zhang, Z.; Zhang, X.; Mohr, M.; Zhang, Y.; Fecht, H.-J. Flexible piezoelectric nanogenerators based on a fiber/ZnO nanowires/paper hybrid structure for energy harvesting. Nano Res. 2014, 7, 917–928. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Da, P.; Xu, M.; Wu, H.; Zheng, G. Silicon nanowires for biosensing, energy storage, and conversion. Adv. Mater. 2013, 25, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Dianatdar, A.; Gładysz, M.Z.; Hemmatpour, H.; Hendriksen, M.; Rudolf, P.; Włodarczyk-Biegun, M.K.; Kamperman, M.; Kottapalli, A.G.P.; Bose, R.K. Electrically Conductive and Highly Stretchable Piezoresistive Polymer Nanocomposites via Oxidative Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2023, 15, 31899–31916. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, L.; Wang, X. Flexible and wearable healthcare sensors for visual reality health-monitoring. Virtual Real. Intell. Hardw. 2019, 1, 411–427. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.-K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixao, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Venugopal, S.; Jung, R. Engaging biological oscillators through second messenger pathways permits emergence of a robust gastric slow-wave during peristalsis. PLoS Comput. Biol. 2021, 17, e1009644. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.; Guitron, S.; Yoshida, K.; Li, S.; Damian, D.D.; Rus, D. Ingestible, controllable, and degradable origami robot for patching stomach wounds. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 909–916. [Google Scholar]
- Ahmed, M.A.; Jung, R. Modeling of Slow Waves in the Stomach. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2020; pp. 1–9. [Google Scholar]
- Roy, S.; Azad, A.N.M.W.; Baidya, S.; Alam, M.K.; Khan, F.H. Powering Solutions for Biomedical Sensors and Implants Inside the Human Body: A Comprehensive Review on Energy Harvesting Units, Energy Storage, and Wireless Power Transfer Techniques. IEEE Trans. Power Electron. 2022, 37, 12237–12263. [Google Scholar] [CrossRef]
- Alkhalaf, H.Y.; Ahmad, M.Y.; Ramiah, H. Self-Sustainable Biomedical Devices Powered by RF Energy: A Review. Sensors 2022, 22, 6371. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.-Z.; Yuan, M. Requirements, challenges, and novel ideas for wearables on power supply and energy harvesting. Nano Energy 2023, 115, 108715. [Google Scholar] [CrossRef]
- Ijemaru, G.K.; Ang, K.L.-M.; Seng, J.K. Wireless power transfer and energy harvesting in distributed sensor networks: Survey, opportunities, and challenges. Int. J. Distrib. Sens. Netw. 2022, 18, 15501477211067740. [Google Scholar] [CrossRef]
- Feng, Q.; Xin, M.; Xu, C.; Zhang, Y.; Liu, Z.; Tan, Z.; Lv, Q. Design and Optimization of the Energy Harvesting Device for Wireless Sensors. In Proceedings of the 2023 IEEE 4th International Conference on Electrical Materials and Power Equipment (ICEMPE), Shanghai, China, 7–10 May 2023; pp. 1–4. [Google Scholar]
- Zhang, A.; Zhu, L. A Promising Way of Energy Harvesting for Implantable Medical Devices—Thermoelectric Generator (TEG). In Proceedings of the 2021 International Conference on Smart City and Green Energy (ICSCGE), Da Nang, Vietnam, 20–22 November 2021; pp. 22–25. [Google Scholar]
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A Wearable Supercapacitor Based on Conductive PEDOT:PSS-Coated Cloth and a Sweat Electrolyte. Adv. Mater. 2020, 32, e1907254. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Hwang, S.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Deterre, M.; Lefeuvre, E.; Zhu, Y.; Woytasik, M.; Bosseboeuf, A.; Boutaud, B.; Molin, R.D. Micromachined piezoelectric spirals and ultra-compliant packaging for blood pressure energy harvesters powering medical implants. In Proceedings of the 2013 IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013; pp. 249–252. [Google Scholar]
- Safaei, M.; Meneghini, R.M.; Anton, S.R. Energy Harvesting and Sensing with Embedded Piezoelectric Ceramics in Knee Implants. IEEE/ASME Trans. Mechatron. 2018, 23, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Kymissis, J.; Kendall, C.; Paradiso, J.; Gershenfeld, N. Parasitic power harvesting in shoes. Digest of Papers. In Proceedings of the Second International Symposium on Wearable Computers (Cat. No.98EX215), Pittsburgh, PA, USA, 19–20 October 1998; pp. 132–139. [Google Scholar]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2018, 9, 1802906. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Zhu, G.; Yang, J.; Bai, P.; Su, Y.; Jing, Q.; Cao, X.; Wang, Z.L. Harvesting energy from the natural vibration of human walking. ACS Nano 2013, 7, 11317–11324. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Flores, A. Implantable Antennas for Endoscopy Applications: Current Review and Challenges. In Proceedings of the 2023 24th International Conference on Applied Electromagnetics and Communications (ICECOM), Dubrovnik, Croatia, 27–29 September 2023; pp. 1–4. [Google Scholar]
- Li, M.; Lou, W.; Ren, K. Data security and privacy in wireless body area networks. IEEE Wirel. Commun. 2010, 17, 51–58. [Google Scholar] [CrossRef]
- Juanola-Feliu, E.; Miribel-Català, P.L.; Avilés, C.P.; Colomer-Farrarons, J.; González-Piñero, M.; Samitier, J. Design of a Customized Multipurpose Nano-Enabled Implantable System for In-Vivo Theranostics. Sensors 2014, 14, 19275–19306. [Google Scholar] [CrossRef] [PubMed]
- Ukkonen, L.; Sydänheimo, L.; Ma, S.; Björninen, T. Backscattering-Based Wireless Communication and Power Transfer to Small Biomedical Implants (SPIE BiOS); SPIE: San Francisco, CA, USA, 2020. [Google Scholar]
- Zhao, B.; Kuo, N.-C.; Liu, B.; Li, Y.-A.; Iotti, L.; Niknejad, A.M. A 5.8GHz power-harvesting 116 μm × 116 μm “dielet” near-field radio with on-chip coil antenna. In Proceedings of the 2018 IEEE International Solid-State Circuits Conference (ISSCC), San Francisco, CA, USA, 11–15 February 2018; pp. 456–458. [Google Scholar]
- Guennoun, M.; Zandi, M.; El-Khatib, K. On the Use of Biometrics to Secure Wireless Biosensor Networks. In Proceedings of the Communication Technologies: From Theory to Applications (ICTTA), Damascus, Syria, 7–11 April 2008; pp. 1–5. [Google Scholar]
- Zhang, F.; Liu, X.; Hackworth, S.A.; Sclabassi, R.J.; Sun, M. Wireless energy delivery and data communication for biomedical sensors and implantable devices. In Proceedings of the 2009 IEEE 35th Annual Northeast Bioengineering Conference, Boston, MA, USA, 3–5 April 2009; pp. 1–2. [Google Scholar]
- Ramaswamy, S.; Gandhi, U.D. Trust-Based Data Communication in Wireless Body Area Network for Healthcare Applications. Big Data Cogn. Comput. 2022, 6, 148. [Google Scholar] [CrossRef]
- Boutry, C.M.; Chandrahalim, H.; Streit, P.; Schinhammer, M.; Hänzi, A.C.; Hierold, C. Characterization of miniaturized RLC resonators made of biodegradable materials for wireless implant applications. Sens. Actuators A Phys. 2013, 189, 344–355. [Google Scholar] [CrossRef]
- Palmroth, A.; Salpavaara, T.; Vuoristo, P.; Karjalainen, S.; Kääriäinen, T.; Miettinen, S.; Massera, J.; Lekkala, J.; Kellomäki, M. Materials and Orthopedic Applications for Bioresorbable Inductively Coupled Resonance Sensors. ACS Appl. Mater. Interfaces 2020, 12, 31148–31161. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Liang, Q.; Sun, X.; Yu, D.; Huang, X.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Intrinsically Electron Conductive, Antibacterial, and Anti-swelling Hydrogels as Implantable Sensors for Bioelectronics. Adv. Funct. Mater. 2022, 32, 2208024. [Google Scholar] [CrossRef]
- Nie, Z.; Kwak, J.W.; Han, M.; Rogers, J.A. Mechanically Active Materials and Devices for Bio-Interfaced Pressure Sensors—A Review. Adv. Mater. 2023, e2205609. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, L.; Liu, S.; Sheng, X.; Yin, L. Recent Development of Implantable Chemical Sensors Utilizing Flexible and Biodegradable Materials for Biomedical Applications. ACS Nano 2024, 18, 3969–3995. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Heidari, H.; Farina, D.; Nazarpour, K. Miniaturized Magnetic Sensors for Implantable Magnetomyography. Adv. Mater. Technol. 2020, 5, 2000185. [Google Scholar] [CrossRef]
- Chang, J.-K.; Fang, H.; Bower, C.A.; Song, E.; Yu, X.; Rogers, J.A. Materials and processing approaches for foundry-compatible transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, E5522–E5529. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hwang, S.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.; Huang, Y.; Rogers, J.A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Ha, D.; de Vries, W.N.; Kim, B.; Chlebowski, A.; John, S.W.; Irazoqui, P.P.; Chappell, W.J. Ultra-thin tag fabrication and sensing technique using third harmonic for implantable wireless sensors. In Proceedings of the 2011 IEEE MTT-S International Microwave Symposium, Baltimore, MD, USA, 5–10 June 2011; pp. 1–4. [Google Scholar]
- Herbert, R.; Lim, H.; Park, S.; Kim, J.; Yeo, W. Recent Advances in Printing Technologies of Nanomaterials for Implantable Wireless Systems in Health Monitoring and Diagnosis. Adv. Health Mater. 2021, 10, 2100158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, Y.-S.; Tillman, B.W.; Yeo, W.-H.; Chun, Y. Advances in Materials for Recent Low-Profile Implantable Bioelectronics. Materials 2018, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tourovskaia, A.; Folch, A. Biology on a Chip: Microfabrication for Studying the Behavior of Cultured Cells. Crit. Rev. Biomed. Eng. 2003, 31, 66. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, B.K.; Ludwig, B.; Shou, W.; Yu, X.; Fregene, E.; Xu, H.; Pan, H.; Huang, X. Aerosol printing and photonic sintering of bioresorbable zinc nanoparticle ink for transient electronics manufacturing. Sci. China Inf. Sci. 2018, 61, 060412. [Google Scholar] [CrossRef]
- Yin, L.; Huang, X.; Xu, H.; Zhang, Y.; Lam, J.; Cheng, J.; Rogers, J.A. Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries. Adv. Mater. 2014, 26, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless Technologies for Implantable Devices. Sensors 2020, 20, 4604. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Legassey, A.; Qiang, L.; Wang, Y.; Burgess, D.J.; Papadimitrakopoulos, F. Enhancing the Sensitivity of Needle-Implantable Electrochemical Glucose Sensors via Surface Rebuilding. J. Diabetes Sci. Technol. 2013, 7, 441–451. [Google Scholar] [CrossRef]
- Vickers, O.G.; Culmer, P.R.; Isaac, G.H.; Kay, R.W.; Shuttleworth, M.P.; Board, T.; Williams, S. Is in vivo sensing in a total hip replacement a possibility? A review on past systems and future challenges. Prog. Biomed. Eng. 2021, 3, 042004. [Google Scholar] [CrossRef]
- Wang, Y.; Vaddiraju, S.; Gu, B.; Papadimitrakopoulos, F.; Burgess, D.J. Foreign Body Reaction to Implantable Biosensors: Effects of Tissue Trauma and Implant Size. J. Diabetes Sci. Technol. 2015, 9, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.W.; Brooker, S.C.; McDonald; Hyett, J. Sensors for Implants: Real-Time Failure Detection on the Arabin Pessary. In Modern Sensing Technologies; Mukhopadhyay, S.C., Jayasundera, K.P., Postolache, O.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 17–28. [Google Scholar]
- Vanhoestenberghe, A. Implantable electronic devices technology challenges for long-term human implantation. Sens. Rev. 2009, 29, 345–348. [Google Scholar] [CrossRef]
- Nagpal, A.; Baddour, L.M.; Sohail, M.R. Microbiology and Pathogenesis of Cardiovascular Implantable Electronic Device Infections. Circ. Arrhythmia Electrophysiol. 2012, 5, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Hall-Stoodley, L.; Stoodley, P. 2.2.8—Biofilms, Biomaterials, and Device-Related Infections. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 823–840. [Google Scholar]
- Trohman, R.G.; Kim, M.H.; Pinski, S.L. Cardiac pacing: The state of the art. Lancet 2004, 364, 1701–1719. [Google Scholar] [CrossRef] [PubMed]
- Hartl, H.; Li, W.; Michl, T.D.; Anangi, R.; Speight, R.; Vasilev, K.; Ostrikov, K.K.; MacLeod, J. Antimicrobial adhesive films by plasma-enabled polymerisation of m-cresol. Sci. Rep. 2022, 12, 7560. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Reinisch, G.; Trettenhahn, G.; Zweymüller, K.; Lintner, F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009, 5, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 100B, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kim, B.J.; Meng, E. Chronically Implanted Pressure Sensors: Challenges and State of the Field. Sensors 2014, 14, 20620–20644. [Google Scholar] [CrossRef] [PubMed]
- Campmier, M.J.; Gingrich, J.; Singh, S.; Baig, N.; Gani, S.; Upadhya, A.; Agrawal, P.; Kushwaha, M.; Mishra, H.R.; Pillarisetti, A.; et al. Seasonally optimized calibrations improve low-cost sensor performance: Long-term field evaluation of PurpleAir sensors in urban and rural India. Atmos. Meas. Tech. 2023, 16, 4357–4374. [Google Scholar] [CrossRef]
- Garg, S.K.; Liljenquist, D.; Bode, B.; Christiansen, M.P.; Bailey, T.S.; Brazg, R.L.; Denham, D.S.; Chang, A.R.; Akturk, H.K.; Dehennis, A.; et al. Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study. Diabetes Technol. Ther. 2022, 24, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Cleven, N.J.; Müntjes, J.A.; Fassbender, H.; Urban, U.; Görtz, M.; Vogt, H.; Gräfe, M.; Göttsche, T.; Penzkofer, T.; Schmitz-Rode, T.; et al. A Novel Fully Implantable Wireless Sensor System for Monitoring Hypertension Patients. IEEE Trans. Biomed. Eng. 2012, 59, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Koley, G.; Liu, J.; Nomani, W.; Yim, M.; Wen, X.; Hsia, T.-Y. Miniaturized implantable pressure and oxygen sensors based on polydimethylsiloxane thin films. Mater. Sci. Eng. C 2009, 29, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Huang, C.-C.; Liou, J.-S.; Ciou, Y.-J.; Huang, I.-Y.; Li, C.-P.; Lee, Y.-C.; Wu, W.-J. A Mini-Invasive Long-Term Bladder Urine Pressure Measurement ASIC and System. IEEE Trans. Biomed. Circuits Syst. 2008, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; Gomes, N.O.; de Oliveira, T.V.; Fortes-Da-Silva, P.; Soares, N.d.F.F.; Raymundo-Pereira, P.A. Review and Perspectives of sustainable, biodegradable, eco-friendly and flexible electronic devices and (Bio)sensors. Biosens. Bioelectron. X 2023, 14, 100371. [Google Scholar] [CrossRef]
- Bathaei, M.J.; Singh, R.; Istif, E.; Beker, L. 25—Biodegradable sensor platforms. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 775–801. [Google Scholar]
- Tian, X.; Zeng, Q.; Kurt, S.A.; Li, R.R.; Nguyen, D.T.; Xiong, Z.; Li, Z.; Yang, X.; Xiao, X.; Wu, C.; et al. Implant-to-implant wireless networking with metamaterial textiles. Nat. Commun. 2023, 14, 4335. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Chan, S.Y.; Du, Z.; Yan, Y.; Wang, T.; Li, P.; Huang, W. Hydrogel-based flexible materials for diabetes diagnosis, treatment, and management. Npj Flex. Electron. 2021, 5, 26. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, D.S.; Kumari, R.; Malode, S.J.; Shetti, N.P.; Chandra, P. Integrated lab-on-a-chip devices: Fabrication methodologies, transduction system for sensing purposes. J. Pharm. Biomed. Anal. 2023, 223, 115120. [Google Scholar] [CrossRef] [PubMed]
- Morris, A. Smart, dissolving pacemaker communicates with body-area sensor and control network. In 1603 Orrington Avenue, 2nd ed.; Northwestern Now, Ed.; Northwestern University: Evanston, IL, USA, 2022. [Google Scholar]
- Morris, A. First-ever transient pacemaker harmlessly dissolves in body. In 1603 Orrington Avenue, 2nd ed.; Northwestern Now, Ed.; Northwestern University: Evanston, IL, USA, 2021. [Google Scholar]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.; Cobian, K. Polyether polyurethanes for implantable pacemaker leads. Biomaterials 1982, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.T.; Choi, Y.S.; Aras, K.K.; Knight, H.S.; Miniovich, A.N.; Efimov, I.R. Chapter 36—Innovation in Cardiovascular Bioelectronics. In Advances in Cardiovascular Technology; Karimov, J.H., Fukamachi, K., Gillinov, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 587–602. [Google Scholar]
- Abdul-Jabbar, H.M.; Abed, J.K. Real Time Pacemaker Patient Monitoring System Based on Internet of Things. IOP Conf. Series Mater. Sci. Eng. 2020, 745, 012093. [Google Scholar] [CrossRef]


| Sensor Type | Analyte | Physiological Range | Detection Limit | Application | In Vivo/In Vitro | Highlight |
|---|---|---|---|---|---|---|
| Resistive Sensor [137] | Acetone | Under 0.6 mmol/L (177 ppb to 2441 ppb) | 0.1 to 2 ppm | Detecting acetone directly in the exhaled breath | Exhaled breath | Detects acetone directly in the exhaled breath. |
| Resistive Sensor [138] | Ethanol | Less than 50 mg/dL, or 0.05% concentration | 5 ppm–1000 ppm | Gas sensing | Homes and industrial sites | Alcohol sensor with high selectivity and stability. |
| Resistive Sensor [160] | Pressure | Flexible | 10–500 kPa | Sensing subtle pressure levels like pulse pressure and high-pressure levels like fingertip pressure | Conformal contact with the skin | Ultrathin, biocompatible, and flexible pressure sensor. |
| Resistive Sensor [161] | Ammonia | 29–688 ppb, Avg 265 ppb | 0.2 to 10 ppm | Detecting ammonia directly in the exhaled breath | Exhaled breath | Molecularly modified. SnO2 sensors for sensing polar gases such as ammonia. |
| Resistive Sensor [162] | Avidin | 0.05% of total protein (1800 μg/egg) | 0.1 nM (6.8 ng⁄ml) | Detection of protein (Avidin) | Lab based | Detection of protein in low concentrations. |
| Capacitive Sensor [163] | Static pressure | Flexible | Compression 0.09/N, Sheer 0.06/(a.u), Bending 0.06/(a.u) | Detection of static pressure | Measures static pressure (cannot be done by piezoelectric sensors) | Highly flexible pressure sensors. |
| Capacitive Sensor [164] | Gram-force | Stretchable | GF = 0.7 Pressure sensitivity 1.62 MPa−1 | Simultaneously detects stretch, pressure, temperature, or touch | Finger and knee | Highly sensitive and wearable sensors. |
| Capacitive Sensor [165] | Gram-force | Stretchable | GF = 1 | Strain gauges to detect human motion | Onto smart clothes or directly onto the body | Date glove, monitoring of balloon inflation and chest movement. |
| Capacitive Sensor [166] | Pressure | Stretchable | 0.7 kPa−1 (0–1 kPa), 0.14 kPa−1 (1–5 kPa), 0.005 kPa−1 (5–20 kPa) | Pressure sensing | Electronic Skin | The first stretchable energy-harvesting electronic skin device. |
| Capacitive Sensor [167] | Pressure | Flexible | 5.54 kPa−1 (0–30 Pa), 0.88 kPa−1 (30–70 Pa) | Monitoring of knee/finger bending, forearm muscular movement and air blow | Finger, arms, and knee | The sensor has been readily integrated into an adhesive bandage and has been successful in detecting human movements. |
| Piezoelectric Sensor [168] | Pressure | Flexible | 0.1 Pa–2 KPa | Pressure sensing | With the human body or with advanced robotic systems | The ability to bend and stretch is attractive for pressure/force sensors. |
| Piezoelectric Sensor [146] | Pressure | Flexible | 0–18 kPa | Pressure sensing | Monitoring of pressure in various parts of the body such as the brain, lungs, eyes, and heart | A biodegradable implantable pressure sensor using PLLA. |
| Piezoelectric Sensor [155] | Pressure | Flexible | 5–60 kPa | Pressure sensing | Measuring pressure under wound bandages | Amino acid glycine and chitosan polymer-based biodegradable pressure sensors. |
| Piezoelectric Sensor [169] | Pressure | Flexible | 0.23 to 10 kPa | Pressure sensing | Human skin | Force-sensing resistors and field effect transistor (FET) sensors for monitoring biological pressure and force-sensing. |
| Piezoelectric Sensor [170] | Piezoelectric Coefficient | Flexible | 4.7–6.4 pC/N | Piezoelectric response | Ambient condition | Potential applications in the fields of electronics, sensors, and biomedical diagnostics. |
| Triboelectric Sensor [158] | Ammonia | 29 to 688 ppb | 50–10,000 ppm | Detection of ammonia in breath | Breath analysis | Monitoring of exhaled gases in human breath for disease diagnosis. |
| Triboelectric Sensor [171] | Acetone, Toluene | 0.3–1.0 ppm Acetone, 6.5 ± 1.5 ppm Toluene | 35 ppb, 3.0 ppm for acetone 1 ppb,10 ppm for toluene | Detection of acetone and toluene in breath | Breath analysis | Volatilome analyzer consisting of polymer nanofiber-MWCNT composite that responds to acetone and toluene. |
| Triboelectric Sensor [172] | Alcohol | 0.01–0.5% (40–500 mg/dL) | 10–2000 ppm | Detection of alcohol in breath. | Breath analysis | Breathed-out alcohol concentration detection regardless of the blow speed and quality of airflow. |
| Triboelectric Sensor [173] | Lactate | 6.5 mM (forehead) to 13 mM (foot) with an avg 5.9 mM for the whole body | 10 µM–20 mM | Detection of lactate in sweat | Sweat analysis | Lightweight and fully self-powered electrochemical sensing system to manage and self-monitor lactate concentration in sweat. |
| Triboelectric Sensor [174] | Glucose | 0.001–5.50 mM | 0.1–1 mM | Onto the clothes | Lab testing | Nonenzymatic glucose detection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, F.; Ashfaq Ahmed, M.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.M.; Pala, N. Recent Progress and Challenges of Implantable Biodegradable Biosensors. Micromachines 2024, 15, 475. https://doi.org/10.3390/mi15040475
Alam F, Ashfaq Ahmed M, Jalal AH, Siddiquee I, Adury RZ, Hossain GMM, Pala N. Recent Progress and Challenges of Implantable Biodegradable Biosensors. Micromachines. 2024; 15(4):475. https://doi.org/10.3390/mi15040475
Chicago/Turabian StyleAlam, Fahmida, Md Ashfaq Ahmed, Ahmed Hasnain Jalal, Ishrak Siddiquee, Rabeya Zinnat Adury, G M Mehedi Hossain, and Nezih Pala. 2024. "Recent Progress and Challenges of Implantable Biodegradable Biosensors" Micromachines 15, no. 4: 475. https://doi.org/10.3390/mi15040475