A Study on TiO2 Surface Texturing Effect for the Enhancement of Photocatalytic Reaction in a Total Phosphorous Concentration Measurement System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanism of Photocatalytic Reaction
2O2− + 2H− → H2O2 + O2
H2O2 + O2− → OH·+ OH− + O2
2h− + 2H2O → 2H+ + H2O2
H2O2 + O2− → OH·+ OH− + O2
2.2. Characteristics of TiO2
2.3. Total Phosphorus Analysis through Photocatalytic Reaction
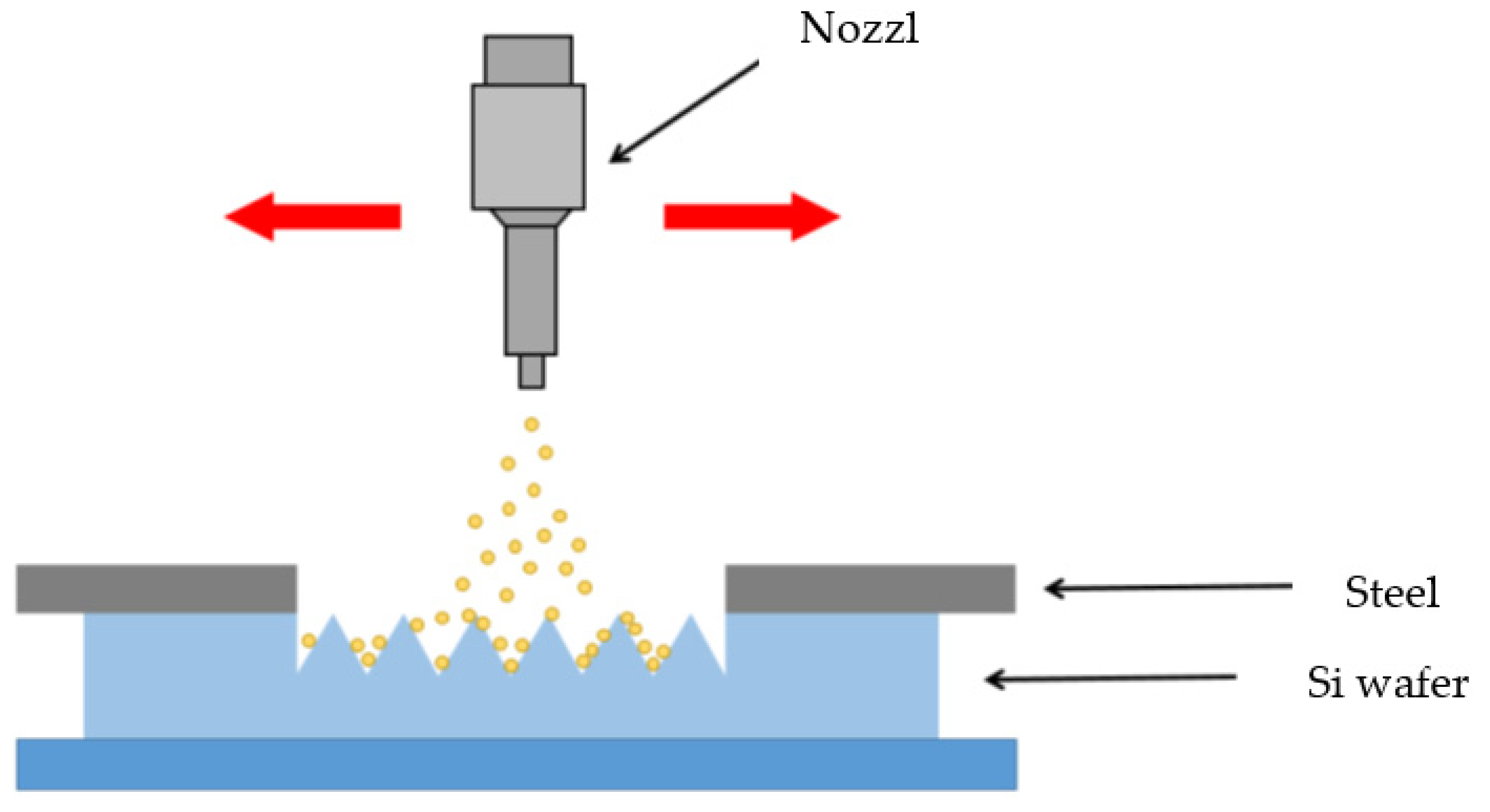
2.4. Sandblasting
2.5. Absorbance
3. Results
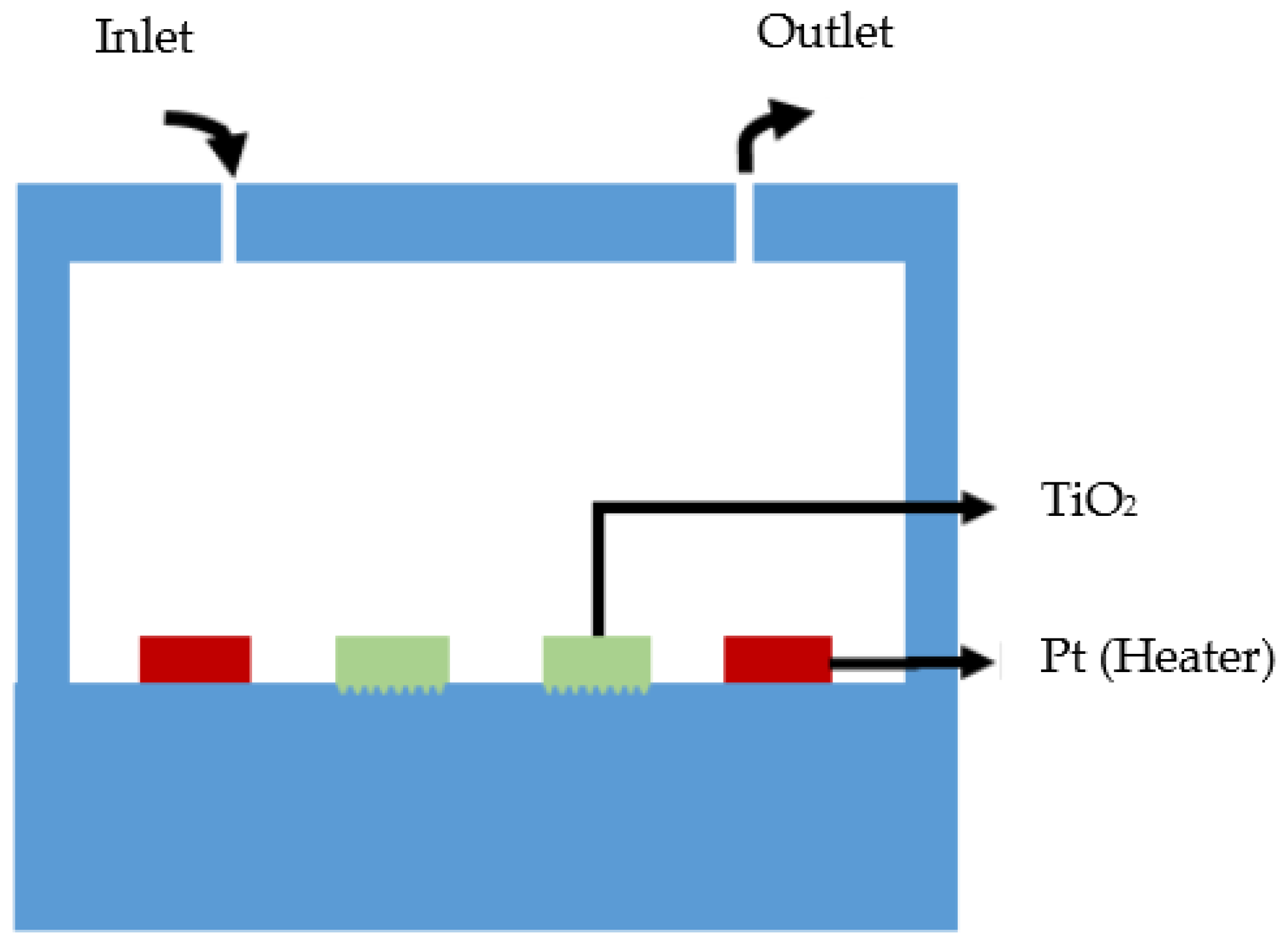
3.1. Design
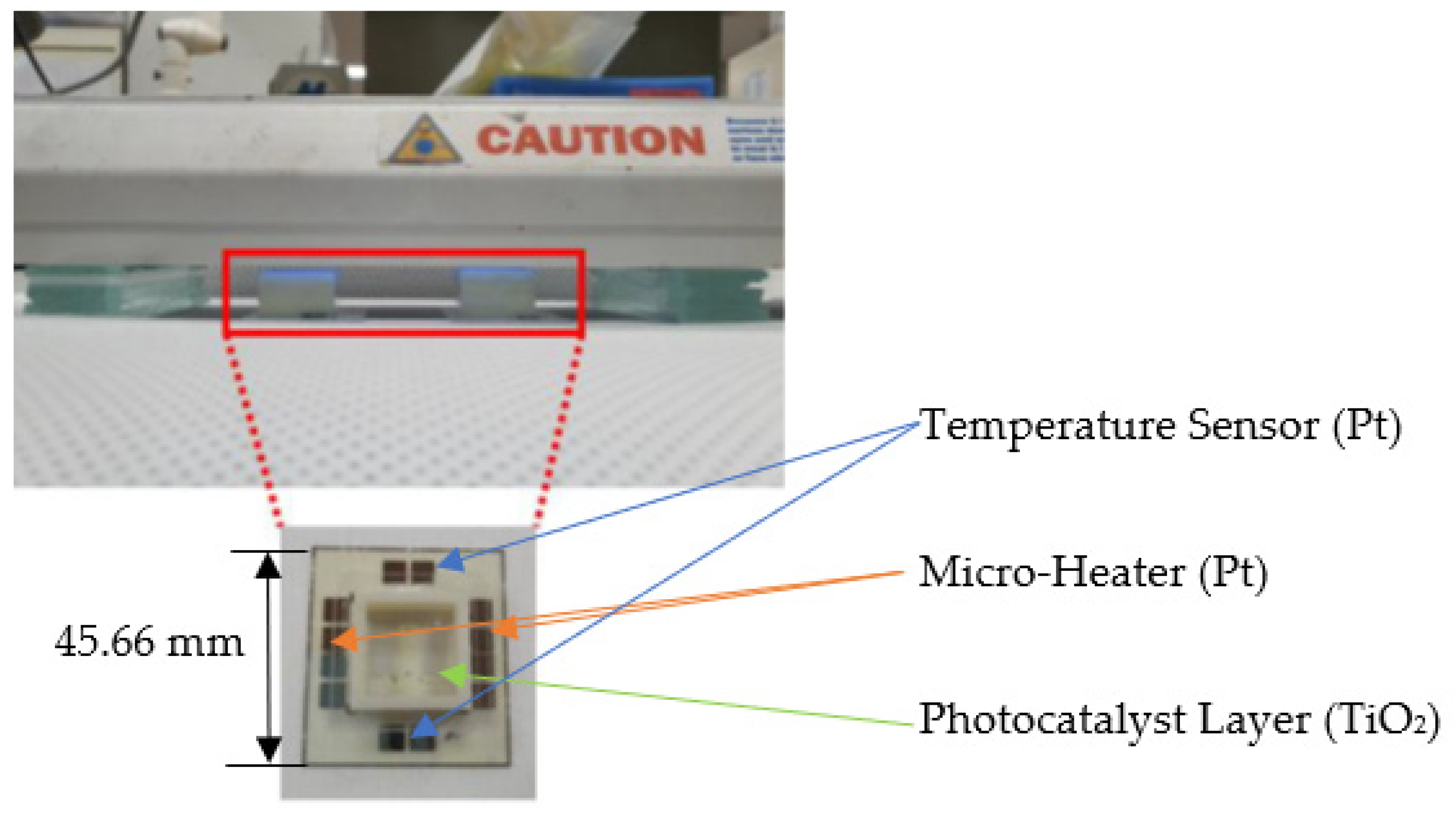
3.2. Fabrication of Total Phosphorus Monitoring Device
3.3. Texturing Process on the Wafer Surface
4. Discussion
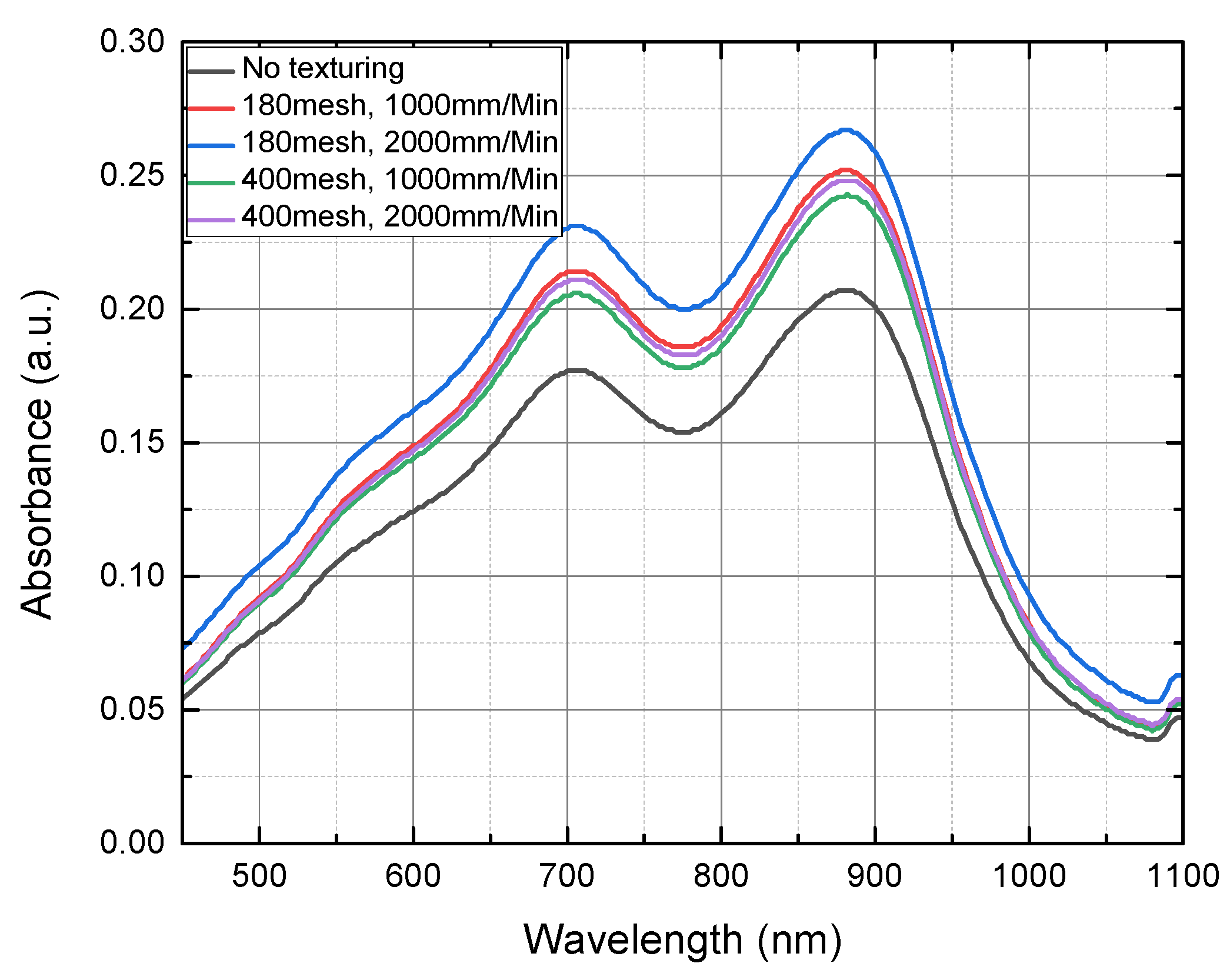
4.1. Analysis of Surface Roughness
4.2. Measurement Result for the Concentration of Total Phosphorus
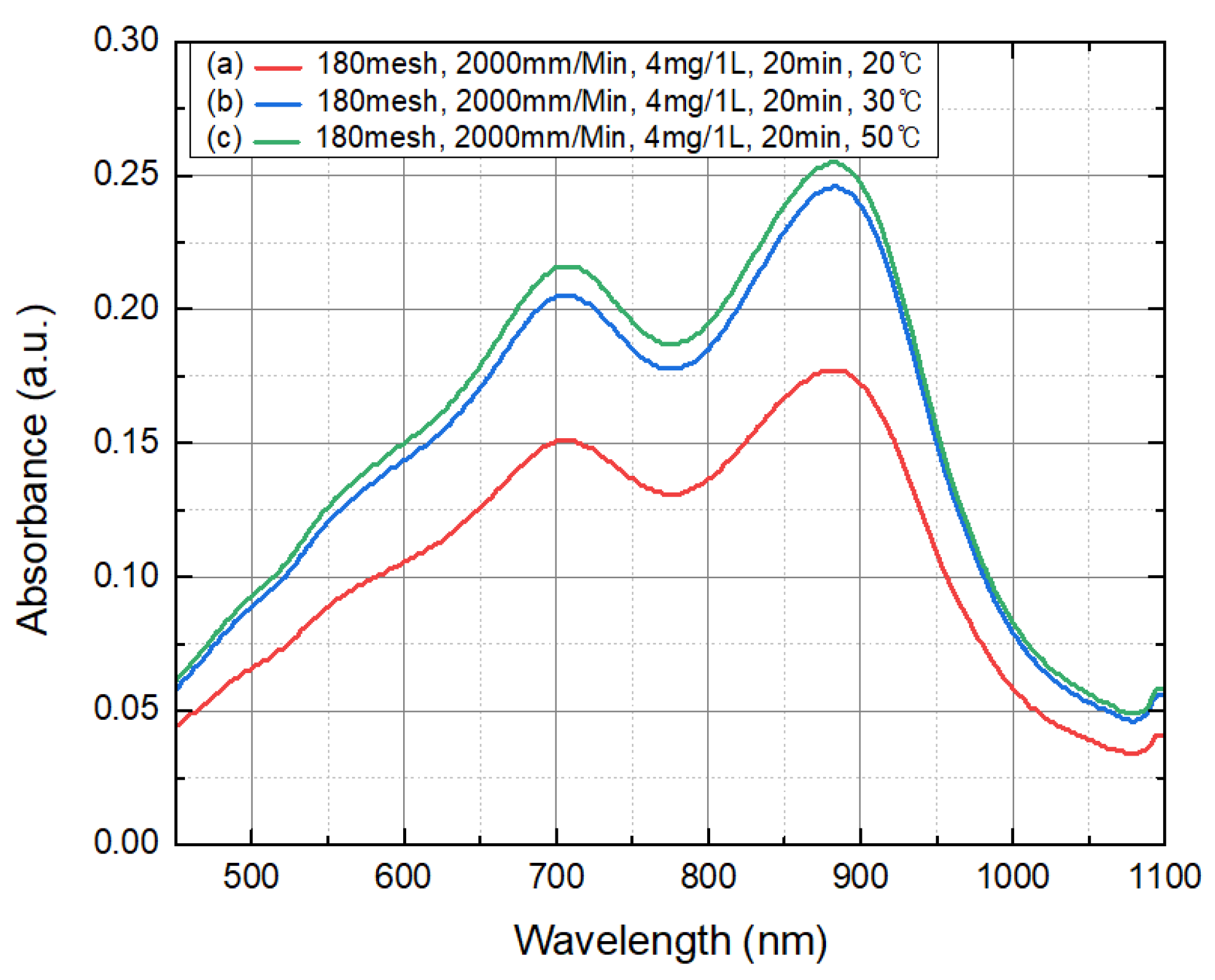
4.3. Absorbance Changes by Temperature
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural phosphorus and eutrophication: A symposium overview. J. Environ. Qual. 1998, 27, 251. [Google Scholar] [CrossRef] [Green Version]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, phosphorus, and eutrophication in streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Carpenter, S.R. Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Song, C.M.; Kim, J.S. Applicability evaluation of the hydrological image and convolution neural network for prediction of the biochemical oxygen demand and total phosphorus loads in agricultural areas. Agriculture 2020, 10, 529. [Google Scholar] [CrossRef]
- Shuvo, A.; O’Reilly, C.M.; Blagrave, K.; Ewins, C.; Filazzola, A.; Gray, D.; Mahdiyan, O.; Moslenko, L.; Quinlan, R.; Sharma, S. Total phosphorus and climate are equally important predictors of water quality in lakes. Aquat. Sci. 2021, 83, 1–11. [Google Scholar] [CrossRef]
- Schilling, K.E.; Streeter, M.T.; Seeman, A.; Jones, C.S.; Wolter, C.F. Total phosphorus export from Iowa agricultural watersheds: Quantifying the scope and scale of a regional condition. J. Hydrol. 2020, 581, 124397. [Google Scholar] [CrossRef]
- Jung, D.G.; Han, M.; Kim, S.D.; Kwon, S.Y.; Kwon, J.B.; Lee, J.; Kong, S.H.; Jung, D. Miniaturized Portable Total Phosphorus Analysis Device Based on Photocatalytic Reaction for the Prevention of Eutrophication. Micromachines 2021, 12, 1062. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Jung, D.G.; Jung, D.; Kong, S.H. Lab-on-a-chip based total-phosphorus analysis device utilizing a photocatalytic reaction. Solid-State Electron. 2018, 140, 100–108. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes–current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar]
- O’Shea, K.E.; Dionysiou, D.D. Advanced oxidation processes for water treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Shieh, D.L.; Lin, Y.S.; Yeh, J.H.; Chen, S.C.; Lin, B.C.; Lin, J.L. N-doped, porous TiO2 with rutile phase and visible light sensitive photocatalytic activity. Chem. Commun. 2012, 48, 2528–2530. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Julkapli, N.M. Mixed-phase TiO2 photocatalysis: Correlation between phase composition and photodecomposition of water pollutants. Rev. Inorg. Chem. 2017, 37, 11–28. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Triani, G.; Sorrell, C.C. Morphology and photocatalytic activity of highly oriented mixed phase titanium dioxide thin films. Surf. Coat. Technol. 2011, 205, 3658–3664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. 2008, 120, 1790–1793. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, K.; Wang, H.; Zhang, W.; Chu, P.K. Influence of structure parameters and crystalline phase on the photocatalytic activity of TiO2 nanotube arrays. J. Nanosci. Nanotechnol. 2011, 11, 11200–11205. [Google Scholar] [CrossRef]
- Yi, J.; Bahrini, C.; Schoemaecker, C.; Fittschen, C.; Choi, W. Photocatalytic decomposition of H2O2 on different TiO2 surfaces along with the concurrent generation of HO2 radicals monitored using cavity ring down spectroscopy. J. Phys. Chem. C 2012, 116, 10090–10097. [Google Scholar] [CrossRef]
- Barakat, M.A.; Tseng, J.M.; Huang, C.P. Hydrogen peroxide-assisted photocatalytic oxidation of phenolic compounds. Appl. Catal. B Environ. 2005, 59, 99–104. [Google Scholar] [CrossRef]
- Ullah, S.; Adeel, M.; Zain, M.; Rizwan, M.; Irshad, M.K.; Jilani, G.; Hameed, A.; Khan, A.; Arshad, M.; Raza, A.; et al. Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: A full life cycle study. J. Environ. Manag. 2020, 263, 110365. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B Environ. 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Alves, S.A.; Rossi, A.L.; Ribeiro, A.R.; Werckmann, J.; Celis, J.P.; Rocha, L.A.; Shokuhfar, T. A first insight on the bio-functionalization mechanisms of TiO2 nanotubes with calcium, phosphorous and zinc by reverse polarization anodization. Surf. Coat. Technol. 2017, 324, 153–166. [Google Scholar] [CrossRef]
- Lapina, O.B.; Shubin, A.A.; Nosov, A.V.; Bosch, E.; Spengler, J.; Knözinger, H. Characterization of V2O5−TiO2 catalysts prepared by milling by ESR and solid state 1H and 51V NMR. J. Phys. Chem. B 1999, 103, 7599–7606. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Kopidakis, N.; Schiff, E.A.; Park, N.G.; Van de Lagemaat, J.; Frank, A.J. Ambipolar diffusion of photocarriers in electrolyte-filled, nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination pathways in the Degussa P25 formulation of TiO2: Surface versus lattice mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef]
- Cho, C.-S.; Bae, I.-S.; Lee, J.-H. Etching Characteristics of Micro Blaster for MEMS Applications. J. Sens. Sci. Technol. 2011, 20, 187–192. [Google Scholar] [CrossRef]
- Swinehart, D.F. The beer-lambert law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Calloway, D. Beer-lambert law. J. Chem. Educ. 1997, 74, 744. [Google Scholar] [CrossRef] [Green Version]
- Mäntele, W.; Deniz, E. UV–VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.G.; Jung, D.; Kong, S.H. Characterization of Total-Phosphorus (TP) Pretreatment Microfluidic Chip Based on a Thermally Enhanced Photocatalyst for Portable Analysis of Eutrophication. Sensors 2019, 19, 3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Nozzle Speed | Ra (μm) | Ramean (μm) | ||
|---|---|---|---|---|
| (mm/min) | 1 | 2 | 3 | |
| 1000 | 1.461 | 1.345 | 1.400 | 1.402 |
| 2000 | 1.470 | 1.340 | 1.442 | 1.417 |
| Nozzle Speed | Ra (μm) | Ramean (μm) | ||
|---|---|---|---|---|
| (mm/min) | 1 | 2 | 3 | |
| 1000 | 2.613 | 2.509 | 2.688 | 2.603 |
| 2000 | 2.793 | 2.657 | 2.403 | 2.617 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.K.; Kim, S.D.; Lee, J.Y.; Kim, C.H.; Lee, H.-S.; Koo, S.M.; Lee, Y.; Paik, J.-H.; Kim, D.Y.; Kong, S.H. A Study on TiO2 Surface Texturing Effect for the Enhancement of Photocatalytic Reaction in a Total Phosphorous Concentration Measurement System. Micromachines 2021, 12, 1163. https://doi.org/10.3390/mi12101163
Kim JK, Kim SD, Lee JY, Kim CH, Lee H-S, Koo SM, Lee Y, Paik J-H, Kim DY, Kong SH. A Study on TiO2 Surface Texturing Effect for the Enhancement of Photocatalytic Reaction in a Total Phosphorous Concentration Measurement System. Micromachines. 2021; 12(10):1163. https://doi.org/10.3390/mi12101163
Chicago/Turabian StyleKim, Jae Keon, Seung Deok Kim, Jae Yong Lee, Chang Hee Kim, Hyeon-Su Lee, Seong Mo Koo, YoungJin Lee, Jong-Hoo Paik, Da Ye Kim, and Seong Ho Kong. 2021. "A Study on TiO2 Surface Texturing Effect for the Enhancement of Photocatalytic Reaction in a Total Phosphorous Concentration Measurement System" Micromachines 12, no. 10: 1163. https://doi.org/10.3390/mi12101163





