A Bi-Directional Acoustic Micropump Driven by Oscillating Sharp-Edge Structures
Abstract
:1. Introduction
2. Design and Working Mechanism
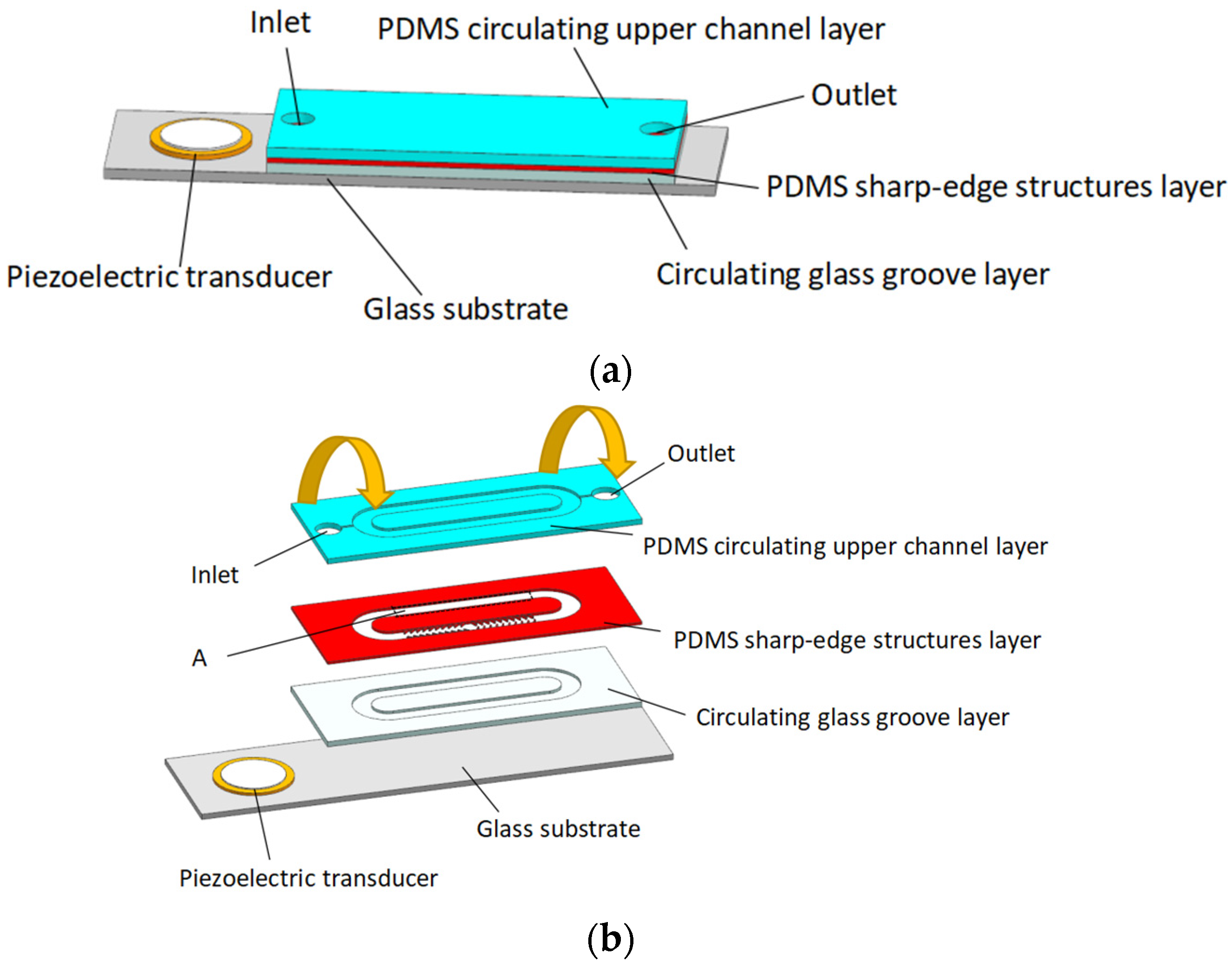
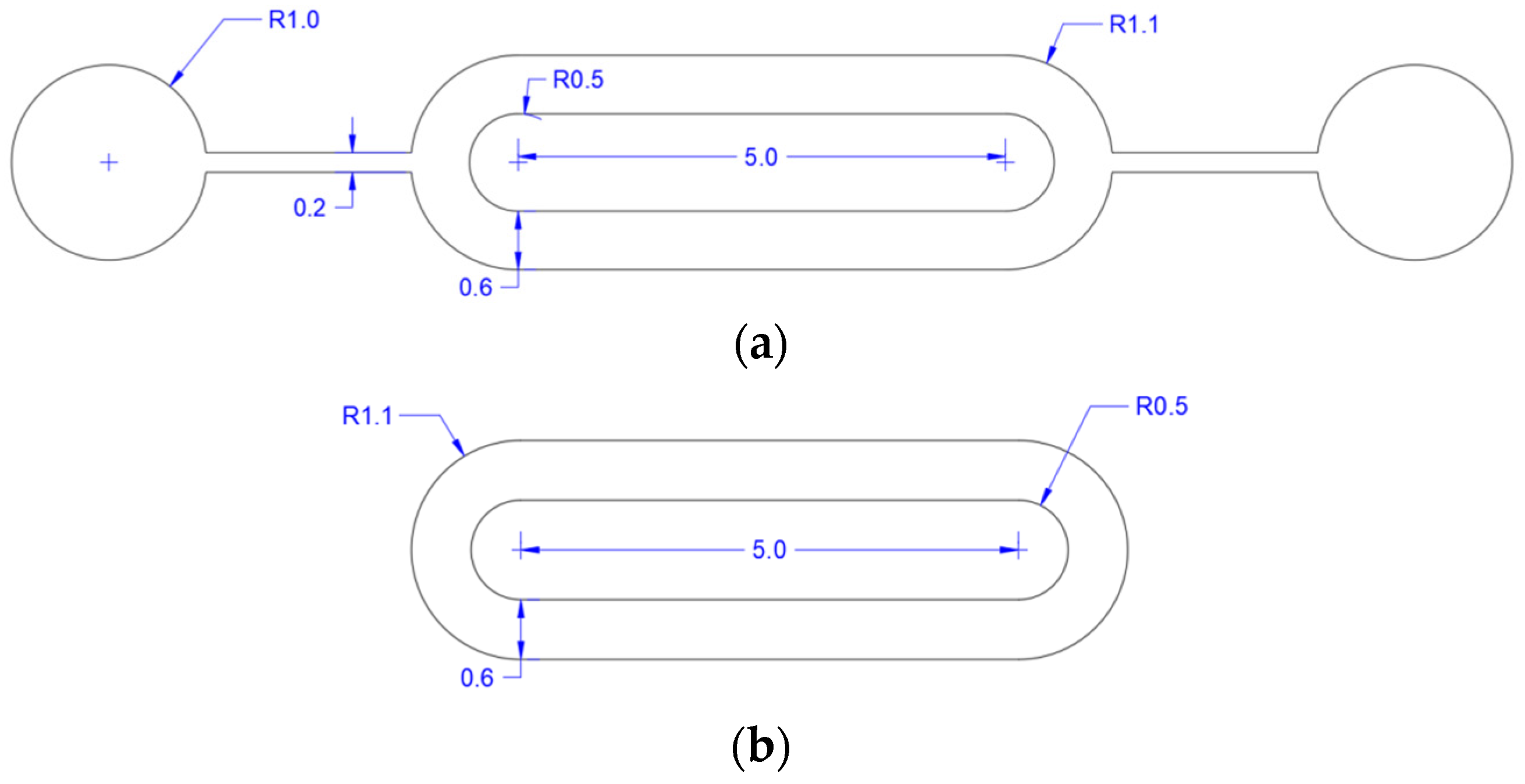
2.1. Design of Acoustic Micropump
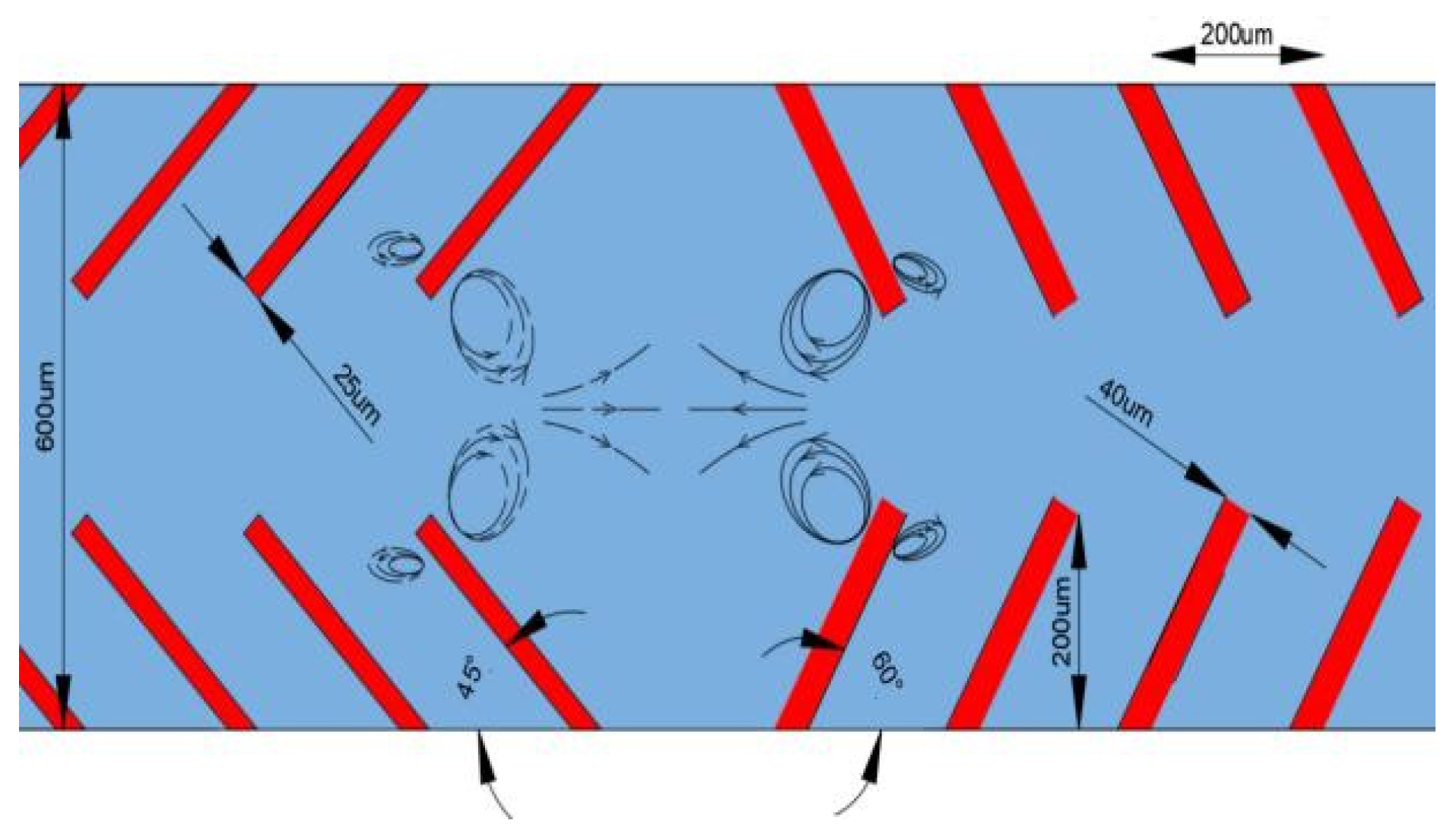
2.2. Working Mechanism of Acoustic Micropump
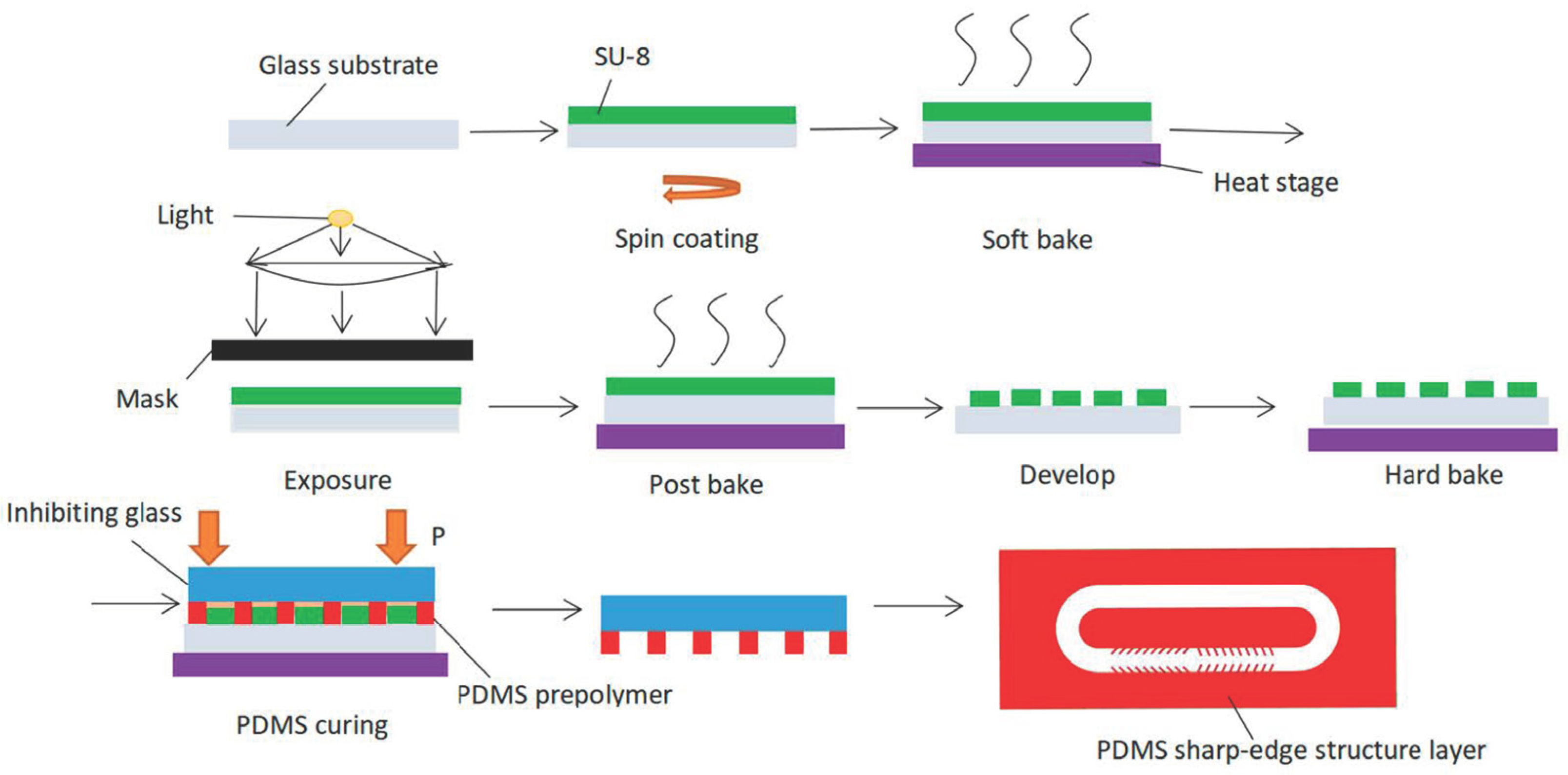
3. Fabrication Process of Acoustic Micropump
4. Results and Discussion
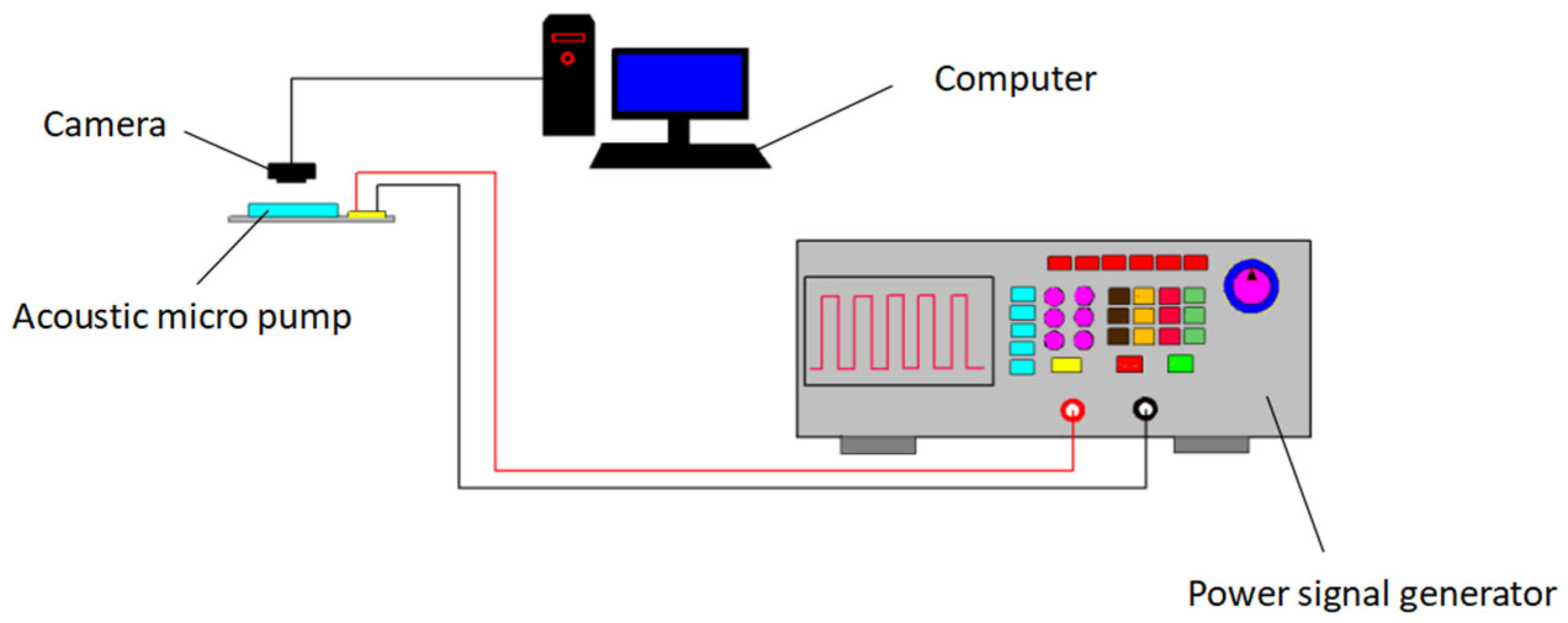
4.1. Composition of Experimental System
4.2. Working Frequency of Acoustic Micropump
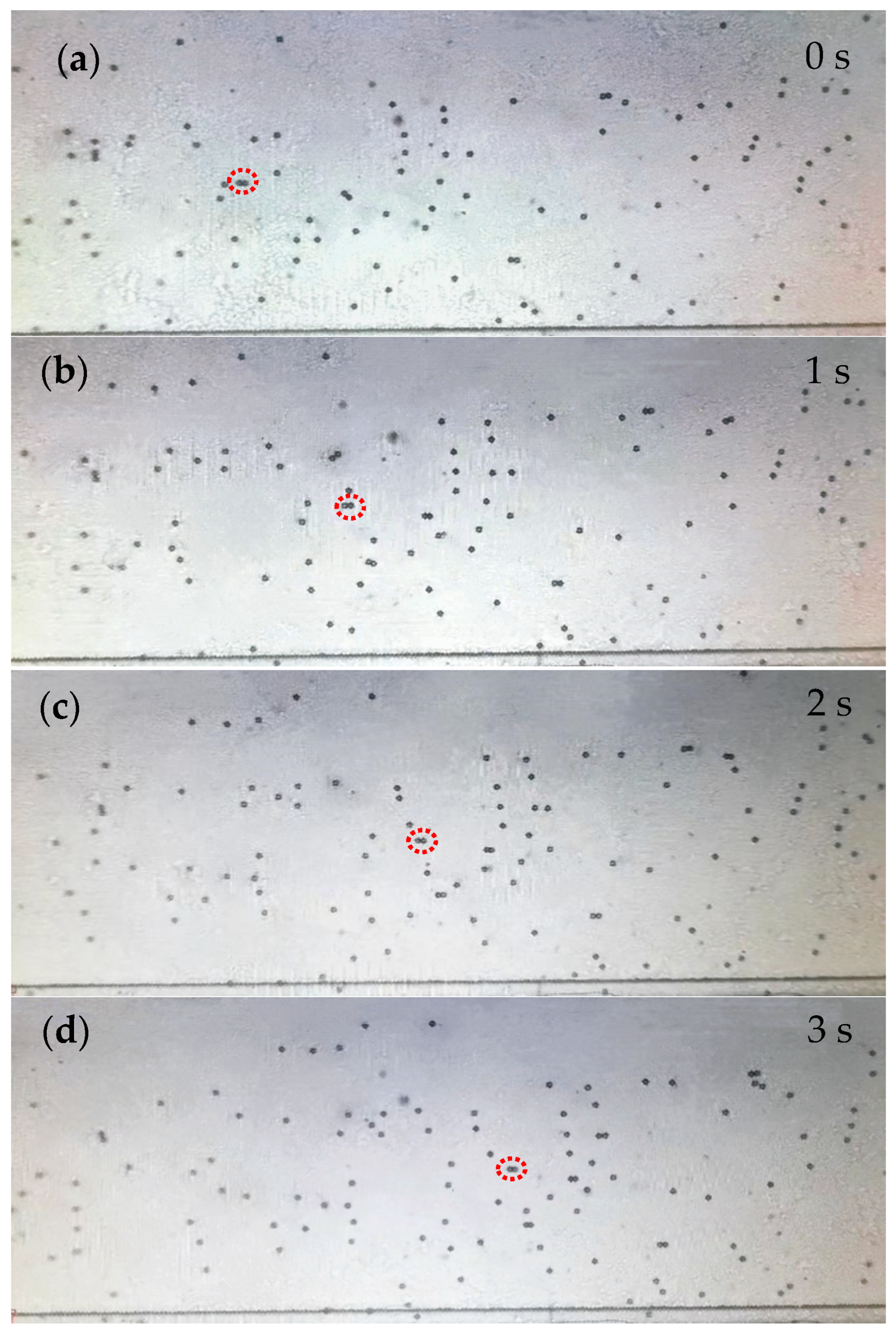
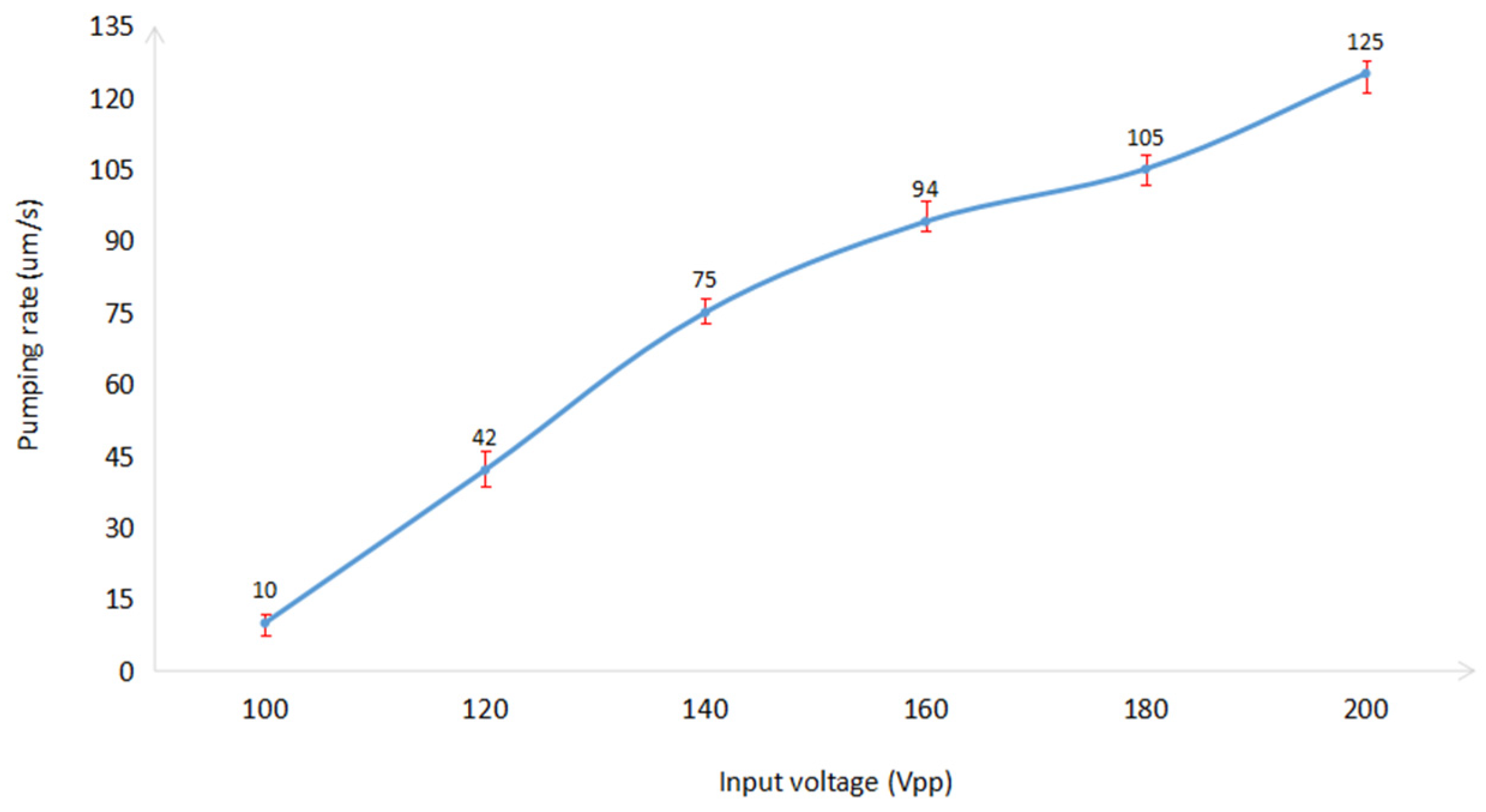
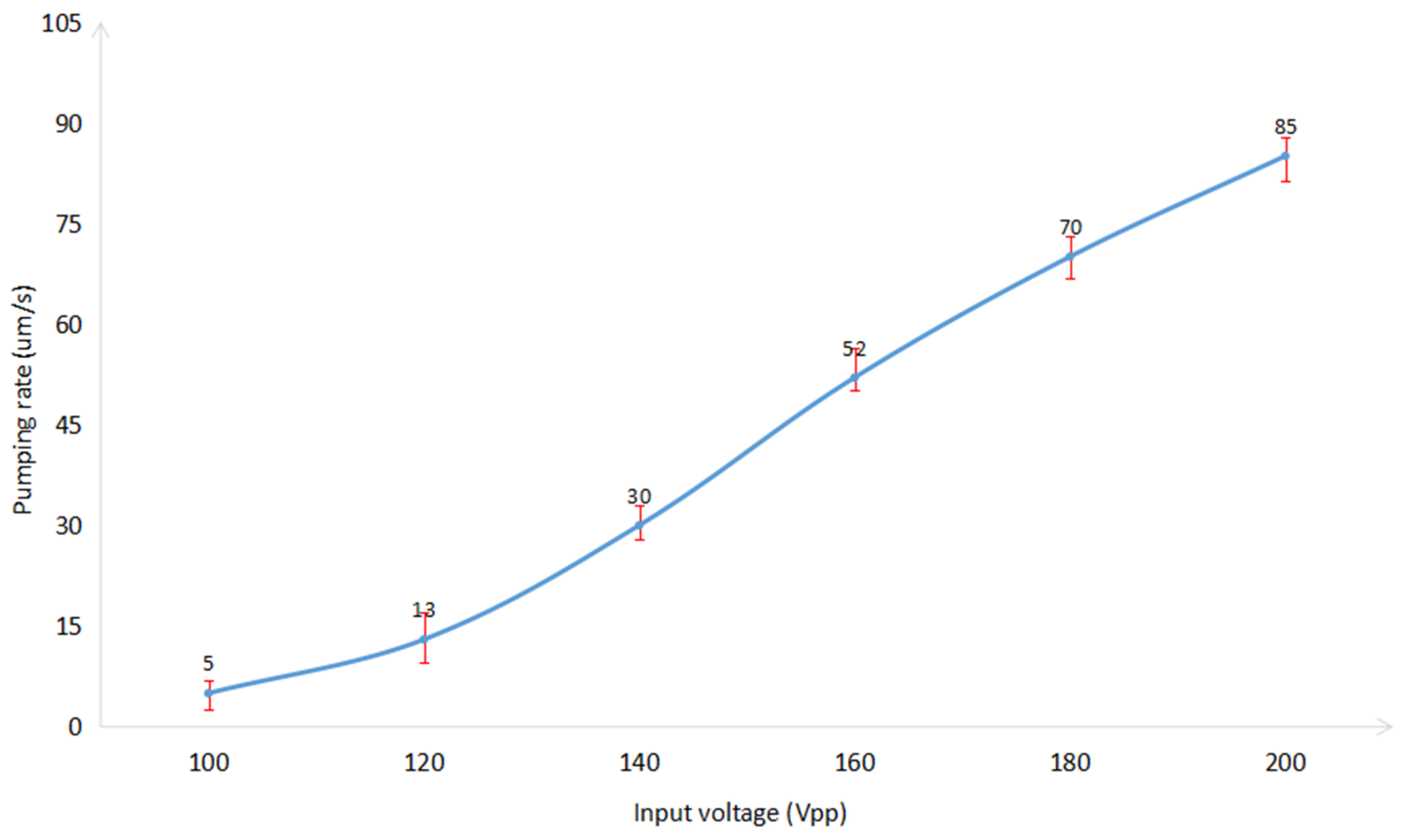
4.3. Pumping Rate of Acoustic Micropump
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.W.; Wang, J.H. The status and application of MEMS technology. Micronanoelectron. Technol. 2023, 40, 29–32. [Google Scholar]
- Gad-el-Hak, M. The fluid mechanics of microdevices—The Freeman scholar lecture. J. Fluids Eng. 1999, 121, 5–33. [Google Scholar] [CrossRef]
- Ho, C.M.; Tai, Y.C. Micro-electro-mechanical-systems (MEMS) and fluid flows. Annu. Rev. Fluid Mech. 1998, 30, 579–612. [Google Scholar] [CrossRef] [Green Version]
- Iverson, B.D.; Garimella, S.V. Recent advances in microscale pumping technologies: A review and evaluation. Microfluid. Nanofluid. 2008, 5, 145–174. [Google Scholar] [CrossRef] [Green Version]
- Au, A.K.; Lai, H.; Utela, B.R.; Folch, A. Microvalves and micropumps for BioMEMS. Micromachines 2011, 2, 179–220. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.; Folch, A. Design and dynamic characterization of “single-stroke” peristaltic PDMS micropumps. Lab Chip 2011, 11, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.; Mushfique, H.; Di Leonardo, R.; Padgett, M.; Cooper, J. An optically driven pump for microfluidics. Lab Chip 2006, 6, 735–739. [Google Scholar] [CrossRef]
- Maruo, S.; Inoue, H. Optically driven viscous micropump using a rotating microdisk. Appl. Phys. Lett. 2007, 91, 084101. [Google Scholar] [CrossRef]
- Badran, M. Modeling and Simulation of a Low Voltage Electroosmotic Micropump for Non-Newtonian Fluids. In Proceedings of the 2021 22nd International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), St. Julian, Malta, 19–21 April 2021. [Google Scholar]
- Sun, J.; Zhang, L.; Li, Z.; Wang, Z.L. A Mobile and Self-Powered Micro-Flow Pump Based on Triboelectricity Driven Electroosmosis. Adv. Mater. 2021, 33, 2102765. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, W.; Ren, Y. A high-throughput electrokinetic micromixer via AC field-effect nonlinear electroosmosis control in 3D electrode configurations. Micromachines 2018, 9, 432. [Google Scholar] [CrossRef]
- Nan, K.; Hu, Z.; Zhao, W.; Wang, K.; Bai, J.; Wang, G. Large-scale flow in micro electrokinetic turbulent mixer. Micromachines 2020, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Vanleeuwen, G.; Coburn, M. Magnetic-Drive Positive-Displacement Pumps for Chemical Processing Applications. Chem. Eng. 2022, 8, 38–41. [Google Scholar]
- Fan, S.K.; Chen, W.J.; Lin, T.H.; Wang, T.T.; Lin, Y.C. Reconfigurable liquid pumping in electric-field-defined virtual microchannels by dielectrophoresis. Lab Chip 2009, 9, 1590–1595. [Google Scholar] [CrossRef]
- You, Q.Y.; Wang, Y.; Zhang, Z. Laser-Driven Optothermal MicroactuatorOperated in the Water. Appl. Opt. 2020, 59, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Malouin, B.A.; Vogel, M.J.; Olles, J.D.; Cheng, L.; Hirsa, A.H. Electromagnetic liquid pistons for capillarity-based pumping. Lab Chip 2011, 11, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, T.H.; Chiou, P.Y. Pulsed laser triggered high speed microfluidic fluorescence activated cell sorter. Lab Chip 2012, 12, 1378–1383. [Google Scholar]
- Tseng, H.Y.; Wang, C.H.; Lin, W.Y.; Lee, G.B. Membrane-activated microfluidic rotary devices for pumping and mixing. Biomed. Microdevices 2007, 9, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Hsiung, S.K.; Lee, G.B. A pneumatic micropump incorporated with a normally closed valve capable of generating a high pumping rate and a high back pressure. Microfluid. Nanofluid. 2008, 6, 823–833. [Google Scholar] [CrossRef]
- Chen, H.; Cornwell, J.; Zhang, H.; Lim, T.; Resurreccion, R.; Port, T.; Rosengarten, G.; Nordon, R.E. Cardiac-like flow generator for long-term imaging of endothelial cell responses to circulatory pulsatile flow at microscale. Lab Chip 2013, 13, 2999–3007. [Google Scholar] [CrossRef]
- Park, J.; Kim, I.C.; Baek, J.; Cha, M.; Kim, J.; Park, S.; Lee, J.; Kim, B. Micro pumping with cardiomyocyte–polymer hybrid. Lab Chip 2007, 7, 1367–1370. [Google Scholar] [CrossRef]
- Andersson, H.; Van Der Wijngaart, W.; Nilsson, P.; Enoksson, P.; Stemme, G. A valve-less diffuser micropump for microfluidic analytical systems. Sens. Actuators B Chem. 2001, 72, 259–265. [Google Scholar] [CrossRef]
- Zahra, G.K.; Bayareh, M.; Kalantar, V. A review on acoustic field-driven micromixers. Int. J. Chem. React. Eng. 2021, 19, 553–569. [Google Scholar]
- Bruus, H.; Dual, J.; Hawkes, J.; Hill, M.; Laurell, T.; Nilsson, J.; Radel, S.; Sadhal, S.; Wiklund, M. Forthcoming Lab on a Chip tutorial series on acoustofluidics: Acoustofluidics-exploiting ultrasonic standing wave forces and acoustic streaming in microfluidic systems for cell and particle manipulation. Lab Chip 2011, 11, 3579–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.C.; Yoo, P.B.; Garg, N.; Lee, A.P.; Rasheed, S. A microfluidic device for blood plasma separation and fluorescence detection of biomarkers using acoustic microstreaming. Sens. Actuators A. Phys. 2021, 317, 112482. [Google Scholar] [CrossRef]
- Huang, P.H.; Xie, Y.; Ahmed, D.; Rufo, J.; Nama, N.; Chen, Y.; Chan, C.Y.; Huang, T.J. An acoustofluidic micromixer based on oscillating sidewall sharp-edges. Lab Chip 2013, 13, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Chan, C.Y.; Lin, S.C.S.; Muddana, H.S.; Nama, N.; Benkovic, S.J.; Huang, T.J. Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles. Lab Chip 2013, 13, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Ian Lapsley, M.; Ahmed, D.; Chen, Y.; Wang, L.; Huang, T.J. A single-layer, planar, optofluidic switch powered by acoustically driven, oscillating microbubbles. Appl. Phys. Lett. 2012, 101, 141101. [Google Scholar] [CrossRef] [Green Version]
- Destgeer, G.; Im, S.; Hang, H.B.; Jung, J.H.; Ahmad, A.M.; Jin, S.H. Adjustable, rapidly switching microfluidic gradient generation using focused travelling surface acoustic waves. Appl. Phys. Lett. 2014, 104, 023506. [Google Scholar] [CrossRef]
- Rogers, P.; Neild, A. Selective particle trapping using an oscillating microbubble. Lab Chip 2011, 11, 3710–3715. [Google Scholar] [CrossRef]
- Schmid, L.; Weitz, D.A.; Franke, T. Sorting drops and cells with acoustics: Acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 2014, 14, 3710–3718. [Google Scholar] [CrossRef]
- Destgeer, G.; Lee, K.H.; Jung, J.H.; Alazzam, A.; Sung, H.J. Continuous separation of particles in a PDMS microfluidic channel via travelling surface acoustic waves (TSAW). Lab Chip 2013, 13, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, P.; Lin, S.C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Huang, T.J. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Lin, S.C.S.; Mao, X.; Huang, T.J. Surface acoustic wave (SAW) acoustophoresis: Now and beyond. Lab Chip 2012, 12, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Schmid, L.; Wixforth, A.; Weitz, D.A.; Franke, T. Novel surface acoustic wave (SAW)-driven closed PDMS flow chamber. Microfluid. Nanofluid. 2012, 12, 229–235. [Google Scholar] [CrossRef]
- Hashmi, A.; Heiman, G.; Yu, G.; Lewis, M.; Kwon, H.J.; Xu, J. Oscillating bubbles in teardrop cavities for microflow control. Microfluid. Nanofluid. 2013, 14, 591–596. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Lin, Y.; Zhao, W.; Xu, J. Acoustic bubble-based bidirectional micropump. Microfluid. Nanofluid. 2020, 24, 29. [Google Scholar] [CrossRef]
- Huang, P.H.; Nama, N.; Mao, Z.M.; Li, P.; Rufo, J.; Chen, Y.C.; Xie, Y.L.; Wei, C.H.; Wang, L.; Huang, T.J. A reliable and programmable acoustofluidic pump powered by oscillating sharp-edge structures. Lab Chip 2014, 14, 4319–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, A.D.; Michael, S.G.; Alen, P.; Jürg, D. Acoustic streaming produced by sharp-edge structures in microfluidic devices. Microfluid. Nanofluid. 2020, 24, 32. [Google Scholar]
- Zhang, C.; Guo, X.; Brunet, P.; Costalonga, M.; Royon, L. Acoustic streaming near a sharp structure and its mixing performance characterization. Microfluid. Nanofluid. 2019, 23, 104. [Google Scholar] [CrossRef]
- Mohanty, S.; Ugo, S.D.C.; Miguel, S.; Sarthak, M. Bi-directional transportation of micro-agents induced by symmetry-broken acoustic streaming. AIP Adv. 2019, 9, 035352. [Google Scholar] [CrossRef] [Green Version]
- Leibacher, I.; Hahn, P.; Dual, J. Acoustophoretic cell and particle trapping on microfluidic sharp edges. Microfluid. Nanofluid. 2015, 19, 923–933. [Google Scholar] [CrossRef]
- Cao, Z.; Lu, C. A microfluidic device with integrated sonication and immunoprecipitation for sensitive epigenetic assays. Anal. Chem. 2016, 88, 1965–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcelik, A.; Nama, N.; Huang, P.H.; Kaynak, M.; McReynolds, M.R.; Hanna-Rose, W.; Huang, T.J. Acoustofluidic rotational manipulation of cells and organisms using oscillating solid structures. Small 2016, 12, 5120–5125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Huang, P.H.; Chen, C.; Bachman, H.; Zhao, S.; Yang, S.; Huang, T.J. Cell lysis via acoustically oscillating sharp edges. Lab Chip 2019, 19, 4021–4032. [Google Scholar] [CrossRef]
- Feng, L.; Song, B.; Chen, Y.; Liang, S.; Dai, Y.; Zhou, Q. On-chip rotational manipulation of microbeads and oocytes using acoustic microstreaming generated by oscillating asymmetrical microstructures. Biomicrofluidics 2019, 13, 064103. [Google Scholar] [CrossRef]
- Berthier, E.; Beebe, D.J. Gradient generation platforms: New directions for an established microfluidic technology. Lab Chip 2014, 14, 3241–3247. [Google Scholar] [CrossRef] [Green Version]
- Jowhar, D.; Wright, G.; Samson, P.C.; Wikswo, J.P.; Janetopoulos, C. Open access microfluidic device for the study of cell migration during chemotaxis. Integr. Biol. 2010, 2, 648–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.I.; Snedeker, J.G. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials 2010, 31, 7695–7704. [Google Scholar] [CrossRef]
- Yu, H.Q. Engineering Fluid Mechanics: Hydraulics; Southwest Jiaotong University Publishing: Chengdu, China, 2007. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Qiao, M.; Zhang, S.; Yang, J. A Bi-Directional Acoustic Micropump Driven by Oscillating Sharp-Edge Structures. Micromachines 2023, 14, 860. https://doi.org/10.3390/mi14040860
Liu B, Qiao M, Zhang S, Yang J. A Bi-Directional Acoustic Micropump Driven by Oscillating Sharp-Edge Structures. Micromachines. 2023; 14(4):860. https://doi.org/10.3390/mi14040860
Chicago/Turabian StyleLiu, Bendong, Meimei Qiao, Shaohua Zhang, and Jiahui Yang. 2023. "A Bi-Directional Acoustic Micropump Driven by Oscillating Sharp-Edge Structures" Micromachines 14, no. 4: 860. https://doi.org/10.3390/mi14040860



