Efficient Cell Impedance Measurement by Dielectrophoretic Cell Accumulation and Evaluation of Chondrogenic Phenotypes
Abstract
1. Introduction
2. Materials and Methods
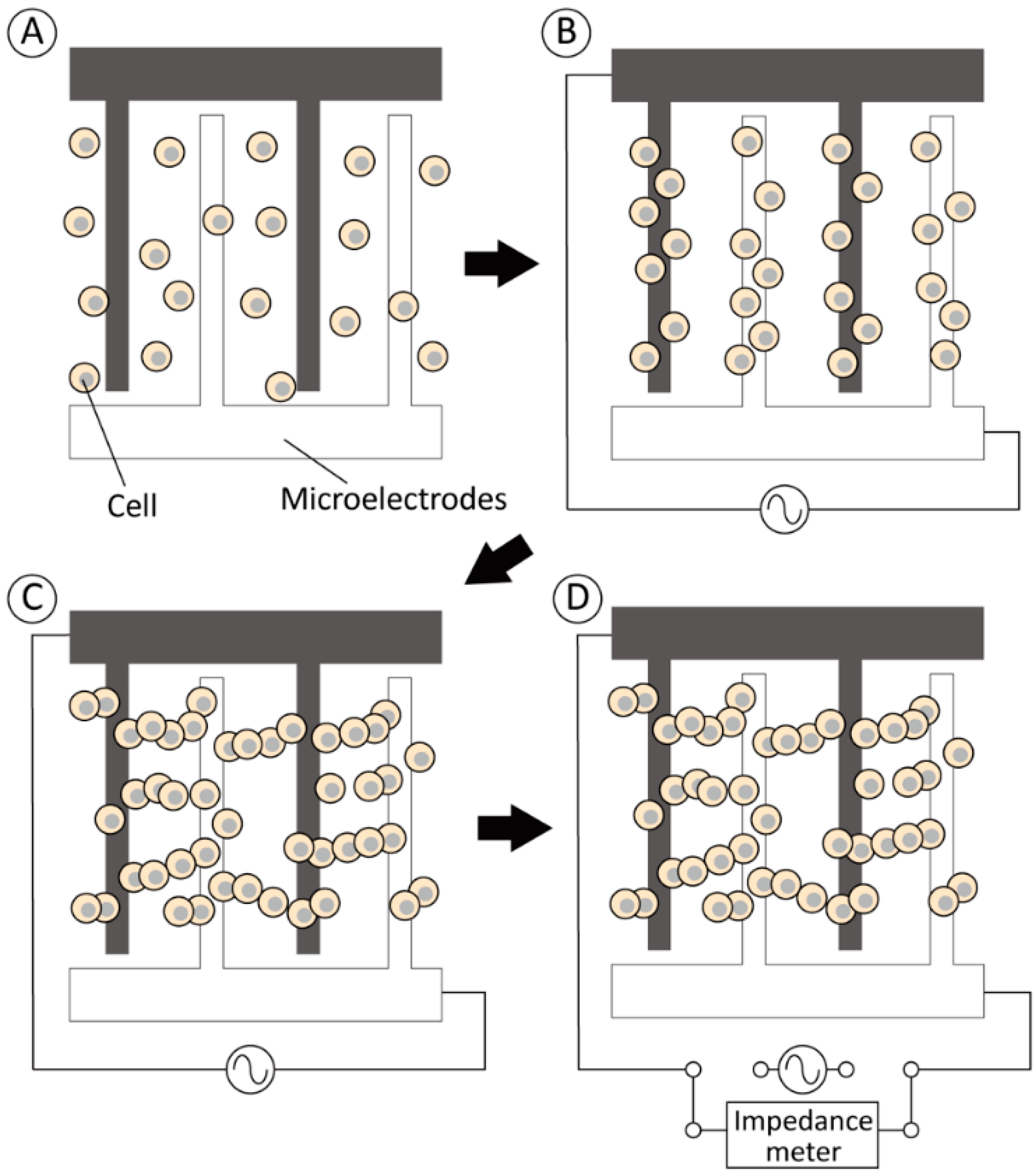
2.1. Experimental Design to Improve the Sensitivity of Electrical Imedance Measurement by Dielectrohpretic Cell Acccumulation
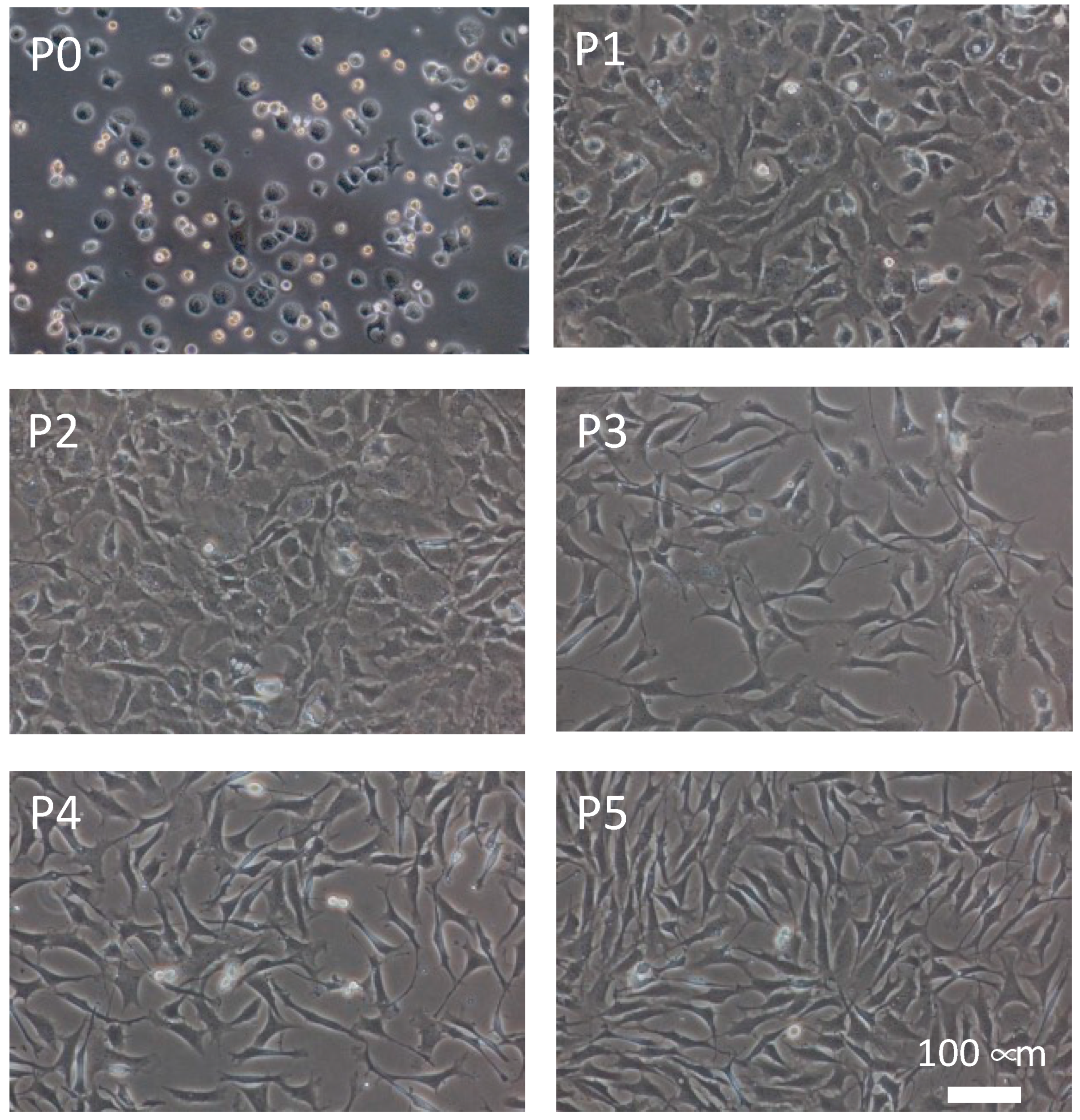
2.2. Chondrocyte Isolation and Culture with Multiple Passages
2.3. Electrical Impedance Measurement Device for Living Cells Supported by Dielectrohpretic Cell Accumulation
2.4. Determination of the Experimental Conditions of the Electrical Impedance Measurement and Dielectrophoresis
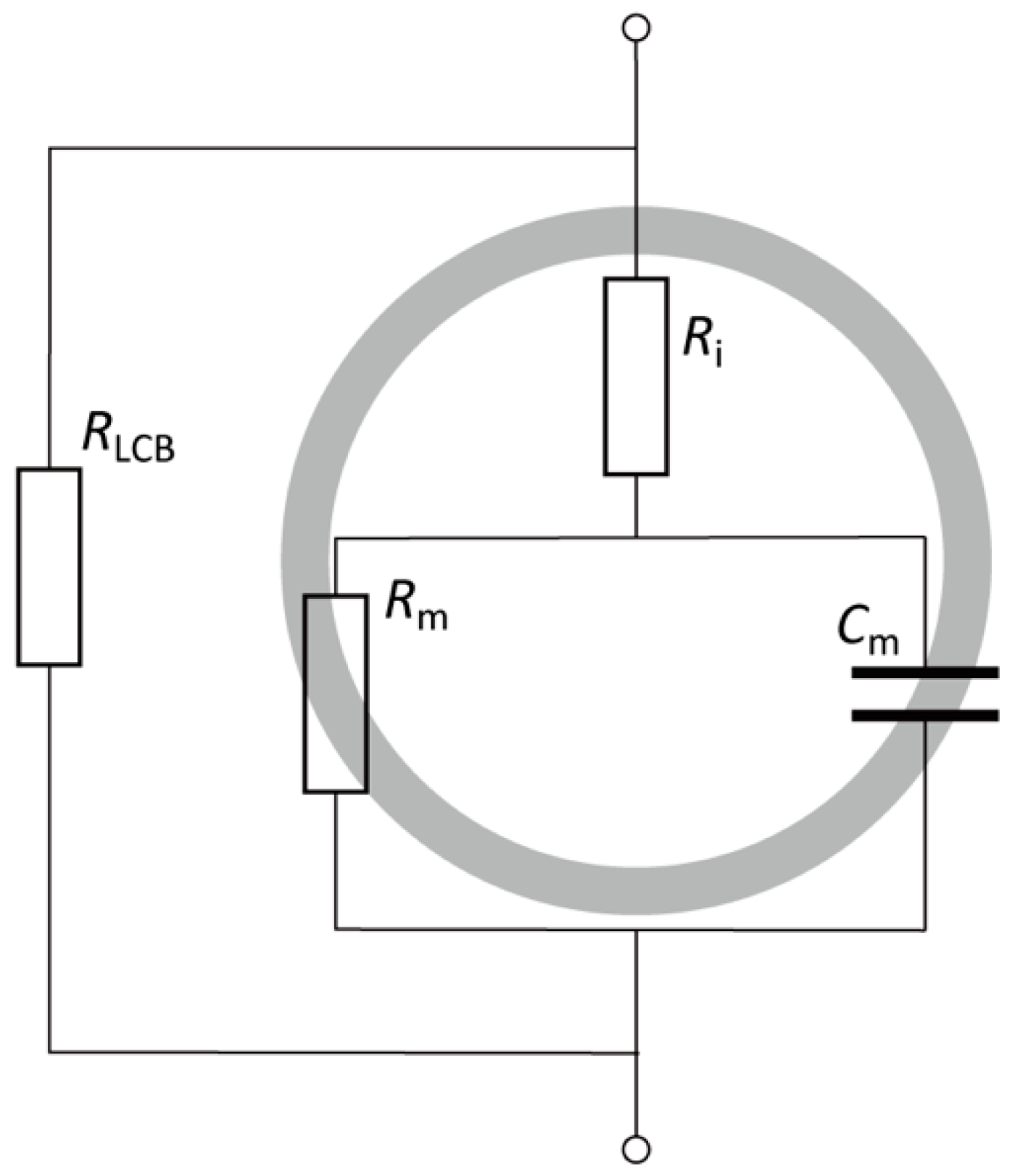
2.5. Characterization of the Relationship between Electrical Impedance and the De-Differentiation Process of Chondrocytes
2.6. Statistical Analysis
3. Results and Discussions
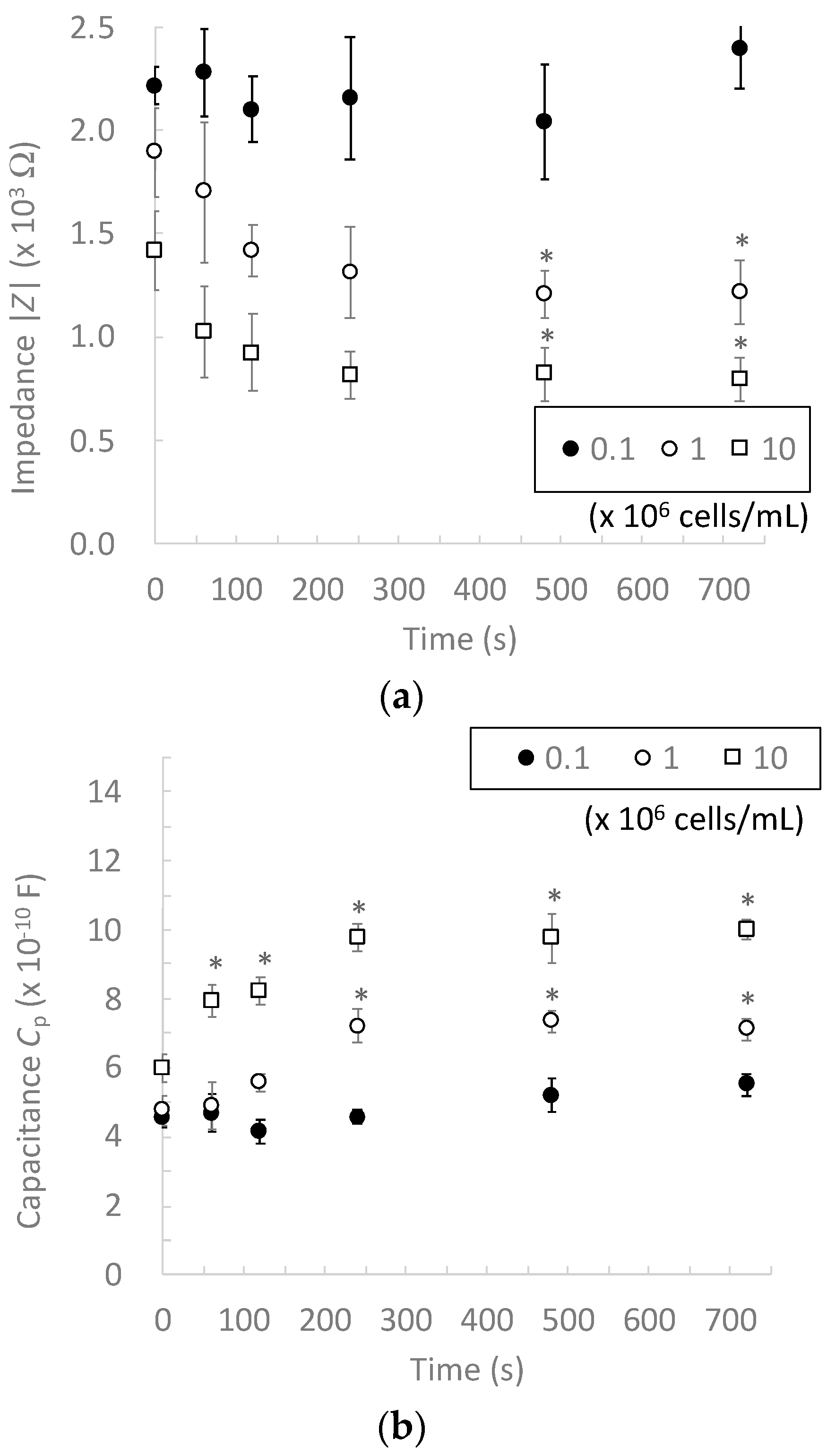
3.1. Effect of Cell Concentration on Impedance Measurement
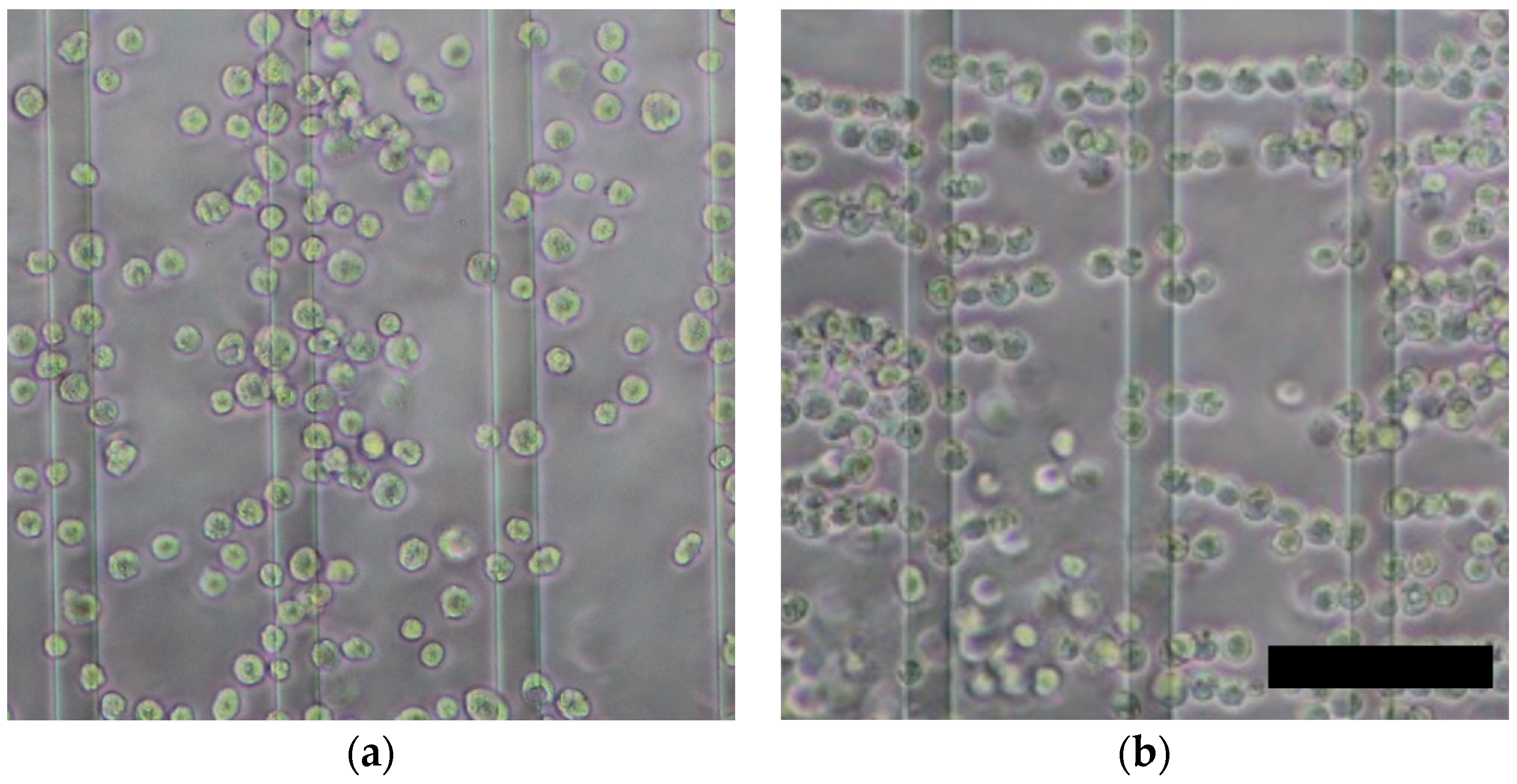
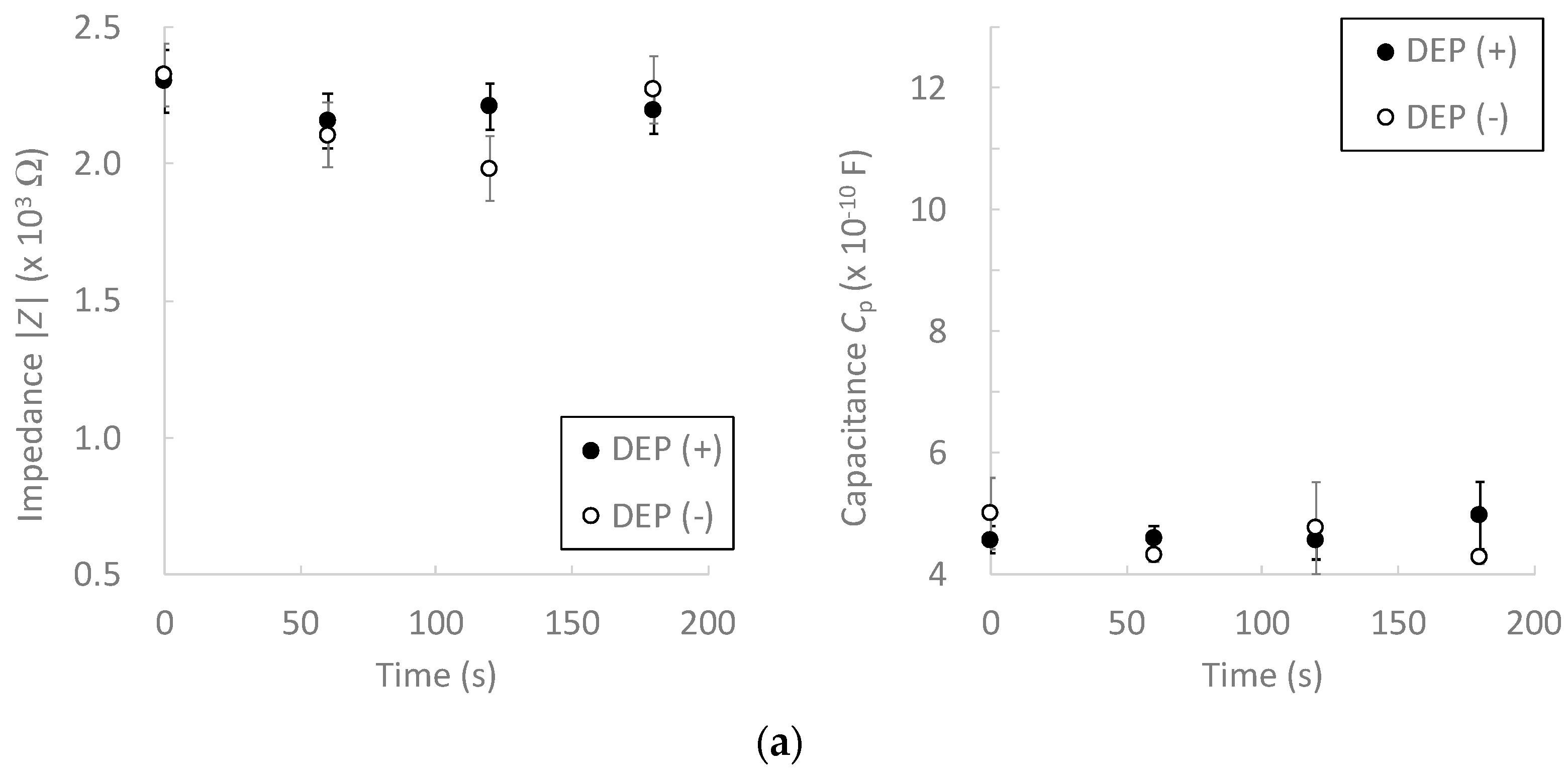
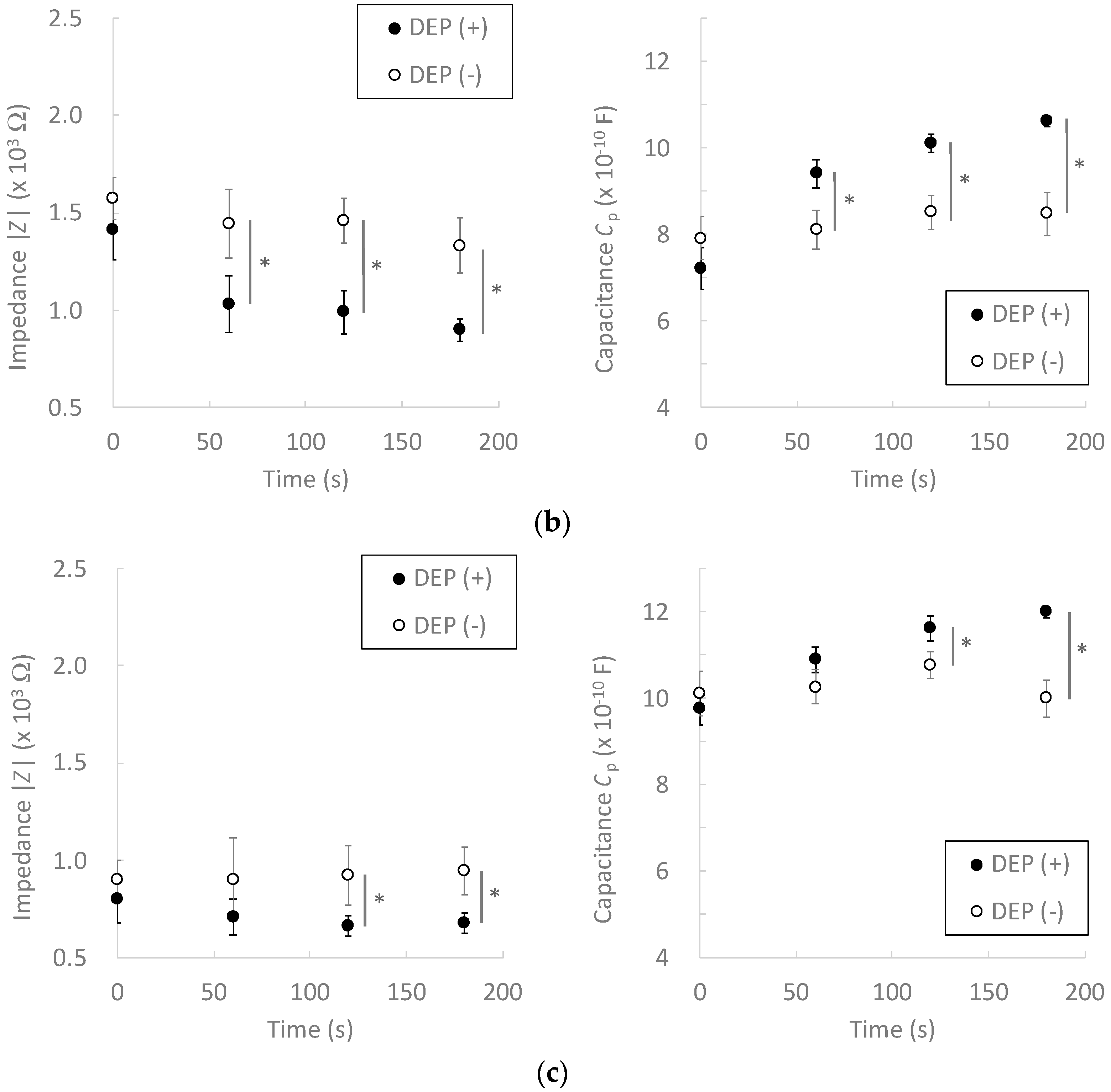
3.2. Effect of Dielectrophoretic Cell Accumulation on Impedance Measurement
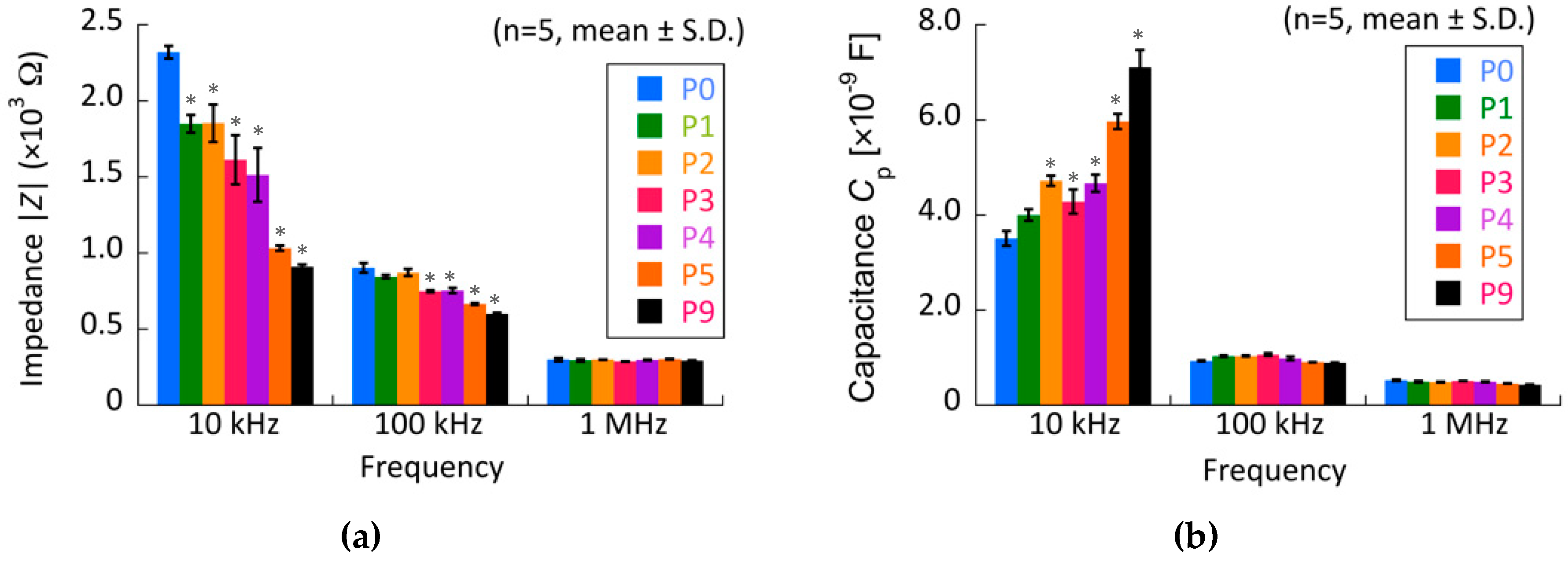
3.3. Relationship between Electrical Impedance and the Phenotypes of Chondrocytes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab A Chip 2001, 1, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sabounchi, P.; Morales, A.M.; Ponce, P.; Lee, L.P.; Simmons, B.A.; Davalos, R.V. Sample concentration and impedance detection on a microfluidic polymer chip. Biomed. Microdevices 2008, 10, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yang, D.; Zhou, Y.; Liang, M.; Ai, Y. Multi-frequency single cell electrical impedance measurement for label-free cell viability analysis. Analyst 2021, 146, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Maji, D.; An, R.; Ahuja, S.P.; Little, J.A.; Suster, M.A.; Mohseni, P.; Gurkan, U.A. Microfluidic electrical impedance assessment of red blood cell mediated microvascular occlusion. Lab A Chip 2021, 21, 1036. [Google Scholar] [CrossRef] [PubMed]
- David, F.; Hebeisen, M.; Schade, G.; Franco-Lara, E.; Di Berardino, M. Viability and membrane potential analysis of Bacillus megaterium cells by impedance flow cytometry. Biotechnol. Bioeng. 2012, 109, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Jendrusch, M.; Constantinou, I. Spatially resolved electrical impedance methods for cell and particle characterization. Electrophoresis 2020, 41, 65–80. [Google Scholar] [CrossRef]
- Schoendube, J.; Wright, D.; Zengerle, R.; Koltay, P. Single-cell printing based on impedance detection. Biomicrofluidics 2015, 9, 014117. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, L.; Zhao, P.; Liang, F.; Wang, W. A Microfluidic Device Integrating Impedance Flow Cytometry and Electric Impedance Spectroscopy for High-Efficiency Single-Cell Electrical Property Measurement. Anal. Chem. 2019, 91, 15204–15212. [Google Scholar] [CrossRef]
- Rosenthal, A.; Voldman, J. Dielectrophoretic traps for single-particle patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Yang, Y.-W.; Chen, Y.-D.; Wang, S.-S.; Chang, Y.-H.; Wu, M.-H. The application of an optically switched dielectrophoretic (ODEP) force for the manipulation and assembly of cell-encapsulating alginate microbeads in a microfluidic perfusion cell culture system for bottom-up tissue engineering. Lab A Chip 2012, 12, 1164–1173. [Google Scholar] [CrossRef]
- Menad, S.; Franqueville, L.; Haddour, N.; Buret, F.; Frenea-Robin, M. nDEP-driven cell patterning and bottom-up construction of cell aggregates using a new bioelectronic chip. Acta Biomater. 2015, 17, 107–114. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyata, S. Continuous ES/feeder cell-sorting device using dielectrophoresis and controlled fluid flow. Micromachines 2020, 11, 734. [Google Scholar] [CrossRef]
- Miyata, S.; Komatsu, Y. Phenotypic analysis of differentiated and de-differentiated chondrocytes based on dielectrophoretic properties. Jap. J. Clin. Biomech. 2011, 32, 21–26. (In Japanese) [Google Scholar]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Albrecht, D.R.; Tsang, V.L.; Sah, R.L.; Bhatia, S.N. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab A Chip 2005, 5, 111–118. [Google Scholar] [CrossRef]
- Albrecht, D.R.; Underhill, G.H.; Wassermann, T.B.; Sah, R.L.; Bhatia, S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods 2006, 3, 369–375. [Google Scholar] [CrossRef]
- Albrecht, D.R.; Underhill, G.H.; Mendelson, A.; Bhatia, S.N. Multiphase electropatterning of cells and biomaterials. Lab A Chip 2007, 7, 702–709. [Google Scholar] [CrossRef]
- Suzuki, M.; Yasukawa, T.; Mase, Y.; Oyamatsu, D.; Shiku, H.; Matsue, T. Dielectrophoretic micropatterning with microparticle monolayers covalently linked to glass surfaces. Langmuir 2004, 20, 11005–11011. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Miyata, S. Multi-layered cell assembling technology using dielectrophoresis and construction of skin tissue microelement. Trans. JSME 2017, 83, 16-00387. (In Japanese) [Google Scholar] [CrossRef]
- Takeuchi, Y.; Miyata, S. Micro cell patterning technology by dielectrophoresis and application to regenerated cartilage. Trans. JSME Part C 2010, 76, 3015–3020. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Takeuchi, Y.; Miyata, S. Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue. Micromachines 2021, 12, 1098. [Google Scholar] [CrossRef]
- Kretschmer, R.; Fritzsche, W. Pearl chain formation of nanoparticles in microelectrode gaps by dielectrophoresis. Langmuir 2004, 20, 11797–11801. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Hunziker, E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 1995, 108, 1497–1508. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef]
- Mizuno, S.; Tateishi, T.; Ushida, T.; Glowacki, J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J. Cell. Physiol. 2002, 193, 319–327. [Google Scholar] [CrossRef]
- Kawanishi, M.; Oura, A.; Furukawa, K.; Fukubayashi, T.; Nakamura, K.; Tateishi, T.; Ushida, T. Redifferentiation of dedifferentiated bovine articular chondrocytes enhanced by cyclic hydrostatic pressure under a gas-controlled system. Tissue Eng. 2007, 13, 957–964. [Google Scholar] [CrossRef]
- Miyata, S.; Numano, T.; Homma, K.; Tateishi, T.; Ushida, T. Feasibility of noninvasive evaluation of biophysical properties of tissue-engineered cartilage by using quantitative MRI. J. Biomech. 2007, 40, 2990–2998. [Google Scholar] [CrossRef]
- Qiao, G.; Wang, W.; Duan, W.; Zheng, F.; Sinclair, A.J.; Chatwin, C.R. Bioimpedance analysis for the characterization of breast cancer cells in suspension. IEEE Trans. Biomed. Eng. 2012, 59, 2321–2329. [Google Scholar] [CrossRef]
- Jiménez, G.; López-Ruiz, E.; Kwiatkowski, W.; Montañez, E.; Arrebola, F.; Carrillo, E.; Gray, P.C.; Belmonte, J.C.I.; Choe, S.; Perán, M.; et al. Activin A/BMP2 chimera AB235 drives efficient redifferentiation of long term cultured autologous chondrocytes. Sci. Rep. 2015, 5, 16400. [Google Scholar] [CrossRef]
- Wozniak, M.J.; Kawazoe, N.; Tateishi, T.; Chen, G. Monitoring of mechanical properties of serially passaged bovine articular chondrocytes by atomic force microscopy. Micron 2009, 40, 870–875. [Google Scholar] [CrossRef]
- Sliogeryte, K.; Botto, L.; Lee, D.A.; Knight, M.M. Chondrocyte dedifferentiation increases cell stiffness by strengthening membrane-actin adhesion. Osteoarthr. Cartil. 2016, 24, 912–920. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, N.; Ishibashi, Y.; Miyata, S. Efficient Cell Impedance Measurement by Dielectrophoretic Cell Accumulation and Evaluation of Chondrogenic Phenotypes. Micromachines 2022, 13, 837. https://doi.org/10.3390/mi13060837
Nakata N, Ishibashi Y, Miyata S. Efficient Cell Impedance Measurement by Dielectrophoretic Cell Accumulation and Evaluation of Chondrogenic Phenotypes. Micromachines. 2022; 13(6):837. https://doi.org/10.3390/mi13060837
Chicago/Turabian StyleNakata, Natsumi, Yuko Ishibashi, and Shogo Miyata. 2022. "Efficient Cell Impedance Measurement by Dielectrophoretic Cell Accumulation and Evaluation of Chondrogenic Phenotypes" Micromachines 13, no. 6: 837. https://doi.org/10.3390/mi13060837
APA StyleNakata, N., Ishibashi, Y., & Miyata, S. (2022). Efficient Cell Impedance Measurement by Dielectrophoretic Cell Accumulation and Evaluation of Chondrogenic Phenotypes. Micromachines, 13(6), 837. https://doi.org/10.3390/mi13060837





