Scalable Processing of Cyclic Olefin Copolymer (COC) Microfluidic Biochips †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microfluidic Structure Fabrication
2.1.1. Hard Mask Fabrication
2.1.2. SU-8 Mould Fabrication
2.1.3. PDMS Structure Fabrication
2.1.4. Epoxy Mould Fabrication
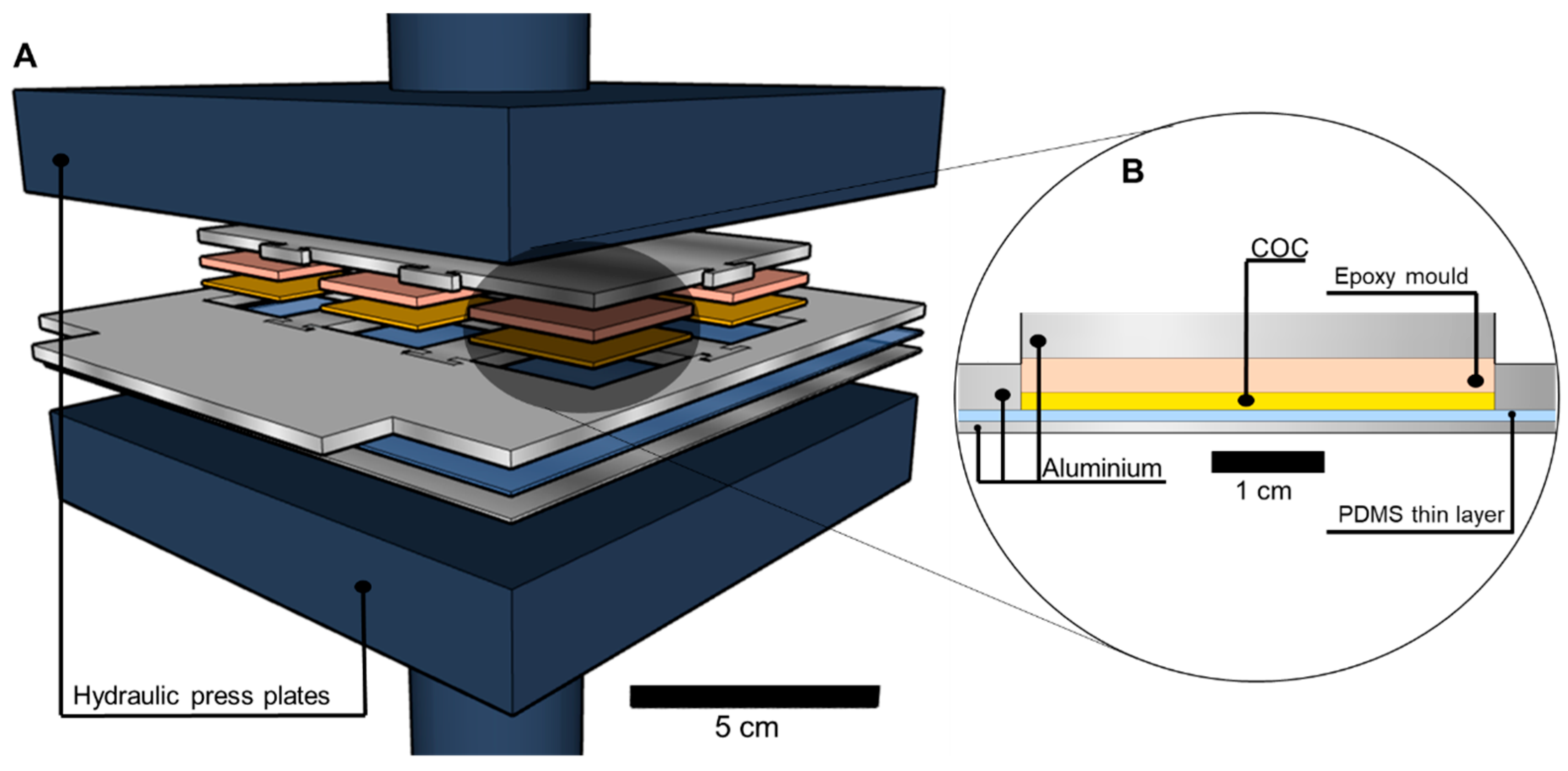
2.1.5. Embossing and De-Embossing of COC
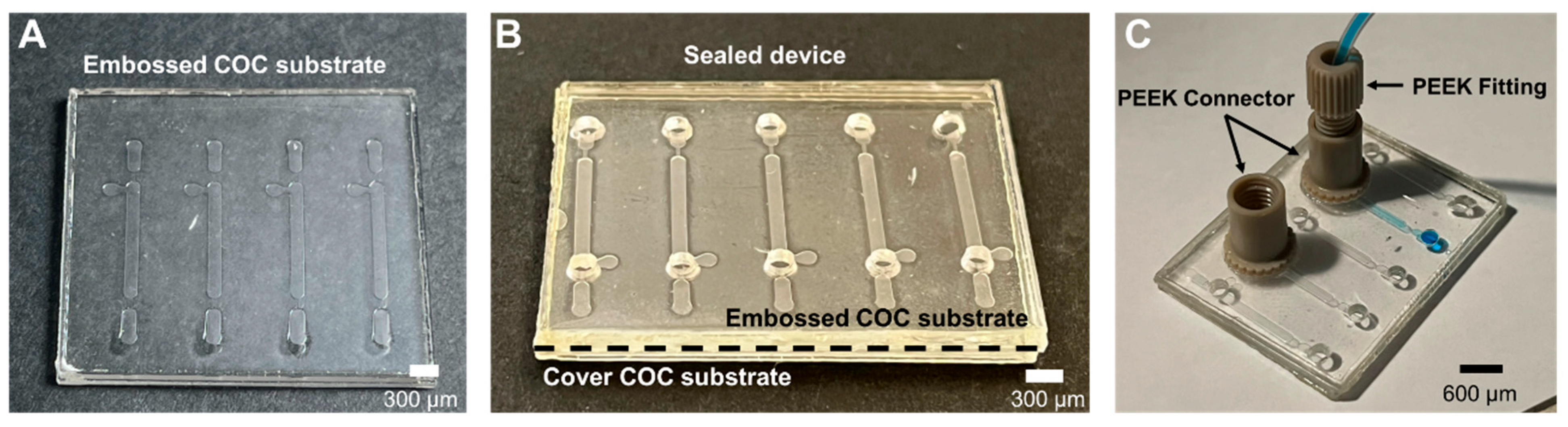
2.1.6. Sealing and Connections
2.2. Methods of Experimental Assays of COC Structures
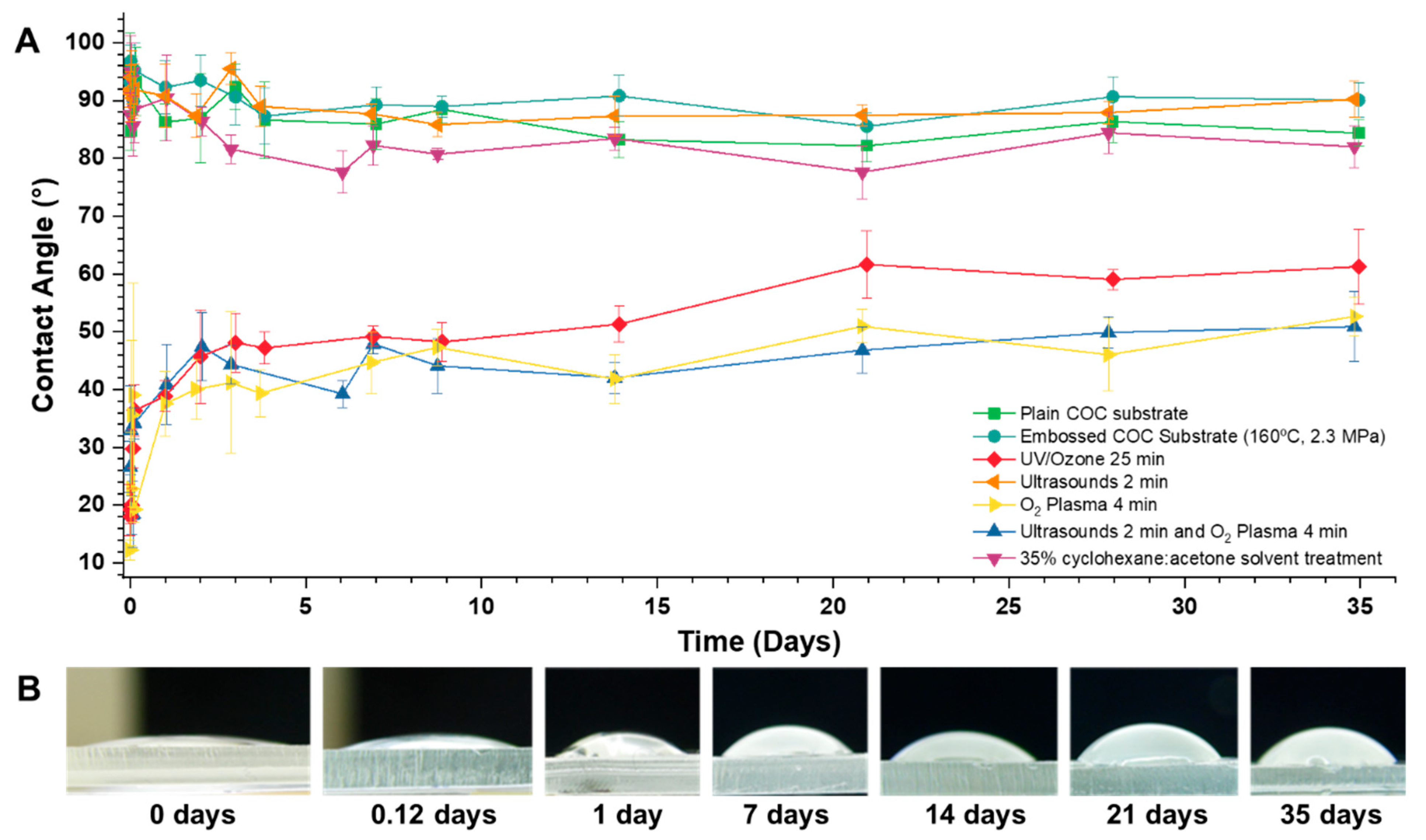
2.2.1. Hydrophilization Surface Treatments
2.2.2. Hydrophilization Surface Treatments—Contact Angle Measurements
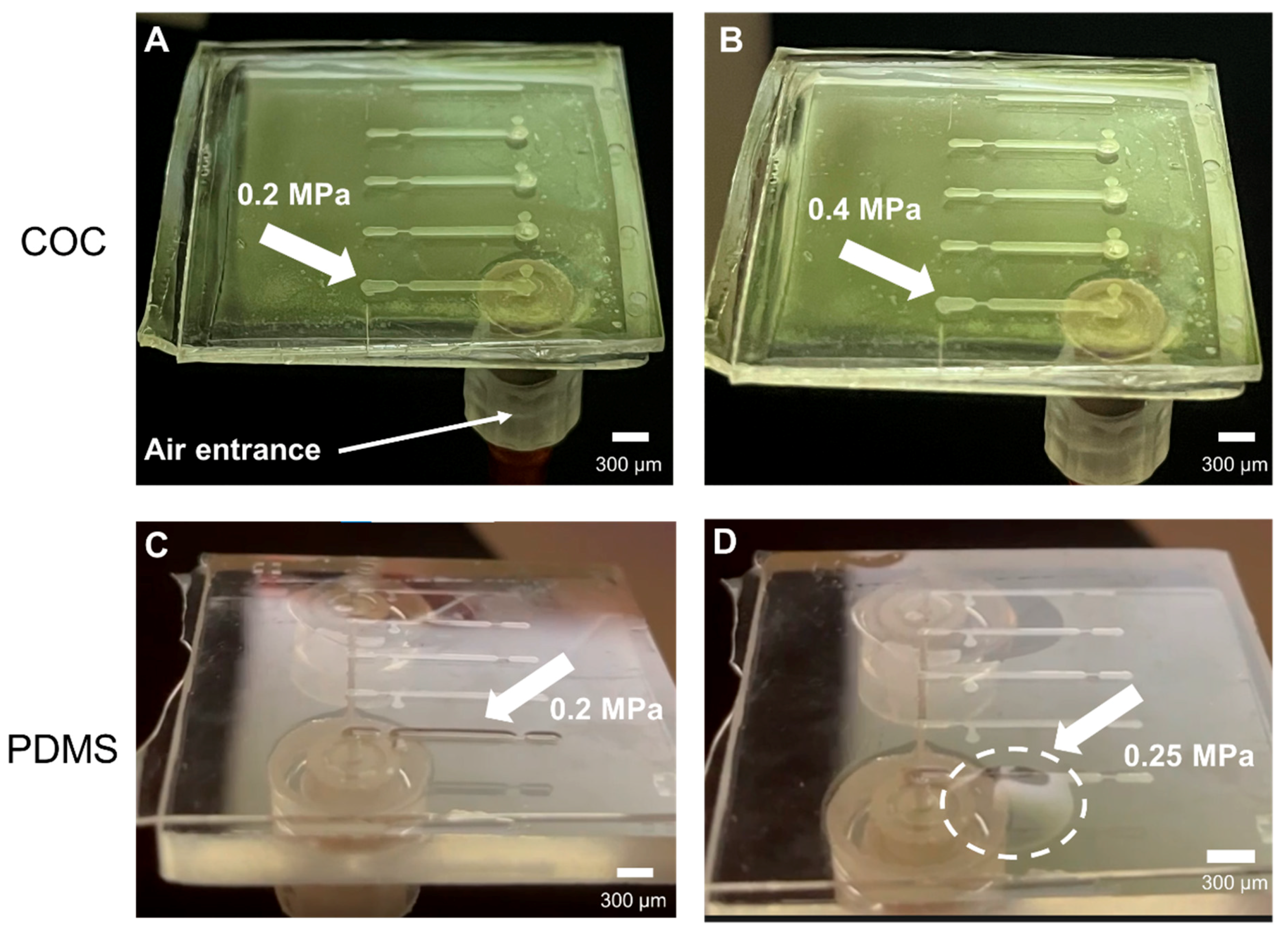
2.2.3. Burst Pressure Tests in Microfluidic Structures
2.2.4. Temperature Tolerance Tests
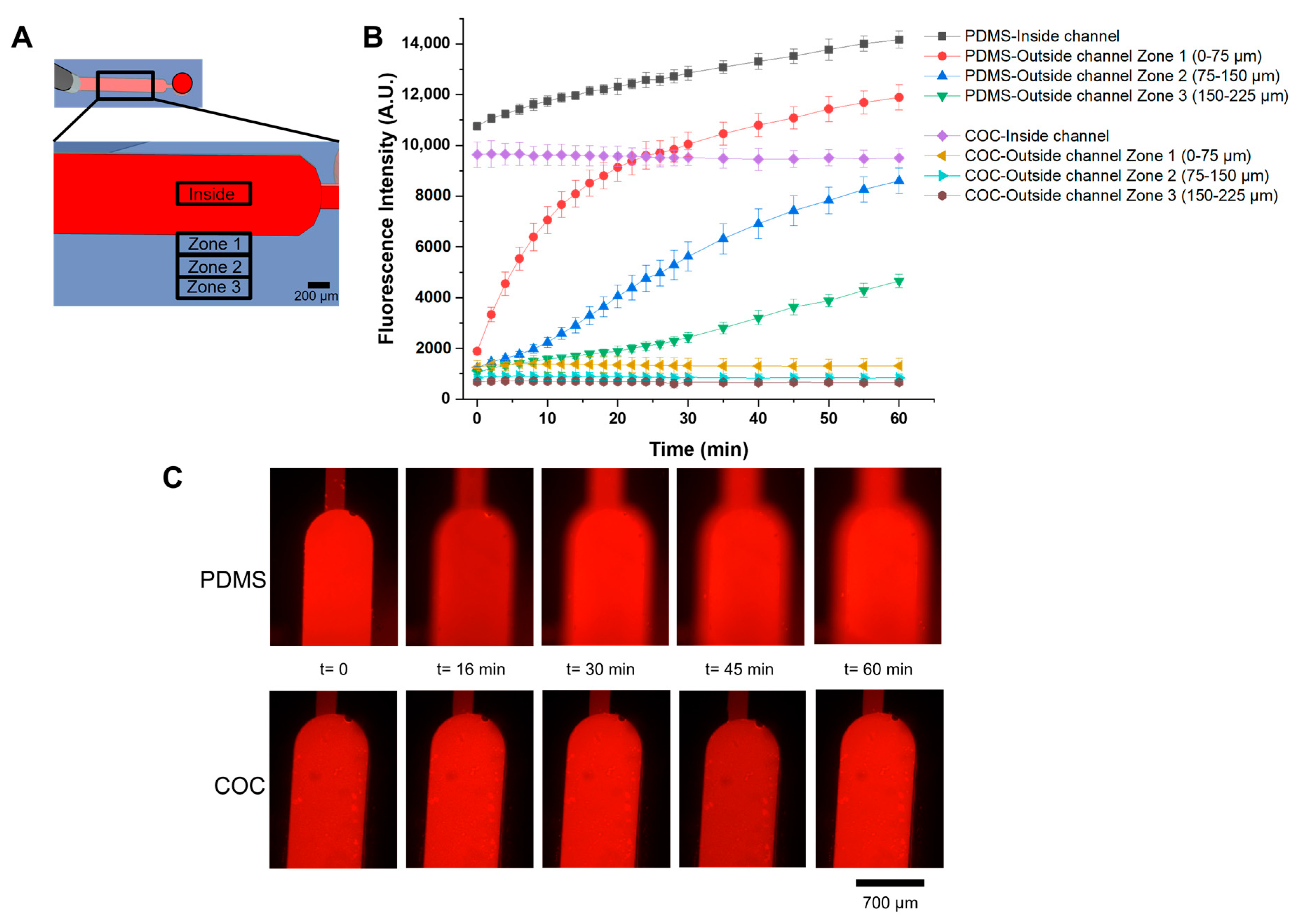
2.2.5. Molecular Diffusion Measurements
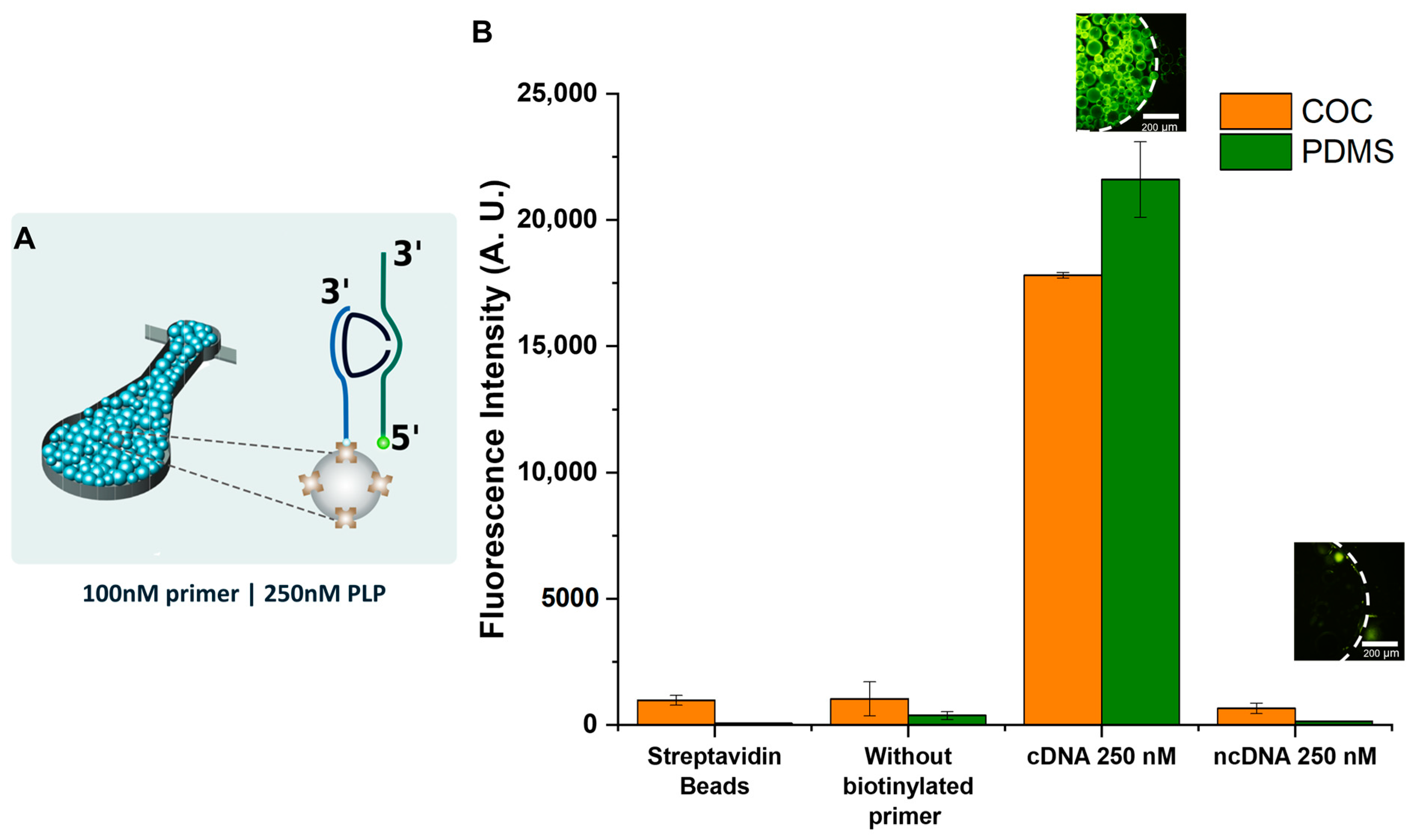
2.2.6. Proof-of-Concept Assay: DNA Capture on Streptavidin Beads and cDNA Hybridization
3. Results and Discussion
3.1. COC Structures Fabrication
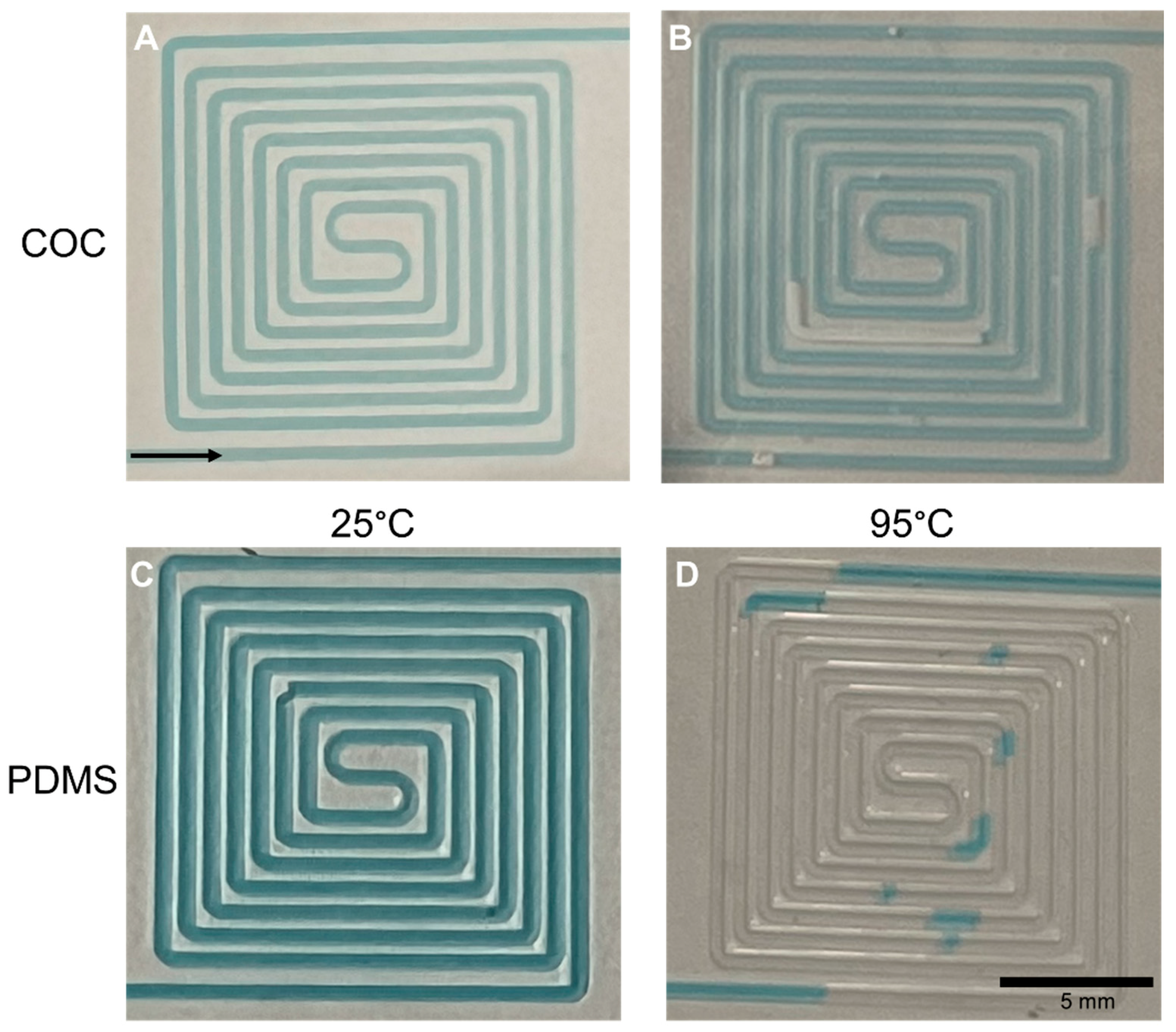
3.1.1. Embossing and De-embossing
3.1.2. Sealing
3.1.3. Connections
3.2. Hydrophilization Surface Treatments
3.3. Burst Pressure Tests in Microfluidic Structures
3.4. Temperature Tolerance Tests
3.5. Molecular Diffusion Measurements
3.6. Proof-of-Concept Assay: DNA Capture on Streptavidin Beads and cDNA Hybridization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, R.G. New Materials for Microfluidic Biochips—Thermoplastic COC. Master’s Thesis, Instituto Superior Técnico, Lisbon, Portugal, 2021. [Google Scholar]
- Convery, N.; Gadegaard, N. 30 Years of Microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Tarn, M.D.; Pamme, N. Microfluidics. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Barnett, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–7. ISBN 978-0-12-409547-2. [Google Scholar]
- Sheidaei, Z.; Akbarzadeh, P.; Kashaninejad, N. Advances in Numerical Approaches for Microfluidic Cell Analysis Platforms. J. Sci. Adv. Mater. Devices 2020, 5, 295–307. [Google Scholar] [CrossRef]
- Ortseifen, V.; Viefhues, M.; Wobbe, L.; Grünberger, A. Microfluidics for Biotechnology: Bridging Gaps to Foster Microfluidic Applications. Front. Bioeng. Biotechnol. 2020, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- Annabestani, M.; Esmaeili-Dokht, P.; Fardmanesh, M. A Novel, Low Cost, and Accessible Method for Rapid Fabrication of the Modifiable Microfluidic Devices. Sci. Rep. 2020, 10, 16513. [Google Scholar] [CrossRef]
- Kuo, J.S.; Chiu, D.T. Disposable Microfluidic Substrates: Transitioning from the Research Laboratory into the Clinic. Lab Chip 2011, 11, 2656. [Google Scholar] [CrossRef]
- Abgrall, P.; Gué, A.-M. Lab-on-Chip Technologies: Making a Microfluidic Network and Coupling It into a Complete Microsystem—A Review. J. Micromech. Microeng. 2007, 17, R15–R49. [Google Scholar] [CrossRef]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A Practical Guide for the Fabrication of Microfluidic Devices Using Glass and Silicon. Biomicrofluidics 2012, 6, 016505. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, L.; Xu, Y.; Zhong, J.; Abedini, A.; Cheng, X.; Sinton, D. Disposable Silicon-Glass Microfluidic Devices: Precise, Robust and Cheap. Lab Chip 2018, 18, 3872–3880. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A Practical Guide to Rapid-Prototyping of PDMS-Based Microfluidic Devices: A Tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- van Midwoud, P.M.; Janse, A.; Merema, M.T.; Groothuis, G.M.M.; Verpoorte, E. Comparison of Biocompatibility and Adsorption Properties of Different Plastics for Advanced Microfluidic Cell and Tissue Culture Models. Anal. Chem. 2012, 84, 3938–3944. [Google Scholar] [CrossRef]
- Paoli, R.; Di Giuseppe, D.; Badiola-Mateos, M.; Martinelli, E.; Lopez-Martinez, M.J.; Samitier, J. Rapid Manufacturing of Multilayered Microfluidic Devices for Organ on a Chip Applications. Sensors 2021, 21, 1382. [Google Scholar] [CrossRef]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-Injection Moulding of Polymer Microfluidic Devices. Microfluid. Nanofluidics 2009, 7, 1–28. [Google Scholar] [CrossRef]
- Ahn, C.H.; Choi, J.-W.; Beaucage, G.; Nevin, J.; Lee, J.-B.; Puntambekar, A.; Lee, R.J.Y. Disposable Smart Lab on a Chip for Point-of-Care Clinical Diagnostics. Proc. IEEE 2004, 92, 154–173. [Google Scholar] [CrossRef]
- Perez-Toralla, K.; Champ, J.; Mohamadi, M.R.; Braun, O.; Malaquin, L.; Viovy, J.-L.; Descroix, S. New Non-Covalent Strategies for Stable Surface Treatment of Thermoplastic Chips. Lab Chip 2013, 13, 4409. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Pourmand, A.; Nabavinia, M.; Avci, H.; Tamayol, A.; Mostafalu, P.; Ghavifekr, H.B.; Aghdam, E.N.; Dokmeci, M.R.; Khademhosseini, A.; et al. Rapid Prototyping of Whole-Thermoplastic Microfluidics with Built-in Microvalves Using Laser Ablation and Thermal Fusion Bonding. Sens. Actuators B Chem. 2018, 255, 100–109. [Google Scholar] [CrossRef]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic Olefin Copolymer Microfluidic Devices for Forensic Applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef]
- Aghvami, S.A.; Opathalage, A.; Zhang, Z.K.; Ludwig, M.; Heymann, M.; Norton, M.; Wilkins, N.; Fraden, S. Rapid Prototyping of Cyclic Olefin Copolymer (COC) Microfluidic Devices. Sens. Actuators B Chem. 2017, 247, 940–949. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Lam, Y.C.; Wang, Z.Y.; Hu, H. Surface Analysis, Hydrophilic Enhancement, Ageing Behavior and Flow in Plasma Modified Cyclic Olefin Copolymer (COC)-Based Microfluidic Devices. Sens. Actuators B Chem. 2010, 150, 537–549. [Google Scholar] [CrossRef]
- Steigert, J.; Haeberle, S.; Brenner, T.; Müller, C.; Steinert, C.P.; Koltay, P.; Gottschlich, N.; Reinecke, H.; Rühe, J.; Zengerle, R.; et al. Rapid Prototyping of Microfluidic Chips in COC. J. Micromech. Microeng. 2007, 17, 333–341. [Google Scholar] [CrossRef]
- Kameoka, J.; Craighead, H.G.; Zhang, H.; Henion, J. A Polymeric Microfluidic Chip for CE/MS Determination of Small Molecules. Anal. Chem. 2001, 73, 1935–1941. [Google Scholar] [CrossRef]
- Mela, P.; van den Berg, A.; Fintschenko, Y.; Cummings, E.B.; Simmons, B.A.; Kirby, B.J. The Zeta Potential of Cyclo-Olefin Polymer Microchannels and Its Effects on Insulative (Electrodeless) Dielectrophoresis Particle Trapping Devices. Electrophoresis 2005, 26, 1792–1799. [Google Scholar] [CrossRef]
- Jena, R.K.; Yue, C.Y.; Lam, Y.C. Micro Fabrication of Cyclic Olefin Copolymer (COC) Based Microfluidic Devices. Microsyst. Technol. 2012, 18, 159–166. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-Bond Axisymmetric Drop Shape Analysis for Surface Tension and Contact Angle Measurements of Sessile Drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Stalder, A.F.; Kulik, G.; Sage, D.; Barbieri, L.; Hoffmann, P. A Snake-Based Approach to Accurate Determination of Both Contact Points and Contact Angles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 286, 92–103. [Google Scholar] [CrossRef]
- Leech, P.W. Hot Embossing of Cyclic Olefin Copolymers. J. Micromech. Microeng. 2009, 19, 55008. [Google Scholar] [CrossRef]
- Kourmpetis, I.; Kastania, A.S.; Ellinas, K.; Tsougeni, K.; Baca, M.; De Malsche, W.; Gogolides, E. Gradient-Temperature Hot-Embossing for Dense Micropillar Array Fabrication on Thick Cyclo-Olefin Polymeric Plates: An Example of a Microfluidic Chromatography Column Fabrication. Micro Nano Eng. 2019, 5, 100042. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, J.; Gao, F.; Jia, G.; Zhuang, J.; Tang, G.; Fan, Y. Rapid Prototyping of Cyclic Olefin Copolymer Based Microfluidic System with CO2 Laser Ablation. Microsyst. Technol. 2017, 23, 5063–5069. [Google Scholar] [CrossRef]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low Temperature Bonding of PMMA and COC Microfluidic Substrates Using UV/Ozone Surface Treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Liga, A.; Morton, J.A.S.; Kersaudy-Kerhoas, M. Safe and Cost-Effective Rapid-Prototyping of Multilayer PMMA Microfluidic Devices. Microfluid. Nanofluidics 2016, 20, 164. [Google Scholar] [CrossRef]
- Roy, S.; Yue, C.Y.; Lam, Y.C. Influence of Plasma Surface Treatment on Thermal Bonding and Flow Behavior in Cyclic Olefin Copolymer (COC) Based Microfluidic Devices. Vacuum 2011, 85, 1102–1104. [Google Scholar] [CrossRef]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Keller, N.; Nargang, T.M.; Runck, M.; Kotz, F.; Striegel, A.; Sachsenheimer, K.; Klemm, D.; Länge, K.; Worgull, M.; Richter, C.; et al. Tacky Cyclic Olefin Copolymer: A Biocompatible Bonding Technique for the Fabrication of Microfluidic Channels in COC. Lab Chip 2016, 16, 1561–1564. [Google Scholar] [CrossRef]
- Šupová, M. Problem of Hydroxyapatite Dispersion in Polymer Matrices: A Review. J. Mater. Sci. Mater. Med. 2009, 20, 1201–1213. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary Microfluidics in Microchannels: From Microfluidic Networks to Capillaric Circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef]
- Temiz, Y.; Lovchik, R.D.; Kaigala, G.V.; Delamarche, E. Lab-on-a-Chip Devices: How to Close and Plug the Lab? Microelectron. Eng. 2015, 132, 156–175. [Google Scholar] [CrossRef]
- Mair, D.A.; Rolandi, M.; Snauko, M.; Noroski, R.; Svec, F.; Fréchet, J.M.J. Room-Temperature Bonding for Plastic High-Pressure Microfluidic Chips. Anal. Chem. 2007, 79, 5097–5102. [Google Scholar] [CrossRef]
- Liu, H.-B.; Gong, H.-Q.; Ramalingam, N.; Jiang, Y.; Dai, C.-C.; Hui, K.M. Micro Air Bubble Formation and Its Control during Polymerase Chain Reaction (PCR) in Polydimethylsiloxane (PDMS) Microreactors. J. Micromech. Microeng. 2007, 17, 2055–2064. [Google Scholar] [CrossRef]
- Wu, W.; Kang, K.T.; Lee, N.Y. Bubble-Free on-Chip Continuous-Flow Polymerase Chain Reaction: Concept and Application. Analyst 2011, 136, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Song, J.; Cho, B.; Hong, S.G.; Hoxha, O.; Kang, T.; Kim, D.; Lee, L.P. Bubble-Free Rapid Microfluidic PCR. Biosens. Bioelectron. 2019, 126, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.-F.; Hsu, M.-C. Preparation and Characterization of Cyclo Olefin Copolymer (COC)/Silica Nanoparticle Composites by Solution Blending. J. Polym. Res. 2007, 14, 373–378. [Google Scholar] [CrossRef]
- Femmer, T.; Kuehne, A.J.C.; Wessling, M. Print Your Own Membrane: Direct Rapid Prototyping of Polydimethylsiloxane. Lab Chip 2014, 14, 2610–2613. [Google Scholar] [CrossRef]
- Sønstevold, L.; Czerkies, M.; Escobedo-Cousin, E.; Blonski, S.; Vereshchagina, E. Application of Polymethylpentene, an Oxygen Permeable Thermoplastic, for Long-Term on-a-Chip Cell Culture and Organ-on-a-Chip Devices. Micromachines 2023, 14, 532. [Google Scholar] [CrossRef]
- Caneira, C.R.F.; Soares, R.R.G.; Pinto, I.F.; Mueller-Landau, H.S.; Azevedo, A.M.; Chu, V.; Conde, J.P. Development of a Rapid Bead-Based Microfluidic Platform for DNA Hybridization Using Single- and Multi-Mode Interactions for Probe Immobilization. Sens. Actuators B Chem. 2019, 286, 328–336. [Google Scholar] [CrossRef]
| COC Type | Tembossing | Pembossing | tembossing (min) | Bonding Lid | Bonding Method | Reference |
|---|---|---|---|---|---|---|
| Substrate—Topas, 1.5 mm thick, Tg = 142 °C | 160 °C | 2.3 Mpa | 10 | Substrate—Topas, 1.5 mm thick, Tg = 142 °C | Thermal bonding: 130 °C; 30 min; 2.3 Mpa | This paper |
| Substrate—Zeonor, 2 mm thick, Tg = 105 °C | 130 °C | 250 psi | 7 | Substrate—Zeonor, 2 mm thick, Tg = 105 °C | Thermal bonding: 85 °C; 10–15 min; 200 psi | [26] |
| Substrate—Zeonor, Tg = 100 °C and Tg = 102 °C | 125 °C | 3400 kg load | 10 | Substrate—Zeonor, Tg = 100 °C and Tg = 102 °C | Thermal bonding: 96 °C; 20 min; 2300 kg load | [27] |
| Substrate—Topas, Tg = 130 °C | Heated under vacuum up to 175 °C and embossed at 175 °C. | 3 kN | 5 | Composite foil made of Topas 8007 (Tg = 75 °C) and Topas 6013 (Tg = 130 °C) (annealed at 75 °C for 1 h) | Thermal bonding in a lamination machine: two cylinders heated up to 120 °C, with a pressure of 5 bar, and a feed rate of 30 cm/min. | [25] |
| Substrate—Topas, 1 mm thick, Tg = 160 °C | 170 °C | 2.94 kN | 3 | Substrate—Topas, 1 mm thick, Tg = 130 °C | Thermal bonding: 125 °C; 6 min; 0.5 kN | [28] |
| Substrate—Topas, 1 mm thick, Tg = 160 °C | 170 °C | 2.94 kPa | 1 h for annealing and 4 min for embossing. | Substrate—Topas, 1 mm thick, Tg = 160 °C | Thermal bonding: 150 °C, 160 °C, and 170 °C; 6 min; 2 Mpa. | [24] |
| Oligonucleotides | Sequence (5′-3′) | 5′mod | 3′mod |
|---|---|---|---|
| Primer | TTTTTTTTTTGTAAGACAC TATTACTGAGGA | Biotin | None |
| Padlock | TGCTTTGTTTCAGGTGTAG TGTATGCAGCTCCTCAGT AATAGTGTCTTACGGCAT CACTGGTTACGTCTGTCT CTACACCTTTTTTAGGA | PO4-phosphorilation | None |
| Complementary target ssDNA | TTAAATTAATGTACAAAGG TCAACCAATGACATTCAGA CTATTATTGGTTGATACAC CTGAAACAAAGCATCCTA AAAAAGGTGTAGAGA | Atto-430LS | None |
| Non-complementary target ssDNA | CGTGTCGTTCACATCTGT CCGT | Atto-430LS | None |
| Tembossing (°C) | Pembossing (MPa) | tembossing (min) | Tde-embossing (min) | Observations |
|---|---|---|---|---|
| 170 | 2.3 | 60 | 30 | Deformed COC structures and deformed epoxy mould |
| 160 | 2.3 | 5/10/20/30 | 15 | Successfully embossed COC structures at all times (98% height replication at 10 min) |
| 150 | 2.3 | 10/20/30 | 15 | Embossed COC structures with 60% height replication |
| 150 | 2.3 | 5 | 15 | Non-uniform embossing |
| 140 | 2.3 | 5/10/20/30 | 15 | No embossing observed |
| Tsealing (°C) | Psealing (MPa) | tsealing (min) | Observations |
|---|---|---|---|
| 150/160 | 2.3 | <10 | Unsealed structures with non-uniform strong bonds |
| 150/160 | 2.3 | >10 | Collapsed microchannels |
| 140 | 2.3 | 10 | Collapsed microchannels |
| 110/120 | 2.3 | 10 | Unsealed structures with non-uniform weak bonds, easy to delaminate |
| 135 | 2.3 | 10 | Collapsed microchannels |
| 130 | 2.3 | 10/20 | Sealed structure with uniform bond |
| 130 | 2.3 | 30 | Sealed structure with uniform bond and strong resistance to delamination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.G.; Condelipes, P.G.M.; Rosa, R.R.; Chu, V.; Conde, J.P. Scalable Processing of Cyclic Olefin Copolymer (COC) Microfluidic Biochips. Micromachines 2023, 14, 1837. https://doi.org/10.3390/mi14101837
Rodrigues RG, Condelipes PGM, Rosa RR, Chu V, Conde JP. Scalable Processing of Cyclic Olefin Copolymer (COC) Microfluidic Biochips. Micromachines. 2023; 14(10):1837. https://doi.org/10.3390/mi14101837
Chicago/Turabian StyleRodrigues, Rodolfo G., Pedro G. M. Condelipes, Rafaela R. Rosa, Virginia Chu, and João Pedro Conde. 2023. "Scalable Processing of Cyclic Olefin Copolymer (COC) Microfluidic Biochips" Micromachines 14, no. 10: 1837. https://doi.org/10.3390/mi14101837





