Effect of Microwave Irradiation on the Dielectric Characteristics of Semi-Conductive Nanoparticle-Based Nanofluids: Progress towards the Microwave Synthesis
Abstract
:1. Introduction
2. Material, Methods, and Experimental Setup
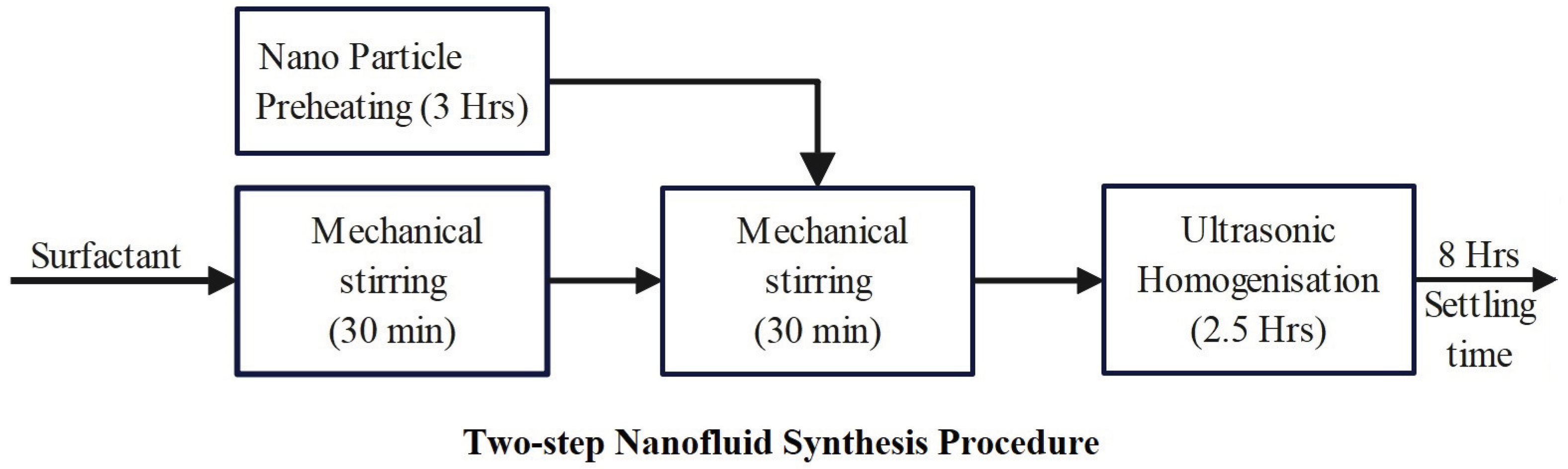
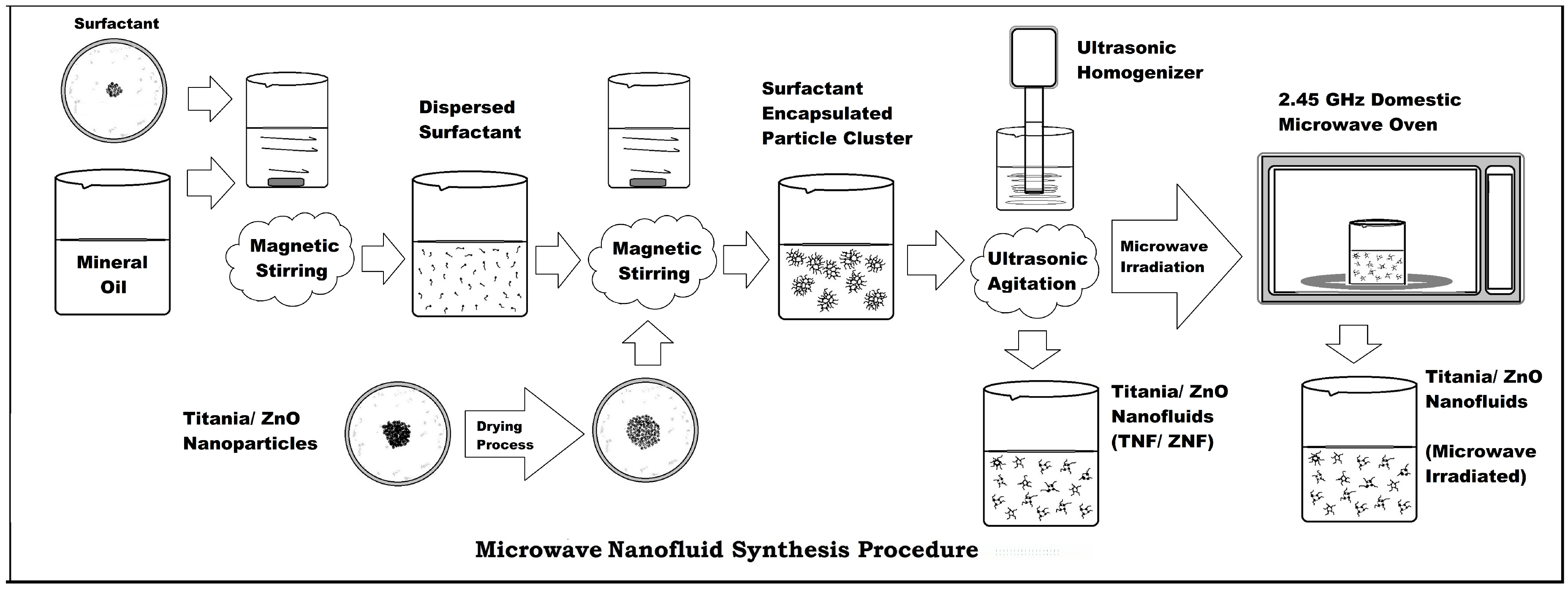
3. SNF Synthesis
4. Dispersivity Evaluation
5. Results and Discussion
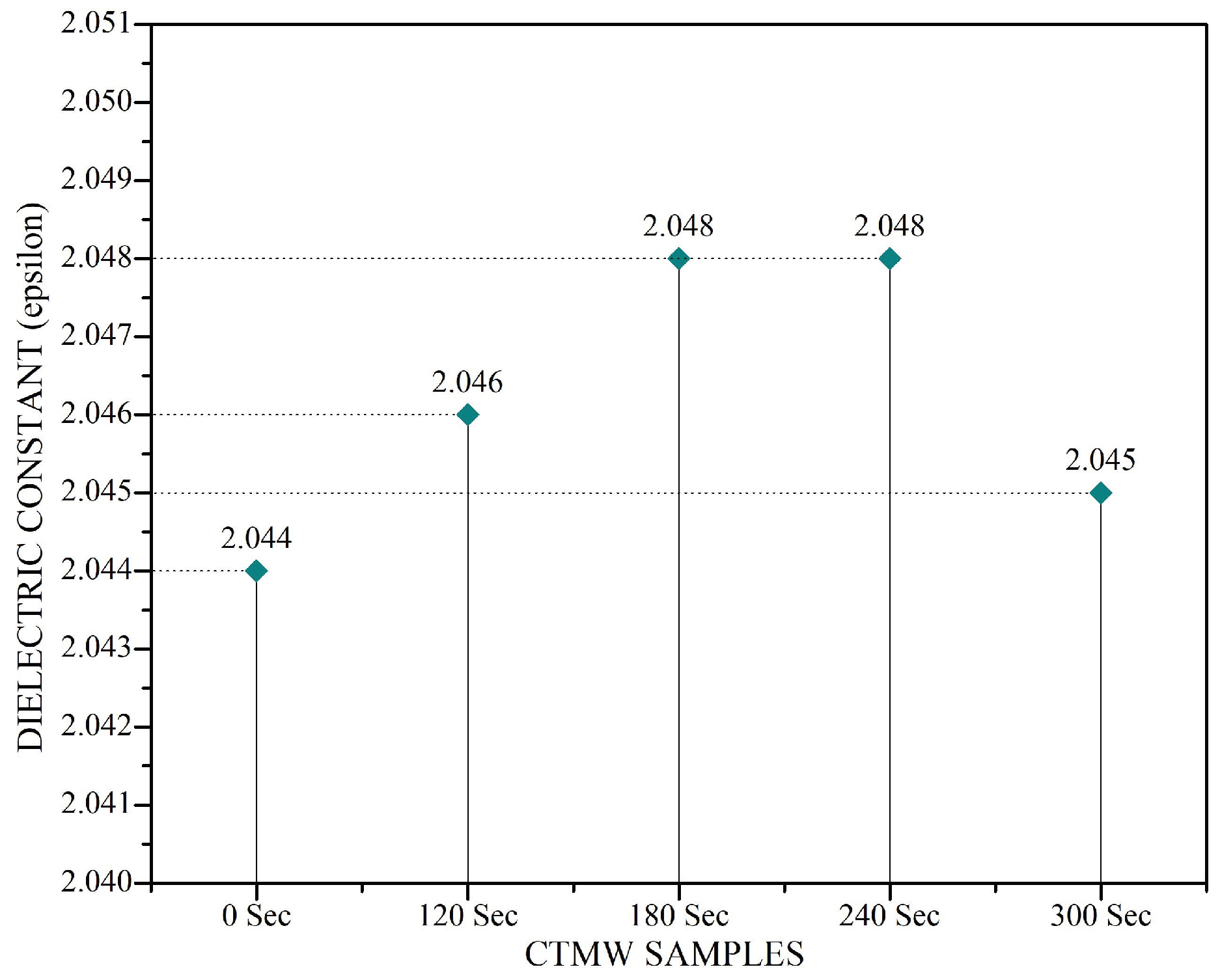
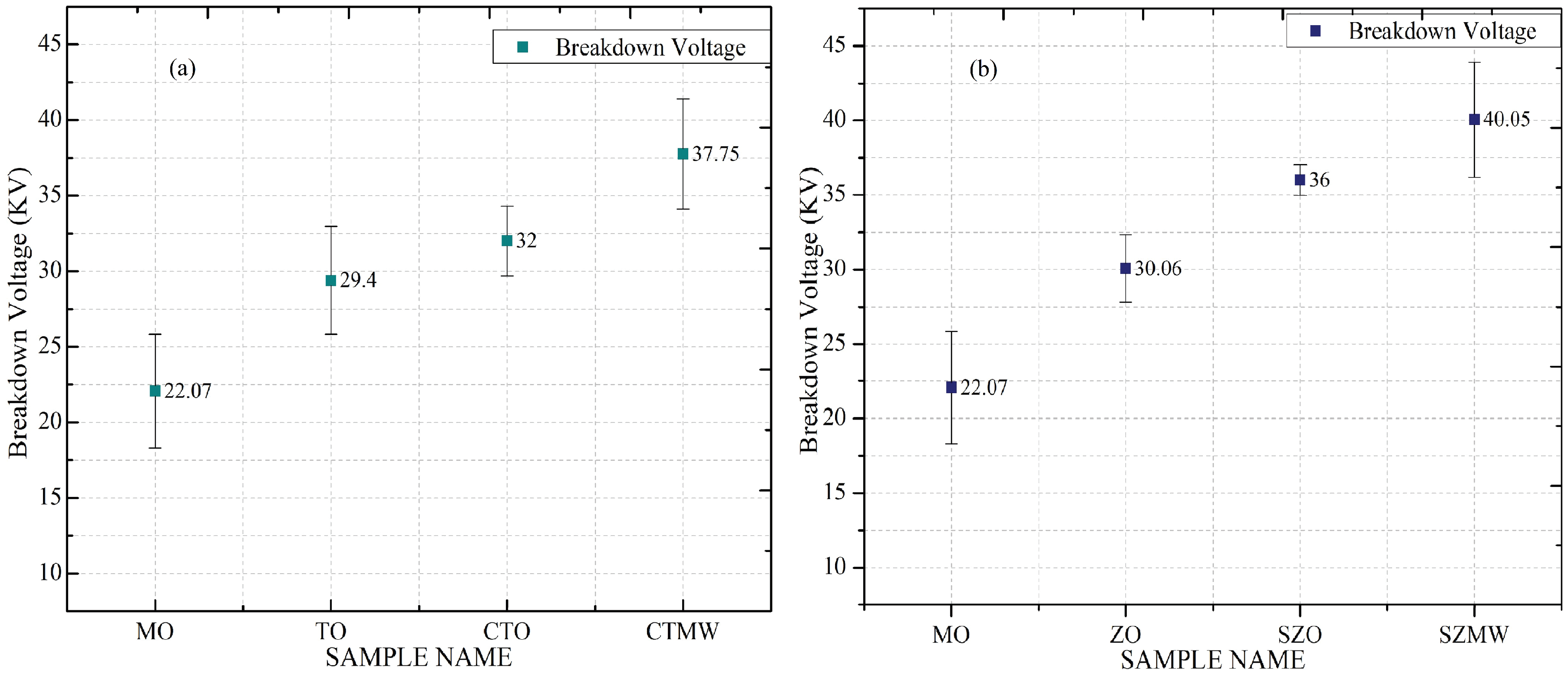
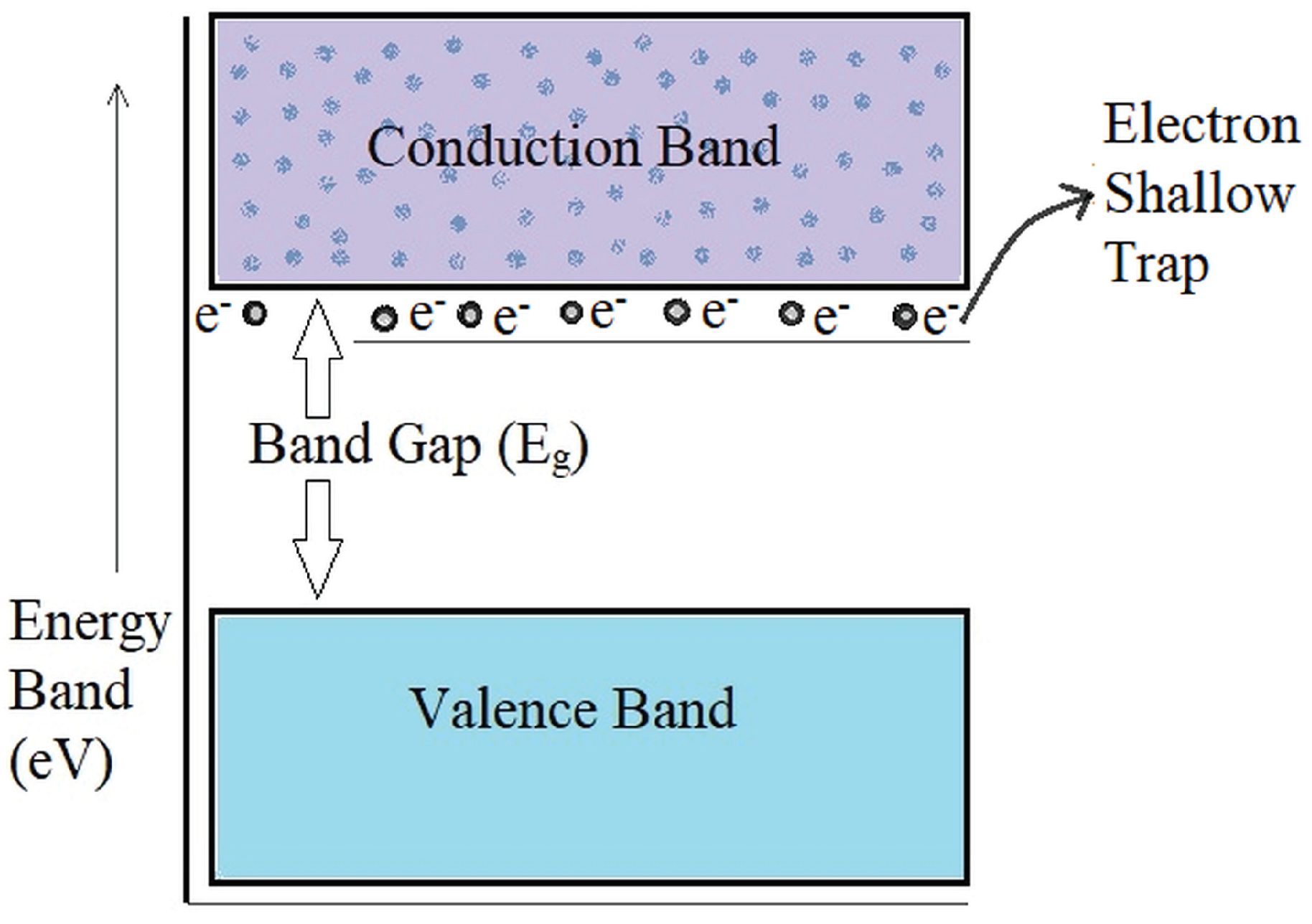
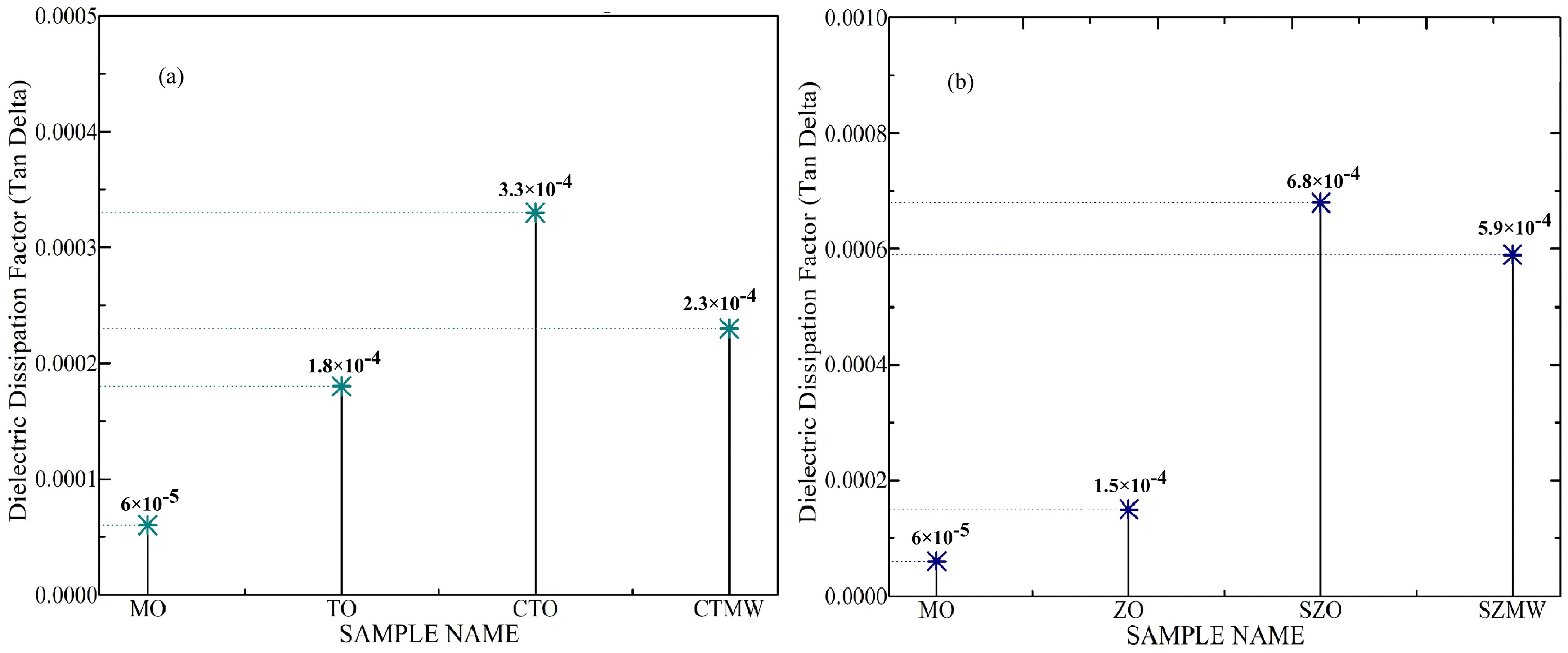
Dielectric and Thermal Studies
6. Conclusions
- The dispersing quantity of ZnO nanoparticles and surfactant Span80 for ZNF preparation are optimized systematically based on BDV enhancement. MO dispersed with 0.04 g/L of ZnO nanoparticles and 4 µL/L of surfactant Span80 provided 63.12% of BDV enhancement compared to mineral oil and is considered an optimal concentration from this study.
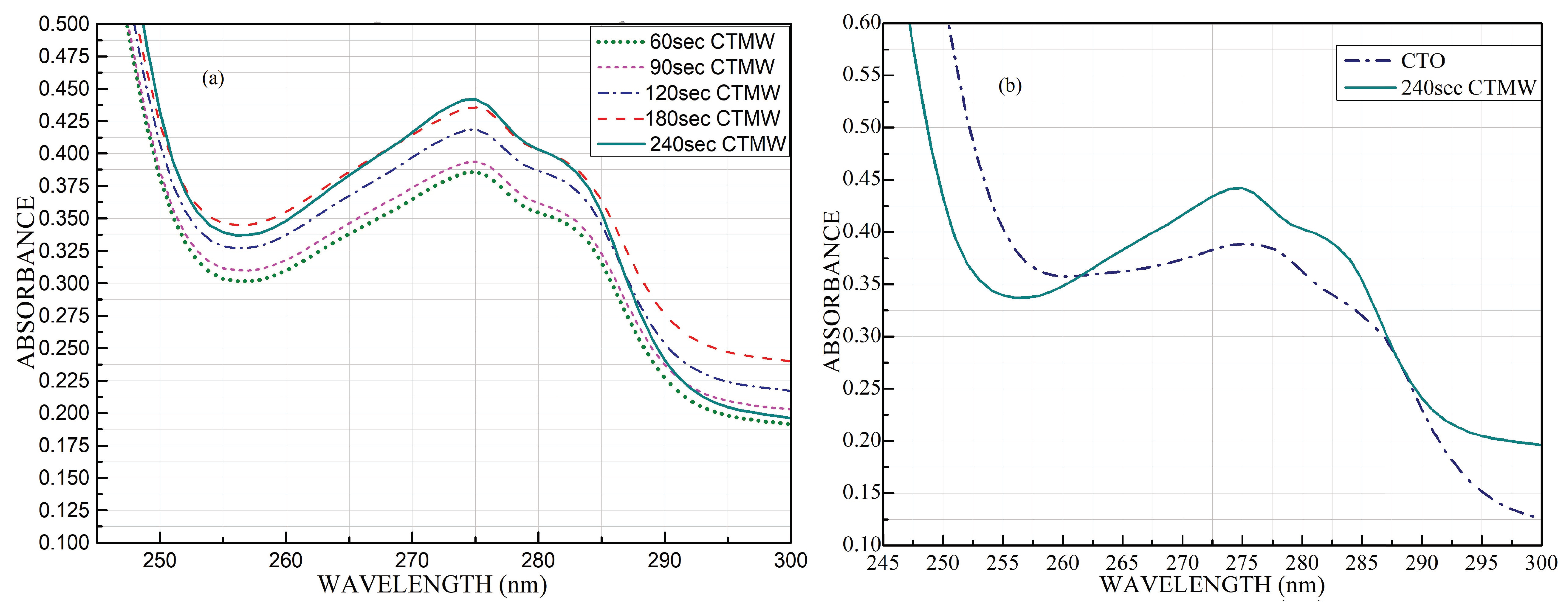
- A dispersion study was conducted for the CTMW sample to verify and fix the microwave irradiation time on the nanofluids. From the higher absorption peak of the UV-Visible absorption spectra of five CTMW samples with different irradiation times, the 240-s sample was inferred as providing a better dispersion; therefore, this measurement was fixed as the optimal irradiation time.
- Dielectric and thermal properties were investigated for the SNFs with and without applying microwaves. TNF and ZNF irradiated with microwaves for 240 s improved their BDV by 16.78% and 11.25%, respectively, which is attributed to their improved dielectric permittivity. Permittivity enhancement is explained in terms of polarization phenomena induced by the microwaves interacting with the dielectric medium.
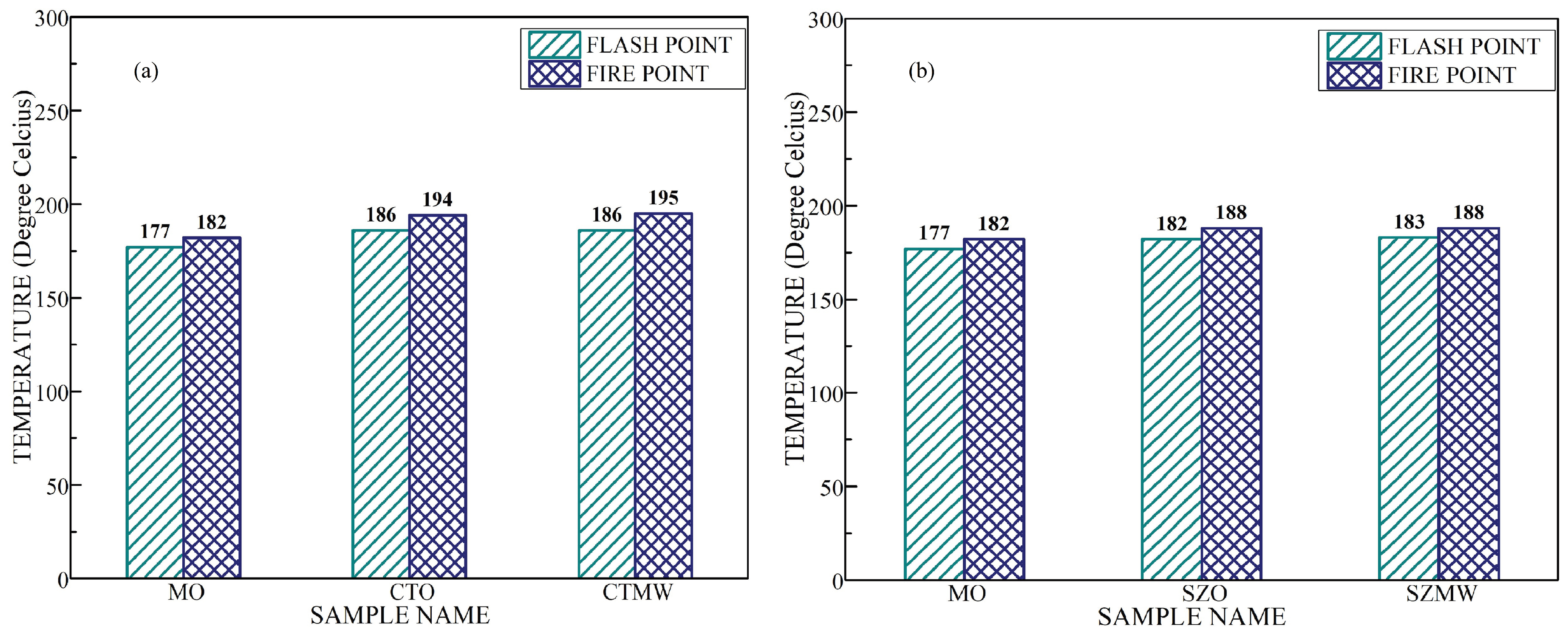
- From the conducted experiments, the flash point and fire point temperature values of the TNF and ZNF did not show any modification with the irradiation of microwaves.
- Increased tan delta values of the SNF with the surfactant addition were slightly reduced with the application of microwaves. However, the modification in tan delta values requires a clearer study owing to the inconsistency in the reported results from various research studies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab.(ANL): Argonne, IL, USA, 1995. [Google Scholar]
- Taghizadeh-Tabari, Z.; Zeinali Heris, S.; Moradi, M.; Kahani, M. The study on application of TiO2/water nanofluid in plate heat exchanger of milk pasteurization industries. Renew. Sustain. Energy Rev. 2016, 58, 1318–1326. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Yao, W.; Wang, F.; Wan, F.; Tan, Y.; Mehmood, M.A. Electrical and thermal properties of insulating oil-based nanofluids: A comprehensive overview. IET Nanodielectr. 2019, 2, 27–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Saleh, R.; Putra, N.; Wibowo, R.E.; Septiadi, W.N.; Prakoso, S.P. Titanium dioxide nanofluids for heat transfer applications. Exp. Therm. Fluid Sci. 2014, 52, 19–29. [Google Scholar] [CrossRef]
- Yang, L.; Du, K.; Niu, X.; Li, Y.; Zhang, Y. An experimental and theoretical study of the influence of surfactant on the preparation and stability of ammonia-water nanofluids. Int. J. Refrig. 2011, 34, 1741–1748. [Google Scholar] [CrossRef]
- Rafiq, M.; Shafique, M.; Azam, A.; Ateeq, M. Transformer oil-based nanofluid: The application of nanomaterials on thermal, electrical and physicochemical properties of liquid insulation-A review. Ain Shams Eng. J. 2021, 12, 555–576. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An overview on the effect of ultrasonication duration on different properties of nanofluids. J. Therm. Anal. Calorim. 2019, 135, 393–418. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Wei, B.; Zou, C.; Li, X. Experimental investigation on stability and thermal conductivity of diathermic oil based TiO2 nanofluids. Int. J. Heat Mass Transf. 2017, 104, 537–543. [Google Scholar] [CrossRef]
- Lv, Y.; Rafiq, M.; Li, C.; Shan, B. Study of Dielectric Breakdown Performance of Transformer Oil Based Magnetic Nanofluids. Energies 2017, 10, 1025. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Chakrabarty, S.; Chaudhuri, P. Effects of Sonication Period on Colloidal Stability and Thermal Conductivity of SiO2–Water Nanofluid: An Experimental Investigation. J. Clust. Sci. 2022, 33, 1763–1771. [Google Scholar] [CrossRef]
- Lv, Y.z.; Li, C.; Sun, Q.; Huang, M.; Li, C.R.; Qi, B. Effect of Dispersion Method on Stability and Dielectric Strength of Transformer Oil-Based TiO2 Nanofluids. Nanoscale Res. Lett. 2016, 11, 515. [Google Scholar] [CrossRef]
- Raja, S.; Koperundevi, G. Titania-based transformer nanofluid: A study on the synthesis for enhanced breakdown strength and its humidity ageing. IET Nanodielectr. 2020, 3, 138–146. [Google Scholar] [CrossRef]
- Moumen, A.; Halim, W.; Jaffal, S.; Abderrafi, K.; Eddahbi, A.; Sebti, S.; Ouaskit, S. Microwave Assisted Synthesis of Palladium Nanoparticles in an Aqueous Emulsion of Copolymer: Application to Catalysis. J. Clust. Sci. 2017, 28, 2817–2832. [Google Scholar] [CrossRef]
- Saloga, P.E.J.; Kästner, C.; Thünemann, A.F. High-Speed but Not Magic: Microwave-Assisted Synthesis of Ultra-Small Silver Nanoparticles. Langmuir 2018, 34, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Riahi-Madvar, S.; Ameri, A.; Amiri-Moghadam, P.; Adeli-Sardou, M.; Forootanfar, H. Microwave Assisted Biosynthesis of Cadmium Nanoparticles: Characterization, Antioxidant and Cytotoxicity Studies. J. Clust. Sci. 2022, 33, 1877–1887. [Google Scholar] [CrossRef]
- Phalswal, P.; Khanna, P. Novel one-pot microwave assisted synthesis of MoO2 nanoparticles. Mater. Lett. 2021, 302, 130445. [Google Scholar] [CrossRef]
- Barani, H.; Mahltig, B. Microwave-Assisted Synthesis of Silver Nanoparticles: Effect of Reaction Temperature and Precursor Concentration on Fluorescent Property. J. Clust. Sci. 2022, 33, 101–111. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Pillai, S.K.; Sinha Ray, S.; Jalama, K.; Krause, R.W.M. Recent Trends in the Microwave-Assisted Synthesis of Metal Oxide Nanoparticles Supported on Carbon Nanotubes and Their Applications. J. Nanomater. 2012, 2012, 691503. [Google Scholar] [CrossRef]
- Tsuji, M. Microwave-Assisted Synthesis of Metallic Nanomaterials in Liquid Phase. ChemistrySelect 2017, 2, 805–819. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef] [PubMed]
- Mayandi, J.; Atchudan, R.; Jayabal, P.; Sasirekha, V.; Pearce, J. Effect of microwave power irradiation on TiO2 nano-structures and binder free paste screen printed dye sensitized solar cells. Ceram. Int. 2018, 45. [Google Scholar] [CrossRef]
- Sreeju., N.; Rufus, A.; Philip, D. Microwave-assisted rapid synthesis of copper nanoparticles with exceptional stability and their multifaceted applications. J. Mol. Liq. 2016, 221, 1008–1021. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Chapter 15—Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Advances in Pharmaceutical Product Development and Research; Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Stuerga, D. Microwave-Material Interactions and Dielectric Properties, Key Ingredients for Mastery of Chemical Microwave Processes. In Microwaves in Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Chapter 1; pp. 1–61. [Google Scholar] [CrossRef]
- Ulloa, R.Z.; Santiago, M.G.H.; Rueda, V.L.V. The Interaction of Microwaves with Materials of Different Properties. In Electromagnetic Fields and Waves; Yeap, K.H., Hirasawa, K., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 6. [Google Scholar] [CrossRef]
- Katiyar, A.; Dhar, P.; Nandi, T.; Das, S.K. Effects of nanostructure permittivity and dimensions on the increased dielectric strength of nano insulating oils. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 235–243. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
- Zhou, T.c.; Chen, G.; Liao, R.j.; Xu, Z. Charge trapping and detrapping in polymeric materials: Trapping parameters. J. Appl. Phys. 2011, 110, 043724. [Google Scholar] [CrossRef]
- Farade, R.A.; Wahab, N.I.B.A.; Mansour, D.E.A.; Azis, N.B.; Jasni, J.; Banapurmath, N.R.; Soudagar, M.E.M. Investigation of the Dielectric and Thermal Properties of Non-Edible Cottonseed Oil by Infusing h-BN Nanoparticles. IEEE Access 2020, 8, 76204–76217. [Google Scholar] [CrossRef]
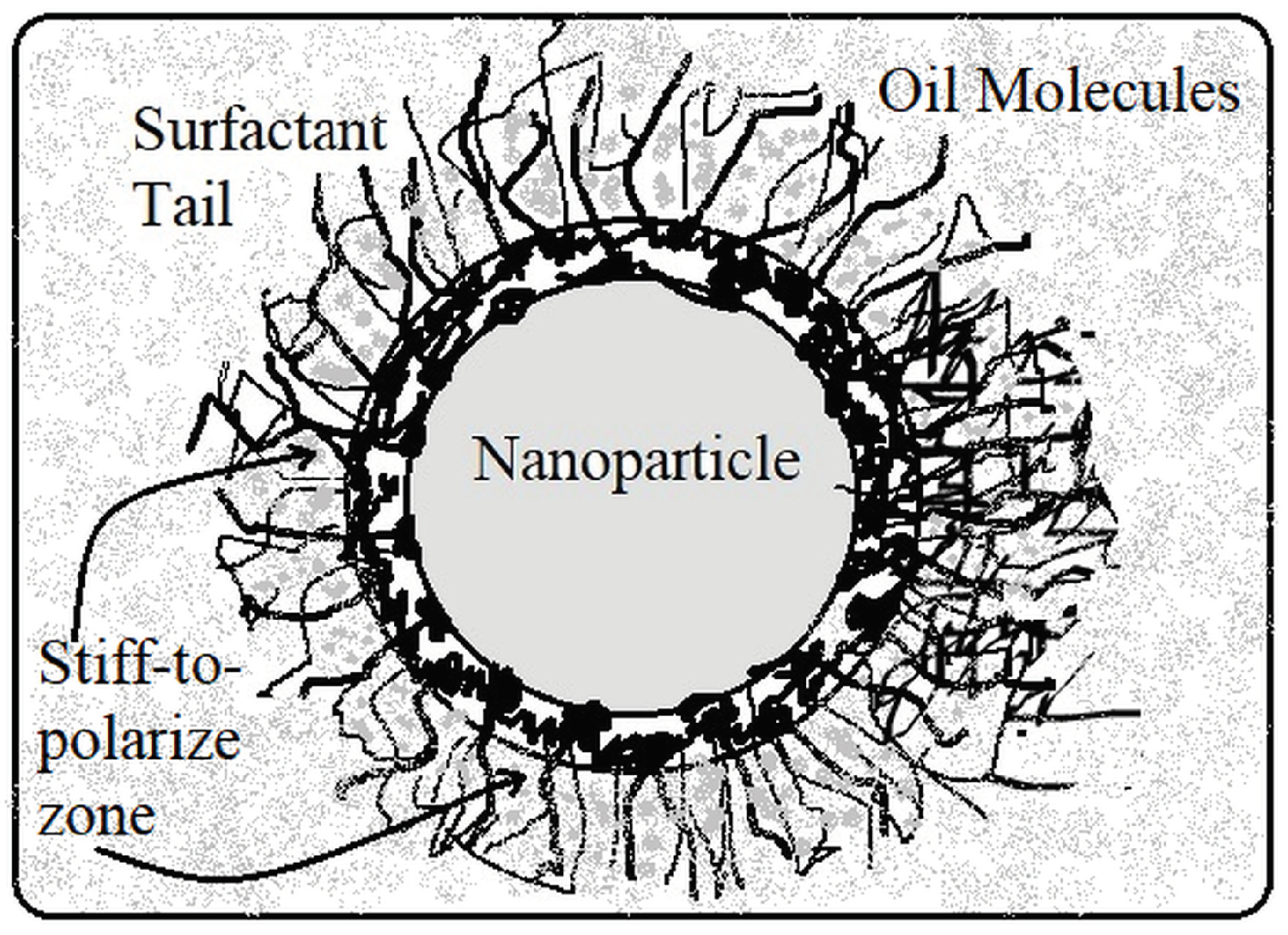
- Mansour, D.E.A.; Elsaeed, A.M.; Izzularab, M.A. The role of interfacial zone in dielectric properties of transformer oil-based nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3364–3372. [Google Scholar] [CrossRef]
- Alafogianni, P.; Dassios, K.; Farmaki, S.; Antiohos, S.; Matikas, T.; Barkoula, N.M. On the efficiency of UV–vis spectroscopy in assessing the dispersion quality in sonicated aqueous suspensions of carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 118–124. [Google Scholar] [CrossRef]
- Dong, M.; Dai, J.; Li, Y.; Xie, J.; Ren, M.; Dang, Z. Insight into the dielectric response of transformer oil-based nanofluids. AIP Adv. 2017, 7, 025307. [Google Scholar] [CrossRef]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q.; Huang, S.; Cao, X. Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: Experiment and theory. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 380–390. [Google Scholar] [CrossRef]
- Primo, V.A.; García, B.; Burgos, J.C.; Pérez, D. AC breakdown voltage of Fe3O4 based nanodielectric fluids. Part 1: Analysis of dry fluids. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 352–359. [Google Scholar] [CrossRef]
- Farade, R.A.; Wahab, N.I.A.; Mansour, D.E.A.; Azis, N.B.; Jasni, J.B.; Veerasamy, V.; Vinayagam, A.; Kotiyal, B.M.; Khan, T.M.Y. The Effect of Interfacial Zone Due to Nanoparticle–Surfactant Interaction on Dielectric Properties of Vegetable Oil Based Nanofluids. IEEE Access 2021, 9, 107033–107045. [Google Scholar] [CrossRef]
- Mahidhar, G.D.P.; Sarathi, R.; Taylor, N.; Edin, H. Dielectric properties of silica based synthetic ester nanofluid. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1508–1515. [Google Scholar] [CrossRef]
- Amalanathan, A.J.; Sarathi, R.; Harid, N.; Griffiths, H. Investigation on flow electrification of ester-based TiO2 nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1492–1500. [Google Scholar] [CrossRef]
- Abid, M.A.; Khan, I.; Ullah, Z.; Ullah, K.; Haider, A.; Ali, S.M. Dielectric and Thermal Performance Up-Gradation of Transformer Oil Using Valuable Nano-Particles. IEEE Access 2019, 7, 153509–153518. [Google Scholar] [CrossRef]
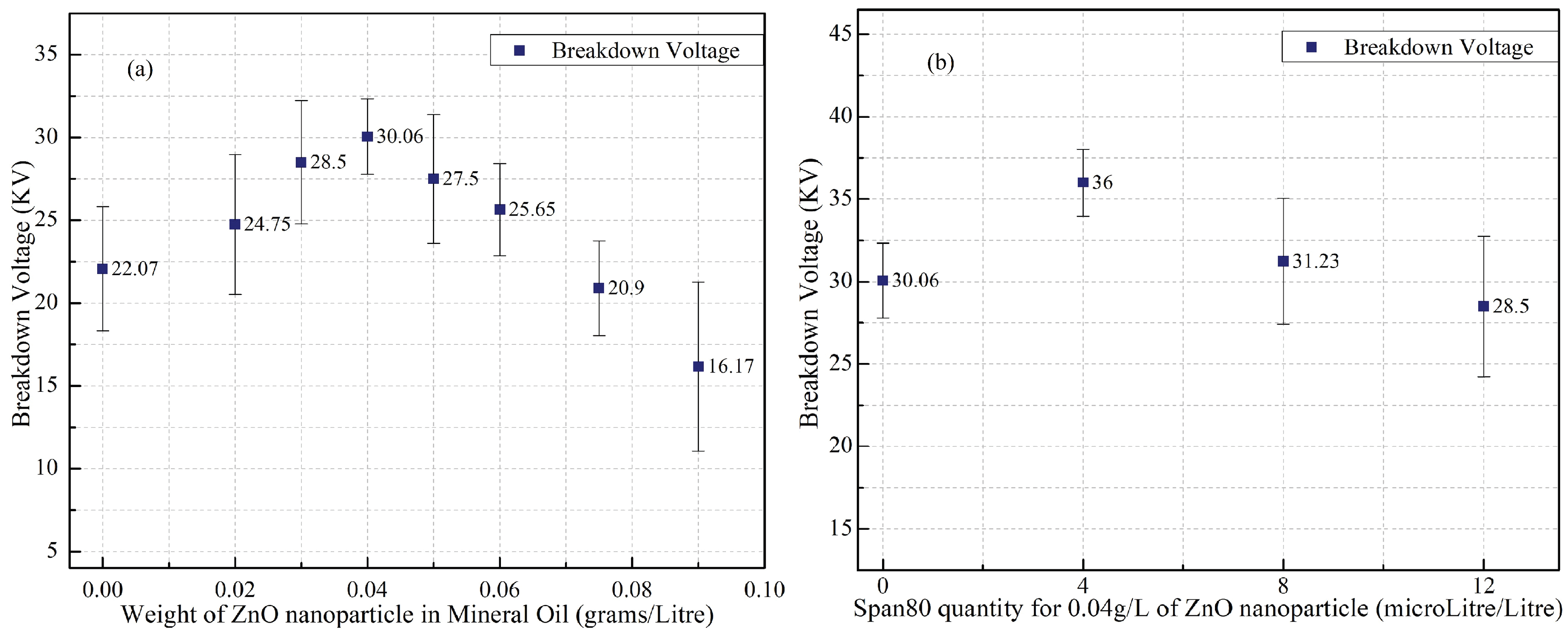

| S. No. | Quantity of ZnO Nanoparticles Dispersed in Mineral Oil (g/L) | Mean AC BDV with Standard Deviation (KV) | BDV Enhancement (in %) |
|---|---|---|---|
| 1 | 0 | 22.07 ± 3.75 | 0 |
| 2 | 0.02 | 24.75 ± 4.23 | 12.14 |
| 3 | 0.03 | 28.5 ± 3.72 | 29.13 |
| 4 | 0.04 | 30.06 ± 2.28 | 36.20 |
| 5 | 0.05 | 27.5 ± 3.89 | 24.60 |
| 6 | 0.06 | 25.65 ± 2.78 | 16.22 |
| 7 | 0.075 | 20.9 ± 2.86 | −5.3 |
| 8 | 0.09 | 16.17 ± 5.1 | −26.73 |
| S. No. | Quantity of Surfactant Span80 with 0.04 g/L ZnO Nanoparticles Dispersed in Mineral Oil (µL/L) | Mean AC BDV with Standard Deviation (KV) | BDV Enhancement (in %) |
|---|---|---|---|
| 1 | 0 µL/L | 30.06 ± 2.28 | 0 |
| 2 | 4 µL/L | 36 ± 2.03 | 19.76 |
| 3 | 8 µL/L | 31.23 ± 3.83 | 3.89 |
| 4 | 12 µL/L | 28.5 ± 4.26 | −6.99 |
| Name of Sample | Sample Type |
|---|---|
| MO | Virgin mineral oil |
| TO | Virgin mineral oil + TiO2 nanoparticles |
| CTO | Virgin mineral oil + surfactant CTAB + TiO2 nanoparticles |
| CTMW | Microwave-irradiated CTO |
| ZO | Virgin mineral oil + ZnO nanoparticles |
| SZO | Virgin mineral oil + Span80 + ZnO nanoparticles |
| SZMW | Microwave-irradiated SZO |
| Name of Sample | Dielectric Properties | Thermal Property | ||||
|---|---|---|---|---|---|---|
| Breakdown Voltage (kV) | Relative Permittivity | Tan Delta | Flash Point (°C) | Fire Point (°C) | ||
| Mean | SD | |||||
| MO | 22.07 | 3.75 | 2.034 | 0.00006 | 177 | 182 |
| TO | 29.4 | 3.57 | 2.04 | 0.00018 | – | – |
| CTO | 32 | 2.3 | 2.044 | 0.00033 | 186 | 194 |
| CTMW | 37.75 | 3.65 | 2.048 | 0.00023 | 186 | 195 |
| ZO | 30.06 | 2.27 | 2.039 | 0.00015 | – | – |
| SZO | 36 | 1.03 | 2.042 | 0.00068 | 182 | 188 |
| SZMW | 40.05 | 3.85 | 2.046 | 0.00059 | 182 | 188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raja, S.; Koperundevi, G.; Eswaran, M. Effect of Microwave Irradiation on the Dielectric Characteristics of Semi-Conductive Nanoparticle-Based Nanofluids: Progress towards the Microwave Synthesis. Micromachines 2023, 14, 1194. https://doi.org/10.3390/mi14061194
Raja S, Koperundevi G, Eswaran M. Effect of Microwave Irradiation on the Dielectric Characteristics of Semi-Conductive Nanoparticle-Based Nanofluids: Progress towards the Microwave Synthesis. Micromachines. 2023; 14(6):1194. https://doi.org/10.3390/mi14061194
Chicago/Turabian StyleRaja, S., G. Koperundevi, and Muthusankar Eswaran. 2023. "Effect of Microwave Irradiation on the Dielectric Characteristics of Semi-Conductive Nanoparticle-Based Nanofluids: Progress towards the Microwave Synthesis" Micromachines 14, no. 6: 1194. https://doi.org/10.3390/mi14061194






