Research on Integrated 3D Printing of Microfluidic Chips
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Preparation of Channel Molds
2.2.2. Dissolution of Channel Molds
- (1)
- Channel mold of PVA material
- (2)
- Dissolution of HIPS channel mold
2.2.3. Hybrid Experiments with Microfluidic Chips
2.2.4. Dimensional Observation of Microchannels
2.2.5. Comparison of Bonding Methods
2.2.6. Statistical Analysis
3. Results and Discussion
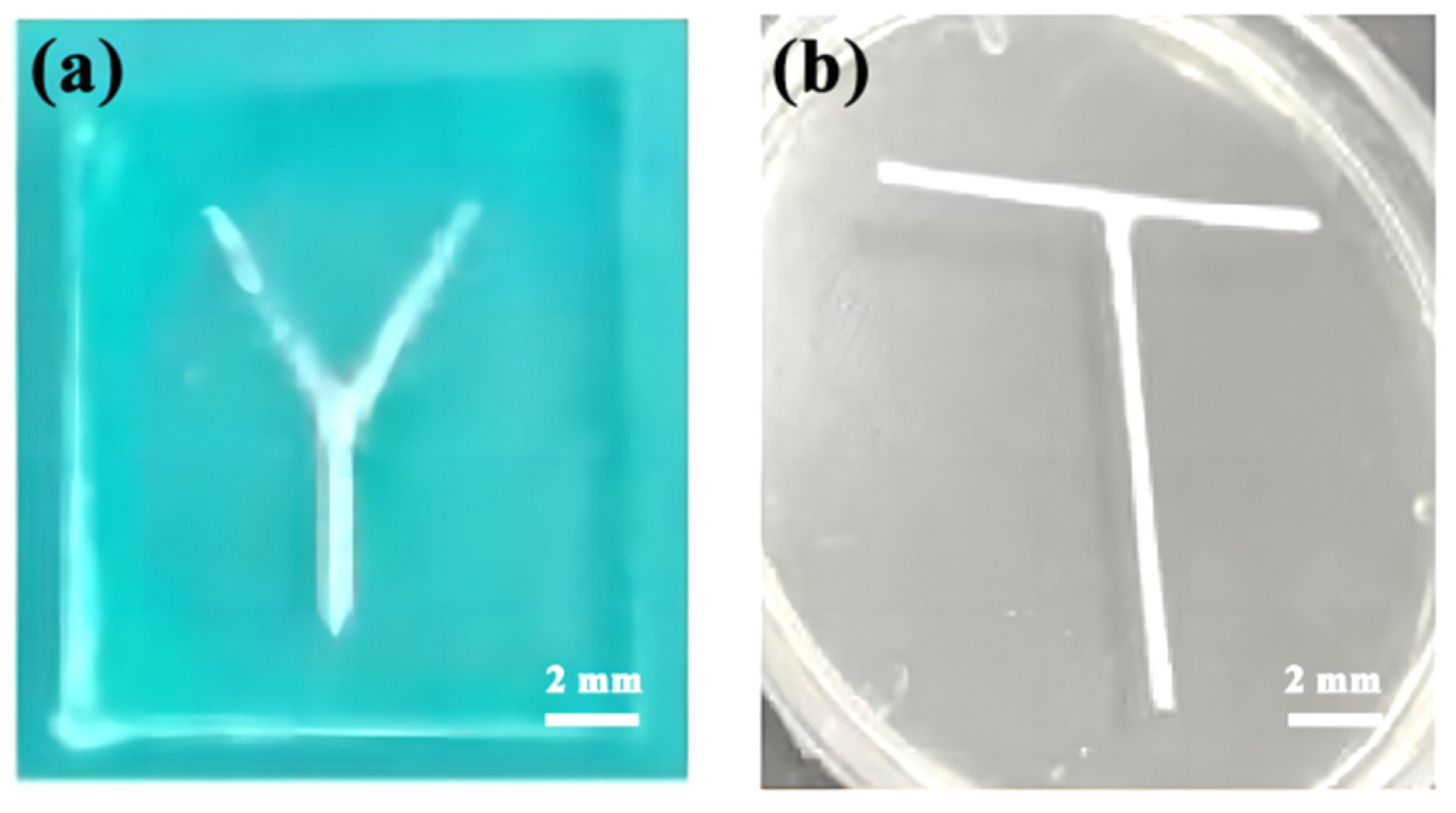
3.1. Channel Mold
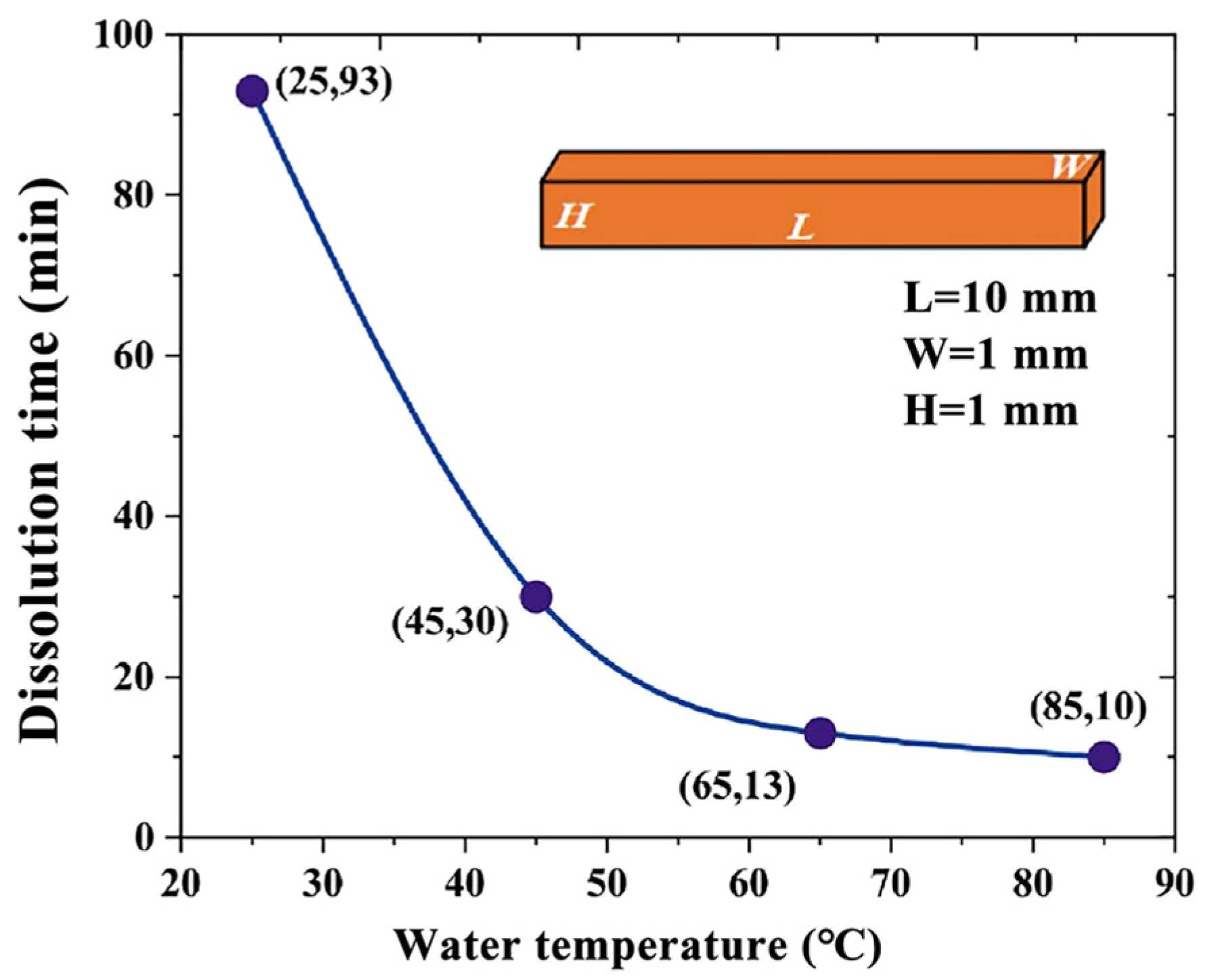
3.2. Dissolution of Mold Channels
- (1)
- Channel mold of PVA
- (2)
- Channel mold of HIPS
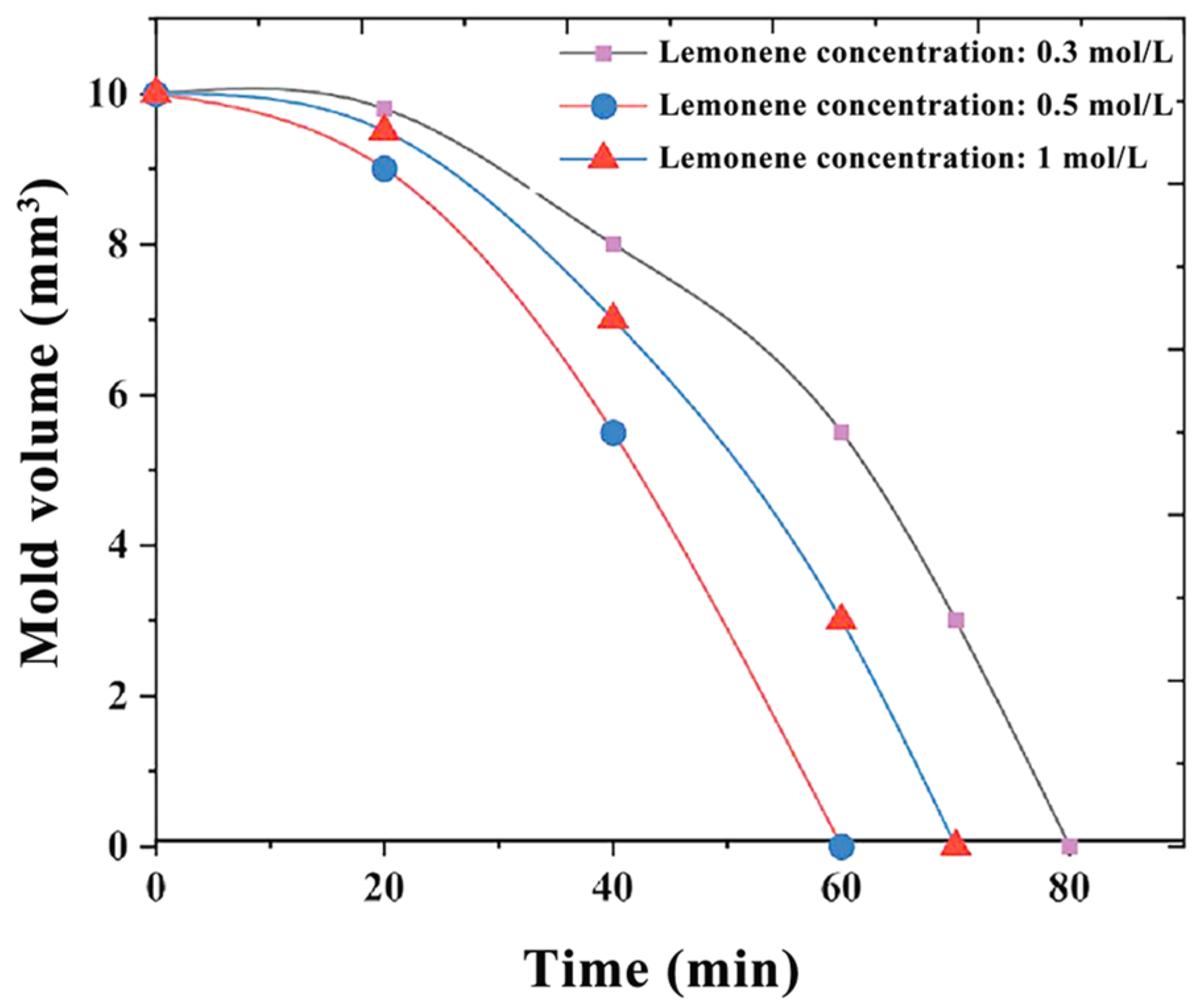
3.3. Hybrid Experiments on Microfluidic Chips
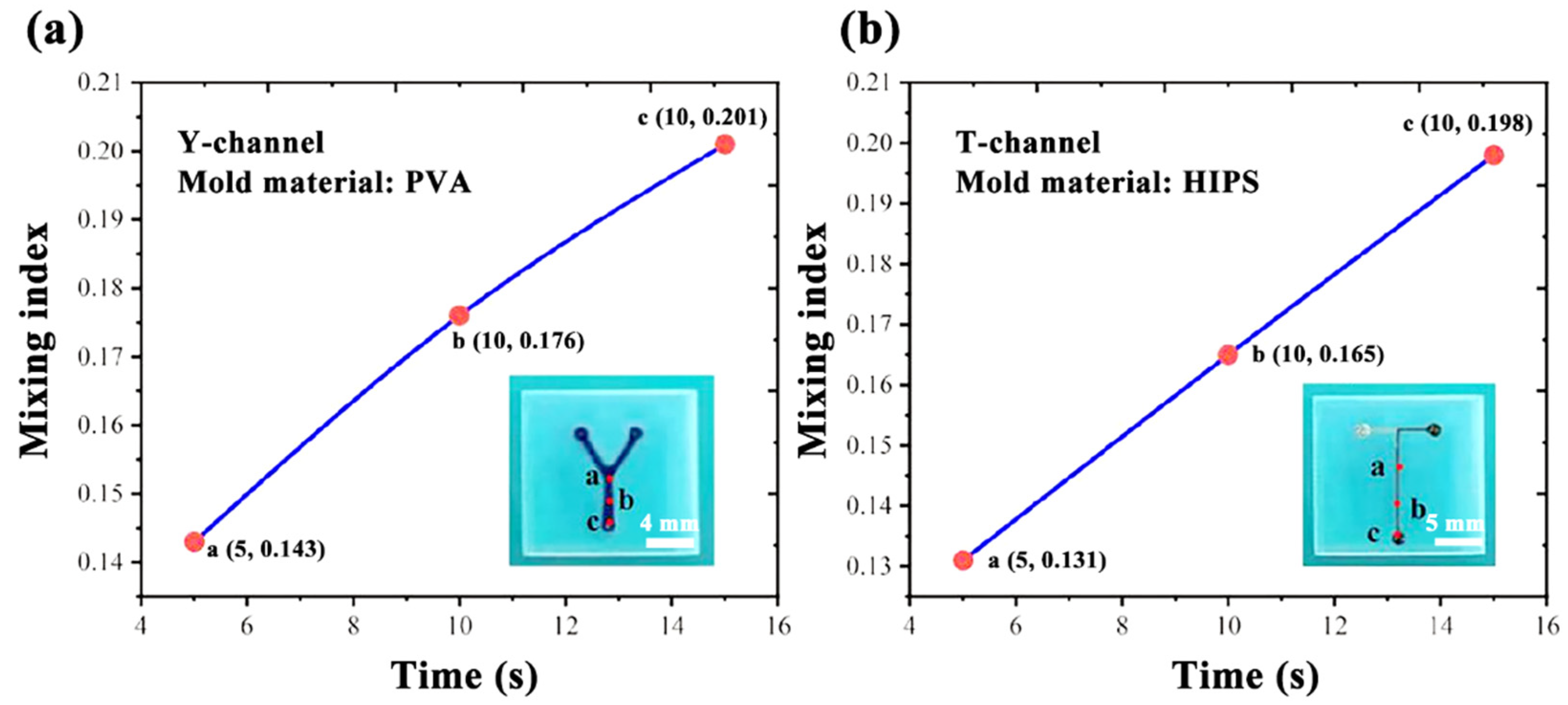
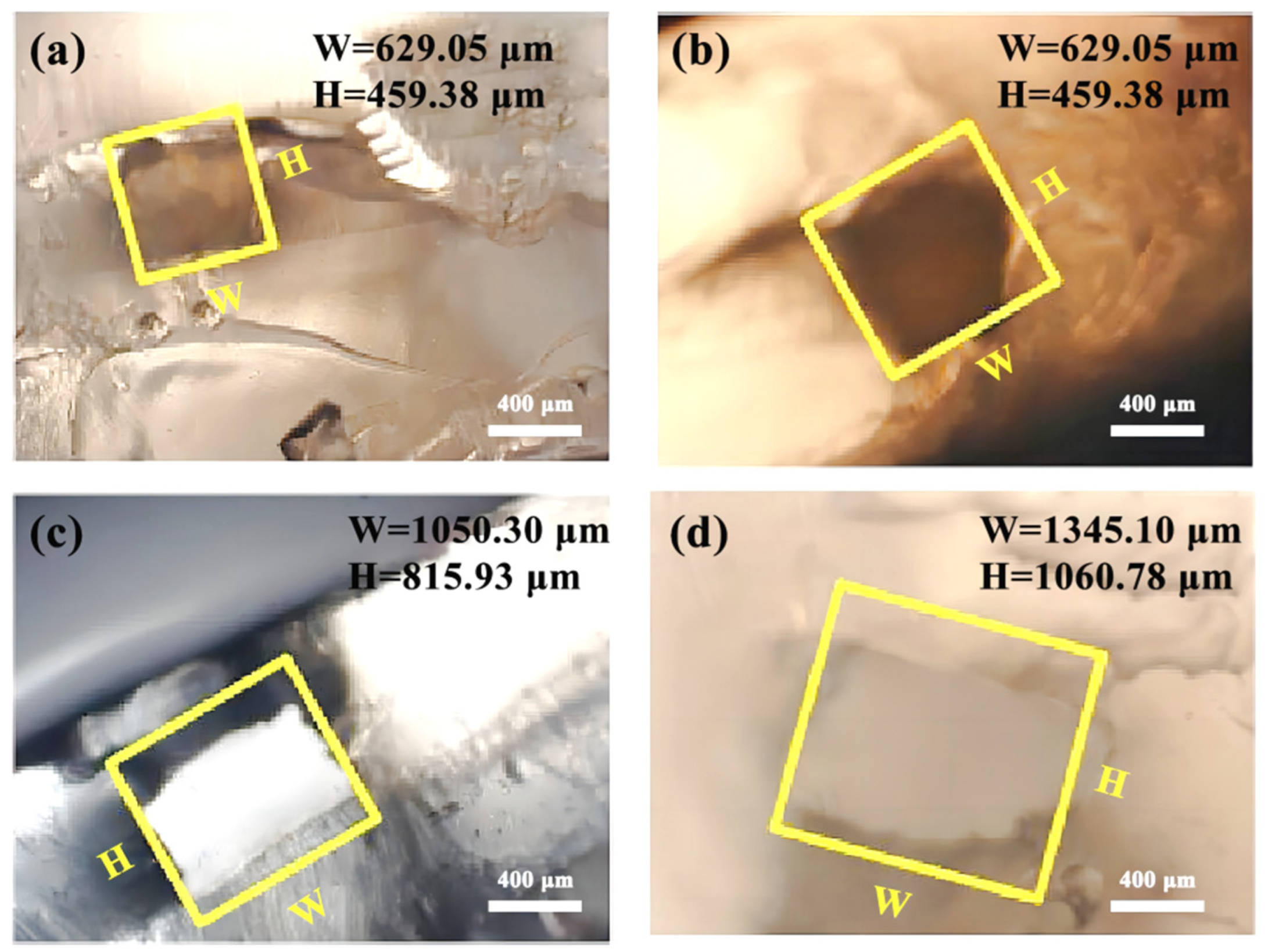
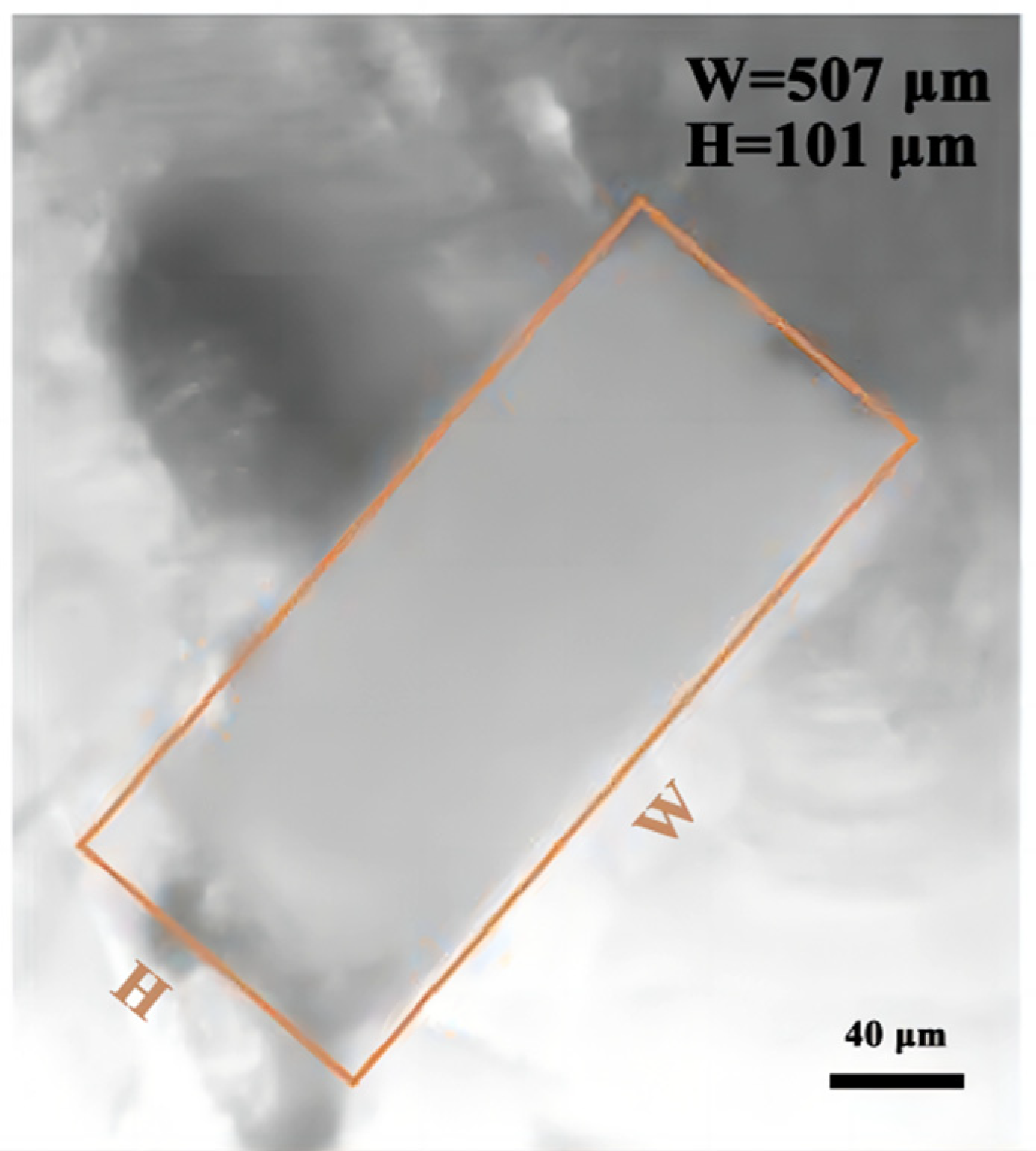
3.4. Size Observation of Microchannels
- (1)
- Channel mold of PVA
- (2)
- Channel mold of HIPS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828 . [Google Scholar] [CrossRef]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Helmer, D.; Rapp, B.E. Emerging Technologies and Materials for High-Resolution 3D Printing of Microfluidic Chips. In Microfluidics in Biotechnology; Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2022; Volume 179, pp. 37–66. [Google Scholar]
- Nie, J.; Fu, J.; He, Y. Hydrogels: The Next Generation Body Materials for Microfluidic Chips? Small 2020, 16, e2003797. [Google Scholar] [CrossRef]
- Li, Y.; Bøtker, J.; Rantanen, J.; Yang, M.; Bohr, A. In silico design and 3D printing of microfluidic chips for the preparation of size-controllable siRNA nanocomplexes. Int. J. Pharm. 2020, 583, 119388. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 3862–3881. [Google Scholar]
- Regan, B.; Kinahan, D.; Daly, P.; O’Kennedy, R.; Collins, D. Design and fabrication of a low-cost wireless camera imaging system for centrifugal microfluidics. HardwareX 2022, 11, e00259. [Google Scholar] [CrossRef] [PubMed]
- Leister, N.; Vladisavljević, G.T.; Karbstein, H.P. Novel glass capillary microfluidic devices for the flexible and simple production of multi-cored double emulsions. J. Colloid Interface Sci. 2022, 611, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and Soft Lithography for Organ-on-Chip Applications. Methods Mol. Biol. 2022, 2373, 1–19. [Google Scholar]
- Ko, J.; Park, D.; Lee, S.; Gumuscu, B.; Jeon, N.L. Engineering Organ-on-a-Chip to Accelerate Translational Research. Micromachines 2022, 13, 1200. [Google Scholar] [CrossRef]
- Weinhart, M.; Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D organ models-Revolution in pharmacological research? Pharmacol. Res. 2019, 139, 446–451. [Google Scholar] [CrossRef]
- Fu, E.; Wentland, L. A survey of 3D printing technology applied to paper microfluidics. Lab Chip 2021, 22, 9–25. [Google Scholar] [CrossRef]
- Hong, N.; Nam, Y. Neurons-on-a-Chip: In Vitro NeuroTools. Mol. Cells 2022, 45, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic devices for bacteria and fungus research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- Ribeiro de Souza, L.; Al-Tabbaa, A. High throughput production of microcapsules using microfluidics for self-healing of cementitious materials. Lab Chip 2021, 21, 4652–4659. [Google Scholar] [CrossRef]
- Clark, A.S.; San-Miguel, A. A bioinspired, passive microfluidic lobe filtration system. Lab Chip 2021, 21, 3762–3774. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.; Zhou, T.; Yue, W. Enzyme Method-Based Microfluidic Chip for the Rapid Detection of Copper Ions. Micromachines 2021, 12, 1380. [Google Scholar] [CrossRef]
- Ansari, A.; Trehan, R.; Watson, C.; Senyo, S. Increasing Silicone Mold Longevity: A Review of Surface Modification Techniques for PDMS-PDMS Double Casting. Soft Mater. 2021, 19, 388–399. [Google Scholar] [CrossRef]
- Yin, B.; Yue, W.; Sohan, A.; Zhou, T.; Qian, C.; Wan, X. Micromixer with Fine-Tuned Mathematical Spiral Structures. ACS Omega 2021, 6, 30779–30789. [Google Scholar] [CrossRef]
- Roshanghias, A.; Bardong, J.; Binder, A. Glass Frit Jetting for Advanced Wafer-Level Hermetic Packaging. Materials 2022, 15, 2786. [Google Scholar] [CrossRef]
- Camli, B.; Altinagac, E.; Kizil, H.; Torun, H.; Dundar, G.; Yalcinkaya, A.D. Gold-on-glass microwave split-ring resonators with PDMS microchannels for differential measurement in microfluidic sensing. Biomicrofluidics 2020, 14, 054102. [Google Scholar] [CrossRef]
- Yin, B.; Qian, C.; Wan, X.; Muhtasim Fuad Sohan, A.S.M.; Lin, X. Tape integrated self-designed microfluidic chip for point-of-care immunoassays simultaneous detection of disease biomarkers with tunable detection range. Biosens. Bioelectron. 2022, 212, 114429. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, S.H. Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective. Polymers 2021, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Conejo, J.; Alammar, A.; Ayub, J. Current Protocols for Resin-Bonded Dental Ceramics. Dent. Clin. North Am. 2022, 66, 603–625. [Google Scholar] [CrossRef]
- Chen, P.C.; Lee, C.Y.; Duong, L.H. Microfabrication of Nonplanar Polymeric Microfluidics. Micromachines 2018, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.J.E.; Uy, C.E.; Plaksina, P.; Ramani, R.S.; Ganjigatti, R.; Waddell, J.N. Bond Strength of Denture Teeth to Heat-Cured, CAD/CAM and 3D Printed Denture Acrylics. J. Prosthodont. 2020, 29, 415–421. [Google Scholar] [CrossRef]
- Chen, Q.; Ying, D.; Chen, Y.; Xie, H.; Zhang, H.; Chang, C. Highly transparent, hydrophobic, and durable anisotropic cellulose films as electronic screen protectors. Carbohydr. Polym. 2023, 311, 120735. [Google Scholar] [CrossRef]
- Chen, P.C.; Zhang, R.H.; Chen, L.T. Using Micromachined Molds, Partial-curing PDMS Bonding Technique, and Multiple Casting to Create Hybrid Microfluidic Chip for Microlens Array. Micromachines 2019, 10, 572. [Google Scholar] [CrossRef] [Green Version]
- Funano, S.I.; Ota, N.; Tanaka, Y. A simple and reversible glass-glass bonding method to construct a microfluidic device and its application for cell recovery. Lab Chip 2021, 21, 2244–2254. [Google Scholar] [CrossRef]
- Zhou, J.; Mei, N.; Leonenko, Z.; Zhou, N.; Mayer, M. Direct glass-to-glass bonding obtained via simplified ammonia-based low-temperature procedure resists high shear stress and powerful CW fiber laser irradiation. RSC Adv. 2022, 12, 31016–31023. [Google Scholar] [CrossRef]
- Yin, B.F.; Wan, X.H.; Yang, M.Z.; Qian, C.C.; Sohan, A. Wave-shaped microfluidic chip assisted point-of-care testing for accurate and rapid diagnosis of infections. Mil. Med. Res. 2022, 9, 8. [Google Scholar] [CrossRef]
- Micheal Raj, P.; Barbe, L.; Andersson, M.; De Albuquerque Moreira, M.; Haase, D.; Wootton, J.; Nehzati, S.; Terry, A.E.; Friel, R.J.; Tenje, M.; et al. Fabrication and characterisation of a silicon-borosilicate glass microfluidic device for synchrotron-based hard X-ray spectroscopy studies. RSC Adv. 2021, 11, 29859–29869. [Google Scholar] [CrossRef]
- Xiang, C.; Lu, Y.; Cheng, C.; Wang, J.; Chen, D.; Chen, J. A Resonant Pressure Microsensor with a Wide Pressure Measurement Range. Micromachines 2021, 12, 382. [Google Scholar] [CrossRef]
- Zeng, S.; Sun, X.; Wan, X.; Qian, C.; Yue, W.; Sohan, A.; Lin, X.; Yin, B. A cascade Fermat spiral microfluidic mixer chip for accurate detection and logic discrimination of cancer cells. Analyst 2022, 147, 3424–3433. [Google Scholar] [CrossRef]
- Miyagawa, S.; Aiba, S.; Kawamoto, H.; Tokunaga, Y.; Kawasaki, T. Absolute asymmetric Strecker synthesis in a mixed aqueous medium: Reliable access to enantioenriched α-aminonitrile. Org. Biomol. Chem. 2019, 17, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Ender, H.; Kierfeld, J. From diffusive mass transfer in Stokes flow to low Reynolds number Marangoni boats. The European physical journal. E Soft Matter 2021, 44, 4. [Google Scholar]
- Bi, W.; Cai, S.; Lei, T.; Wang, L. Implementation of blood-brain barrier on microfluidic chip: Recent advance and future prospects. Ageing Res. Rev. 2023, 87, 101921. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Liu, H.; Li, Y.; Lang, T.; Wang, R.; Cui, B.; Zhu, W. Efficient Mixing of Microfluidic Chip with a Three-Dimensional Spiral Structure. ACS Omega 2022, 7, 1527–1536. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Xiang, D.; Cui, Y.; Wan, Z.; Wang, S.; Peng, L.; Liao, Z.; Chen, C.; Liu, W. Study on swelling, compression property and degradation stability of PVA composite hydrogels for artificial nucleus pulposus. J. Mech. Behav. Biomed. Mater. 2022, 136, 105496. [Google Scholar] [CrossRef]
- Zhao, Z.; Lai, M.; Yang, Y.; Li, J.; Song, H.; He, J.; Zhang, H.; Mao, Y.; Ma, Y.; Liu, B. PVA/Tween 20 thin-film-based fiber optic humidity sensor with enhanced sensing performance. Appl. Opt. 2022, 61, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, B.; Liu, H.; Chen, X.; Li, Y.; Wang, R.; Lang, T.; Yang, H.; Li, L.; Pan, H.; et al. Tesla Valve-Based Flexible Microhybrid Chip with Unidirectional Flow Properties. ACS Omega 2022, 7, 31744–31755. [Google Scholar] [CrossRef] [PubMed]
- Ebciba, C.; Gnanamani, A. Detailed studies on microbial adhesion and degradation of polystyrene foam wastes (PSFW) for clean environment. Environ. Sci. Pollut. Res. Int. 2020, 27, 44257–44266. [Google Scholar] [CrossRef]



| Channel Mold Material | Cleaning Method | Dissolution Time | Width Change before and after the Dissolution | Height Change before and after the Dissolution |
|---|---|---|---|---|
| PVA | Press extrusion | 12 h | 12–25% | 8–12% |
| HIPS | Clean water flushing | 12 h | 0.5–1% | 1–2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Sun, J.; Yin, B. Research on Integrated 3D Printing of Microfluidic Chips. Micromachines 2023, 14, 1302. https://doi.org/10.3390/mi14071302
Wu C, Sun J, Yin B. Research on Integrated 3D Printing of Microfluidic Chips. Micromachines. 2023; 14(7):1302. https://doi.org/10.3390/mi14071302
Chicago/Turabian StyleWu, Chuang, Jiju Sun, and Binfeng Yin. 2023. "Research on Integrated 3D Printing of Microfluidic Chips" Micromachines 14, no. 7: 1302. https://doi.org/10.3390/mi14071302





